NGF/TrkA轴对宫颈癌SiHa细胞增殖、凋亡和侵袭的影响
2023-11-08龙颖黄芳依韦有生
龙颖 黄芳依 韦有生
摘要:目的 探討神经生长因子(NGF)/原肌球蛋白受体激酶A(TrkA)轴对宫颈癌SiHa细胞增殖、凋亡和侵袭的影响。方法 体外培养正常宫颈上皮细胞HUCEC和宫颈癌细胞SiHa,将SiHa细胞分为对照组(正常培养)、L-NGF组(50 μg/L重组人NGF蛋白)、H-NGF组(100 μg/L重组人NGF蛋白)、H-NGF+L-K252a组(100 μg/L重组人NGF蛋白+50 μg/L K252a)、H-NGF+H-K252a组(100 μg/L重组人NGF蛋白+100 μg/L K252a)。Western blot检测NGF、TrkA、E-钙黏蛋白(E-cadherin)、N-钙黏蛋白(N-cadherin)、波形蛋白(Vimentin)表达;CCK-8法检测SiHa细胞增殖情况;流式细胞术检测SiHa细胞凋亡;划痕愈合实验测定细胞迁移;Transwell试验测定细胞侵袭。结果 SiHa细胞较HUCEC细胞NGF、TrkA水平升高(P<0.01)。与对照组相比,L-NGF组、H-NGF组NGF、TrkA水平,增殖活力,迁移率,侵袭细胞数量以及N-cadherin、Vimentin蛋白水平显著增加,凋亡率、E-cadherin蛋白水平显著下降(P<0.05),且H-NGF组较L-NGF组以上指标差异更显著(P<0.05);与H-NGF组相比,H-NGF+L-K252a组、H-NGF+H-K252a组NGF、TrkA水平,增殖活力,迁移率,侵袭细胞数量以及N-cadherin、Vimentin蛋白水平显著降低,凋亡率、E-cadherin蛋白水平显著升高(P<0.05),且H-NGF+H-K252a组较H-NGF+L-K252a组以上指标差异更显著(P<0.05)。结论 下调NGF/TrkA轴可以抑制宫颈癌SiHa细胞增殖、侵袭,促进其凋亡。
关键词:神经生长因子;受体,TrkA;宫颈肿瘤;细胞增殖;细胞凋亡;肿瘤浸润
中图分类号:R737.3,R349.54文献标志码:ADOI:10.11958/20230318
Impacts of NGF/TrkA axis on proliferation, apoptosis and invasion of cervical cancer SiHa cells
LONG Ying, HUANG Fangyi, WEI Yousheng
Department of Gynecology, Affiliated Cancer Hospital of Guangxi Medical University, Nanning 530021, China
Abstract: Objective To investigate the impact of nerve growth factor (NGF)/tropomyosin receptor kinase A (TrkA) axis on the proliferation, apoptosis and invasion of cervical cancer SiHa cells. Methods Normal cervical epithelial cells HUCEC and cervical cancer cells SiHa were cultured in vitro. SiHa cells were grouped into the control group (normal culture), the L-NGF group (50 μg/L recombinant human NGF protein), the H-NGF group (100 μg/L recombinant human NGF protein), the H-NGF+L-K252a group (100 μg/L recombinant human NGF protein+50 μg/L K252a) and H-NGF+H-K252a group (100 μg/L recombinant human NGF protein+100 μg/L K252a). The protein expression levels of NGF, TrkA, E-cadherin, N-cadherin and Vimentin were detected by Western blot assay. The proliferation of SiHa cells was detected by CCK-8 method. Apoptosis of SiHa cells was detected by flow cytometry. Cell migration was determined by scratch healing test. Cell invasion was measured by Transwell assay. Results The levels of NGF and TrkA were obviously higher in SiHa cells than those in HUCEC cells (P<0.01). Compared with the control group, levels of NGF and TrkA, proliferative activity, migration rate, number of invasive cells and N-cadherin and Vimentin proteins were obviously higher in the L-NGF group and the H-NGF group (P<0.05), and the apoptosis rate and the level of E-cadherin protein were obviously lower (P<0.05). The above indexes were more obviously different in the H-NGF group than those of the L-NGF group (P<0.05). Compared with the H-NGF group, levels of NGF, TrkA, proliferative activity, migration rate, number of invasive cells and N-cadherin and Vimentin proteins were obviously lower in the H-NGF+L-K252a group and the H-NGF+H-K252a group (P<0.05), and the apoptosis rate and the level of E-cadherin protein were obviously higher (P<0.05). The above indexes in the H-NGF+H-K252a group were more obviously different than those in the H-NGF+L-K252a group (P<0.05). Conclusion Down-regulation of NGF/TrkA axis can inhibit the proliferation and invasion of cervical cancer SiHa cells and promote their apoptosis.
