The skeleton of 5,7-fused bicyclic imidazole-diazepine for heatresistant energetic materials
2023-10-09XiaoxiaoZhengYubingXueChanghaoDaiHongweiYangGuangbinCheng
Xiaoxiao Zheng,Yubing Xue,Changhao Dai,Hongwei Yang ,Guangbin Cheng
School of Chemistry and Chemical Engineering,Nanjing University of Science and Technology,Nanjing,210094,China
Keywords:Imidazole-diazepine Heat-resistant material 5,7-Fused skeleton Energetic materials
ABSTRACT In light of the low yields and complex reaction routes of some well-known 5,5-fused and 5,6-fused bicyclic compounds,a series of 5,7-fused bicyclic imidazole-diazepine compounds were developed with high yields by only two efficient steps.Significantly,the seven-membered heterocyclic ring has a stable energetic skeleton with multiple modifiable sites.However,the 5,7-fused bicyclic energetic compounds were rarely reported in the area of energetic materials.Three neutral compounds 1,2 and 4 were synthesized in this work.To improve the detonation performances of the 5,7-fused neutral compounds,corresponding perchlorate 1a and 2a were further developed.The physicochemical and energetic performances of all newly developed compounds were experimentally determined.All newly prepared energetic compounds exhibit high decomposition temperatures (Td: 243.8-336 ℃) and low mechanical sensitivities (IS: >15 J, FS: >280 N).Among them,the velocities performances of 1a(Dv=7651 m/s)and 4(Dv=7600 m/s)are comparable to that of typical heat-resistant energetic material HNS(Dv=7612 m/s).Meanwhile,the high decomposition temperature and low mechanical sensitivities(Td=336 ℃; IS=32 J; FS >353 N) of 4 are superior to that of HNS (Td=318 ℃; IS=5 J; FS=250 N).Hence,the 5,7-fused bicyclic compounds with high thermostability,low sensitivities and adjustable detonation performance have a clear tendency to open up a new space for the development of heatresistant energetic materials.
1.Introduction
Energetic materials (EMs) are a class of compounds or formulations with oxidants and combustible components,which can release tremendous energy independently during the chemical reactions [1,2].Generally,to meet the demand for rapid and massive energy release,energetic groups such as nitro (-NO2),nitramine (-NHNO2) and azide (-N3) are installed to decorate the skeleton of Ems[3].However,the preoccupation with energy might have undesirable consequences,giving the material unsatisfactory stabilities.In other words,the two objectives of high detonation performance and excellent stabilities for EMs are mutually incompatible.For a long time,researchers pay more attention to improving detonation performance at the expense of stability.As a result,the heat-resistant materials (Td>300 ℃) account for less than 10% of the reported energetic materials (the samples of EMs>600) [4].In the context of the increasing demand for the exploitation of scarce resources,heat-resistant materials have been concerned gradually in recent years.However,the poor thermostability of EMs can restrict their application in several task-specific such as hypersonic weapons,deep mining and aerospace engineering [5].In this way,the innovation of the design and development for novel heat-resistant energetic materials is of great significance.
Fused-ring energetic skeletons are generally considered to be the polycyclic system composed of two or more heterocycles sharing at least one ring edge [6].In the past few decades,the fused-ring derivatives had been chiefly applied in pharmaceutical chemistry,bioluminescence and photoelectric [7-9].In recent years,fused-ring energetic compounds with high thermostability have been designed gradually [10].The fused-ring energetic compounds could lead to a high decomposition temperature due to their large π-conjugated system,which has a tendency of application in the area of heat-resistant energetic materials [11-13].Additionally,the amino group as an insensitive substituent group plays a vital role in maintaining molecular stability,which can provide hydrogen donors to form intra-and intermolecular hydrogen bond interactions [14-16].For example,DNPP(Td=336 ℃),PTX (Td=246 ℃) and DNPPDA (Td=325 ℃) all demonstrate that the fused energetic skeleton with amino group is an outstanding frame structure to beget the thermostable energetic materials (Fig.1) [17-19].Regrettably,the development of fusedring energetic materials is not plain sailing due to the obstacles of practical factors.The prohibitively high price of the reaction substrates,low yields,time-consuming synthesis steps or complicated reaction routes are some principal causes that hinder the practical application of fused-ring energetic compounds [20,21].