Key words: nerve growth factor; receptor, TrkA; uterine cervical neoplasms; cell proliferation; cell apoptosis; neoplasm invasiveness
宫颈癌是常见的妇科恶性肿瘤之一,严重威胁女性健康[1]。多数宫颈癌是由人乳头瘤病毒(HPV)感染引起的[2]。此外,转移和复发是宫颈癌相关死亡的主要原因,远处转移患者预后较差[3]。因此,明确宫颈癌进展和转移机制并从中找到治疗该病新的生物标志物和治疗靶点至关重要。神经生长因子(NGF)及其高亲和力受体原肌球蛋白受体激酶A(TrkA)是研究癌症的常见通路。Marsland等[4]发现,下调NGF/TrkA轴可抑制黑色素瘤的进展。Gao等[5]也发现NGF和TrkA蛋白水平在肺鳞状细胞癌患者中增加,下调NGF/TrkA轴可抑制癌细胞的增殖。近期,Faulkner等[6]发现,NGF和TrkA在宫颈鳞状细胞癌中过表达。但是,关于NGF/TrkA轴对宫颈癌细胞恶性生物学行为影响的研究鲜有报道,本研究旨在探讨可否通过下调NGF/TrkA轴抑制宫颈癌SiHa细胞的恶性生物学行为。
1 材料与方法
1.1 细胞及主要材料
正常宫颈上皮细胞HUCEC购自深圳市豪地华拓生物科技有限公司;宫颈癌SiHa细胞购自上海雅吉生物科技有限公司;NGF/TrkA轴抑制剂K252a购自无锡云萃生物科技有限公司;兔源NGF一抗、TrkA一抗、E-钙黏蛋白(E-cadherin)一抗、N-钙黏蛋白(N-cadherin)一抗、波形蛋白(Vimentin)一抗、辣根过氧化物酶(HRP)标记的山羊抗兔IgG二抗购自Abcam;重组人NGF蛋白购自伊艾博(武汉)科技股份有限公司;CCK-8细胞增殖及毒性检测试剂盒购自北京索莱宝科技有限公司;膜联蛋白V(Annexin V)-异硫氰酸荧光素(FITC)/碘化丙啶(PI)细胞凋亡试剂盒购自杭州联科生物技术股份有限公司。
1.2 细胞培养及分组
将HUCEC和SiHa用DMEM高糖培养基(含10%胎牛血清、1 000 U/mL氨苄青霉素、100 g/L卡那霉素)在37 ℃、5%CO2细胞培养箱中培养。每2 d用胰酶消化传代。
将SiHa细胞分为对照组(正常培养)、L-NGF组(50 μg/L重组人NGF蛋白)、H-NGF组(100 μg/L重组人NGF蛋白)、H-NGF+L-K252a组(100 μg/L重组人NGF蛋白+50 μg/L K252a)、H-NGF+H-K252a组(100 μg/L重组人NGF蛋白+100 μg/L K252a)。处理24 h后用于后续实验。
1.3 Western blot检测上皮-间充质转化(EMT)相关蛋白表达
通过RIPA裂解液提取总蛋白。二辛可宁酸(BCA)蛋白质检测试剂盒检测蛋白质浓度。将等量的总蛋白质经十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE),然后转移到聚偏二氟乙烯(PVDF)膜。用5%脱脂奶粉在37 ℃下封闭PVDF膜1 h。通过与一抗NGF(1︰1 000)、TrkA(1︰2 000)、E-cadherin(1︰2 000)、N-cadherin(1︰2 000)、Vimentin(1︰1 000)、GAPDH(1︰1 000)在4 ℃下孵育过夜。次日将膜与二抗在常温下继续反应1 h。最后加入电化学发光试剂显色。GAPDH作为内参,并通过Image J软件对蛋白质条带进行灰度分析。
1.4 CCK-8法检测SiHa细胞增殖情况
将各组SiHa细胞以每孔5 × 103个细胞的密度接种在96孔培养板中,每组设3个复孔。孵育4、24、48、72、96、120 h后,向每个孔中加入10 μL CCK-8溶液。在37 ℃孵育2 h后,使用酶标仪在450 nm波长处测量每孔的光密度(OD)。
1.5 流式细胞术检测SiHa細胞凋亡
将每组SiHa细胞收集到离心管中,磷酸盐缓冲液(PBS)洗涤细胞2次后,500 μL Bing Buffer重悬细胞。加入5 μL Annexin V-FITC和5 μL PI,并在室温避光条件下孵育15 min,使用流式细胞仪检测细胞凋亡率。
1.6 划痕愈合实验测定SiHa细胞迁移
当各组SiHa细胞在6孔板中生长达到80%~90%时,使用无菌200 μL移液器吸头在细胞中制造划痕,然后用饥饿培养基洗涤以去除未贴壁的细胞。培养24 h后,用光学显微镜对划痕进行成像,计算细胞迁移率。迁移率=(0 h划痕宽度?24 h划痕宽度)/0 h划痕宽度×100%。
1.7 Transwell实验测定SiHa细胞侵袭
Transwell室预包被基质胶(在培养基中稀释至1 g/L)。SiHa细胞在无血清培养基中重悬后,将6×104个/mL细胞接种在上室,并将600 μL含有15%胎牛血清的培养基加入下室。培养24 h,用棉签擦拭Transwell小室上表面。将Transwell下室中的细胞在甲醇中固定30 min,0.1%结晶紫染色20 min,然后用PBS洗涤。在显微镜下观察染成紫色细胞的数量并进行记录,即为侵袭细胞的数量。
1.8 统计学方法
采用SPSS 25.0软件处理数据。数据经正态分布、方差齐性检验后以均数±标准差(x±s)表示,2组间均数比较采用独立样本t检验,多组间比较用单因素方差分析,组间多重比较用SNK-q检验,P<0.05为差异有统计学意义。
2 结果
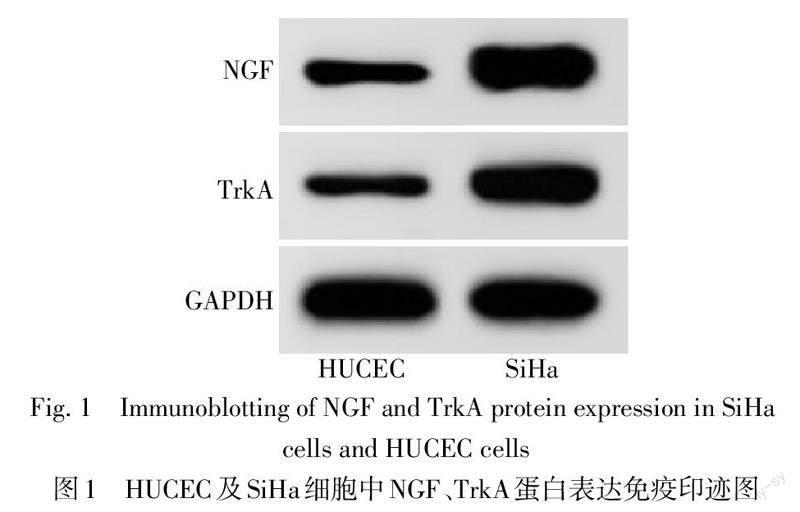
2.1 NGF、TrkA在HUCEC和SiHa细胞中的表达
SiHa細胞较HUCEC细胞NGF、TrkA水平升高(P<0.01),见图1、表1。
2.2 各组SiHa细胞NGF、TrkA蛋白表达水平比较
与对照组相比,L-NGF组、H-NGF组NGF、TrkA水平上调,且H-NGF组NGF、TrkA水平高于L-NGF组(P<0.05);与H-NGF组相比,H-NGF+L-K252a组、H-NGF+H-K252a组NGF、TrkA水平下降,且H-NGF+H-K252a组下降更显著(P<0.05),见表2、图2。
2.3 各组SiHa细胞增殖活力比较
与对照组相比,L-NGF组、H-NGF组24、48、72、96和120 h OD450增加(P<0.05),且H-NGF组OD450值高于L-NGF组(P<0.05);与H-NGF组相比,H-NGF+L-K252a组、H-NGF+H-K252a组24 h、48 h、72 h、96 h、120 h OD450值下降(P<0.05),且H-NGF+H-K252a组下降更显著(P<0.05),见表3。
2.4 各组SiHa细胞凋亡率比较
与对照组相比,L-NGF组、H-NGF组凋亡率下降,且H-NGF组凋亡率低于L-NGF组(P<0.05);与H-NGF组相比,H-NGF+L-K252a组、H-NGF+H-K252a组凋亡率升高,且H-NGF+H-K252a组较H-NGF+L-K252a组上升更显著(P<0.05),见图3、4。
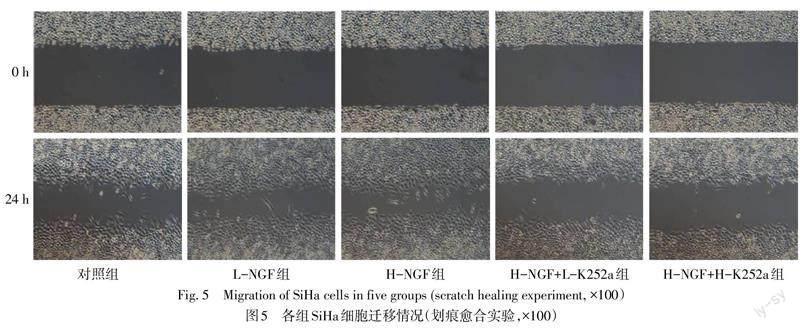
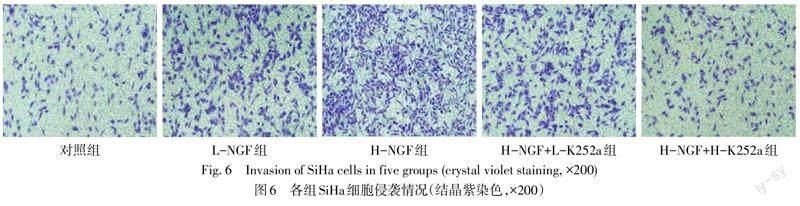
2.5 各组SiHa细胞侵袭、迁移以及EMT相关蛋白水平的比较
与对照组相比,L-NGF组、H-NGF组的迁移率、侵袭细胞数量以及N-cadherin、Vimentin蛋白水平增加,E-cadherin蛋白水平下降(P<0.05),且H-NGF组较L-NGF组差异更显著(P<0.05);与H-NGF组相比,H-NGF+L-K252a组、H-NGF+H-K252a组迁移率、侵袭细胞数量以及N-cadherin、Vimentin蛋白水平降低,E-cadherin蛋白水平升高(P<0.05),且H-NGF+H-K252a组差异更显著(P<0.05),见图5—7,表4、5。
3 讨论
宫颈癌是常见的妇科恶性肿瘤之一,全世界每年约有500万例确诊[7]。宫颈癌复发和治疗效果差主要是由细胞的侵袭和转移引起的[8]。EMT与宫颈癌的发展密切相关,是该病患者预后不良的主要原因之一[9]。因此,研究宫颈癌细胞迁移、侵袭以及EMT发生机制对于治疗宫颈癌至关重要。
NGF因其在神经系统发育中的作用而被广泛研究,NGF通过激活TrkA在神经发育过程中驱动神经元生长(轴突生成)[6]。近年来研究显示,癌细胞表达神经生长因子(如脑源性神经营养因子、胶质细胞源性神经营养因子),提高肿瘤生长速度,刺激肿瘤细胞转移;肿瘤去神经支配可以阻止肿瘤进展[10]。因此,抑制肿瘤微环境中的神经元生成被视为创新疗法的新靶点[5]。在胃癌中,NGF过表达已被证明可以促进肿瘤细胞生长[11]。在结肠癌中,交感神经和副交感神经参与刺激肿瘤生长和转移,下调NGF可以抑制大鼠结肠癌细胞增殖和血管生成,并降低肿瘤体积和质量[12]。本研究通过检测正常宫颈上皮细胞HUCEC和宫颈癌细胞SiHa中NGF、TrkA蛋白水平,结果发现SiHa细胞较HUCEC细胞NGF、TrkA水平显著升高,提示NGF/TrkA轴在宫颈癌细胞中被激活。本研究还发现,NGF处理后SiHa细胞增殖活力升高,细胞凋亡率降低,提示NGF过表达促进宫颈癌的发生,而NGF/TrkA信号轴抑制剂K252a处理SiHa细胞后NGF、TrkA水平下调,且SiHa细胞增殖活力降低,凋亡率升高,表明下调NGF/TrkA信号轴可能抑制宫颈癌的发生与发展。
EMT是上皮肿瘤细胞失去上皮特征并获得间充质表型的过程,是肿瘤细胞获得更高侵袭和转移能力的关键步骤。肿瘤细胞利用EMT作为中间表型来实现自我更新并适应其微环境[13]。在EMT过程中,上皮细胞通过失去细胞极性,上皮标志物E-cadherin表达减少,间充质标志物N-cadherin、Vimentin表达增多,从而获得间充质表型[14];且获得间充质表型已被证明可增强肿瘤细胞对化疗的耐药性并导致预后不良[15]。本研究结果发现,NGF过表达后,SiHa细胞迁移率、侵袭细胞数量以及N-cadherin、Vimentin蛋白水平增加,E-cadherin蛋白水平下降,表明激活NGF/TrkA轴可能促进SiHa细胞EMT过程,进而加快肿瘤细胞的迁移和侵袭,最终促进宫颈癌的发展。为了进一步证实该结论,笔者用H-NGF处理SiHa细胞后加用K252a干预,结果发现,K252a处理后SiHa细胞迁移率、侵袭细胞数量以及N-cadherin、Vimentin蛋白表达减少,E-cadherin蛋白表达升高,且高剂量K252a抑制SiHa细胞迁移、侵袭以及EMT过程更明显,表明通过抑制NGF/TrkA轴来抑制SiHa细胞迁移和侵袭可能是治疗宫颈癌的潜在治疗策略。
综上所述,下调NGF/TrkA信号通路可抑制SiHa细胞EMT过程,进而抑制肿瘤细胞的迁移和侵袭。
参考文献
[1] MANRRIQUEZ E N,ZAKHOUR M,SALANI R. Precision medicine for cervical cancer[J]. Curr Opin Obstet Gynecol,2022,34(1):1-5. doi:10.1097/GCO.0000000000000755.
[2] BAHRAMABADI R,DABIRI S,IRANPOUR M,et al. TLR4:an important molecule participating in either anti-human papillomavirus immune responses or development of its related cancers[J]. Viral Immunol,2019,32(10):417-423. doi:10.1089/vim.2019.0061.
[3] COLEMAN R L,LORUSSO D,GENNIGENS C,et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6):a multicentre,open-label,single-arm,phase 2 study[J]. Lancet Oncol,2021,22(5):609-619. doi:10.1016/S1470-2045(21)00056-5.
[4] MARSLAND M,DOWDELL A,JIANG C C,et al. Expression of NGF/proNGF and their receptors TrkA,p75NTR and sortilin in melanoma[J]. Int J Mol Sci,2022,23(8):4260-4274. doi:10.3390/ijms23084260.
[5] GAO F,GRIFFIN N,FAULKNER S,et al. The neurotrophic tyrosine kinase receptor TrkA and its ligand NGF are increased in squamous cell carcinomas of the lung[J]. Sci Rep,2018,8(1):8135-8145. doi:10.1038/s41598-018-26408-2.
[6] FAULKNER S,GRIFFIN N,ROWE C W,et al. Nerve growth factor and its receptor tyrosine kinase TrkA are overexpressed in cervical squamous cell carcinoma[J]. FASEB Bioadv,2020,2(7):398-408. doi:10.1096/fba.2020-00016.
[7] SUNG H,FERLAY J,SIEGEL R L,et al. Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3):209-249. doi:10.3322/caac.21660.
[8] YANG S,LONG Q,CHEN M,et al. CAF-1/p150 promotes cell proliferation,migration,invasion and predicts a poor prognosis in patients with cervical cancer[J]. Oncol Lett,2020,20(3):2338-2346. doi:10.3892/ol.2020.11775.
[9] LIAO Y,HUANG J,LIU P,et al. Downregulation of LNMAS orchestrates partial EMT and immune escape from macrophage phagocytosis to promote lymph node metastasis of cervical cancer[J]. Oncogene,2022,41(13):1931-1943. doi:10.1038/s41388-022-02202-3.
[10] SILVERMAN D A,MARTINEZ V K,DOUGHERTY P M,et al. Cancer-associated neurogenesis and nerve-cancer cross-talk[J]. Cancer Res,2021,81(6):1431-1440. doi:10.1158/0008-5472.CAN-20-2793.
[11] DOU N,YANG D,YU S,et al. SNRPA enhances tumour cell growth in gastric cancer through modulating NGF expression[J]. Cell Prolif,2018,51(5):e12484. doi:10.1111/cpr.12484.
[12] SADIGHPARVAR S,DARBAND S G,GHADERI-PAKDEL F,et al. Parasympathetic,but not sympathetic denervation,suppressed colorectal cancer progression[J]. Eur J Pharmacol,2021,913:174626. doi:10.1016/j.ejphar.2021.174626.
[13] NACHIYAPPAN A,GUPTA N,TANEJA R. EHMT1/EHMT2 in EMT,cancer stemness and drug resistance:emerging evidence and mechanisms[J]. FEBS J,2022,289(5):1329-1351. doi:10.1111/febs.16334.
[14] MIRZAEI S,SAGHARI S,BASSIRI F,et al. NF-κB as a regulator of cancer metastasis and therapy response:a focus on epithelial-mesenchymal transition[J]. J Cell Physiol,2022,237(7):2770-2795. doi:10.1002/jcp.30759.
[15] JIANG X,LIU F,WANG Y,et al. Secreted protein acidic and rich in cysteine promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells and acquisition of cancerstem cell phenotypes[J]. J Gastroenterol Hepatol,2019,34(10):1860-1868. doi:10.1111/jgh.14692.
(2023-03-10收稿 2023-04-13修回)
(本文編辑 李鹏)