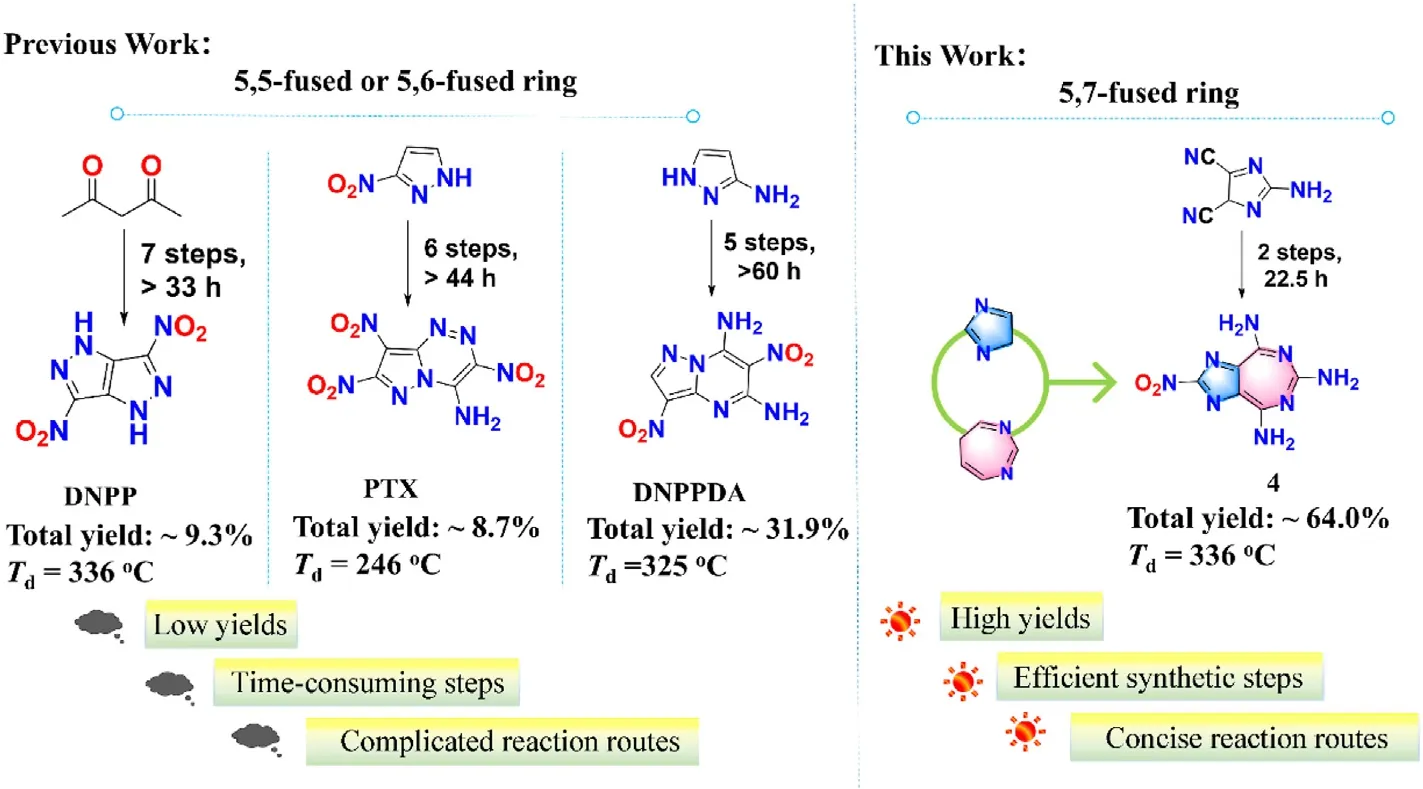
Fig.1.Comparison of previous work (5,5-fused and 5,6-fused bicyclic energetic compounds) and current work (5,7-fused bicyclic energetic compound 4).
At present,the bicyclic fused-ring framework used in the field of EMs is dominated by 5,5-fused and 5,6-fused bicyclic skeletons[22].The 5,7-fused bicyclic energetic skeleton was rarely reported.Considering that the seven-membered heterocyclic ring is a stable energetic skeleton with multiple modifiable sites[23],we focus our attention on the design and development of 5,7-fused bicyclic compounds.In this study,a facile synthetic method of 5,7-fused bicyclic compounds in high yields via only a two-step reaction was reported.Furthermore,in view of the costly price of reaction substrates for a certain number of reported 5,5-fused and 5,6-fused compounds,we selected the commercially available and economical starting materials for the design of 5,7-fused compounds.Here,compounds1and2were developed based on the skeleton of 5,7-fused bicyclic imidazole-diazepine.To obtain the compound with high energy properties,the 5,7-fused compound4with a nitro group was further synthesized [24].Significantly,the unusual skeleton of 5,7-fused bicyclic imidazole-diazepine has unique advantages in heat-resistant energetic materials due to its powerful π-delocalization and symmetric modifiable sites.All the compounds in this work gather the performances of high thermostability and low mechanical sensitivity.Among them,the decomposition temperatures of 2 and 4 are higher than 300 ℃.Overall,the high decomposition temperatures and facile synthesis of 5.7-fused bicyclic compounds are competitive and attractive in the field of heat-resistant materials.
2.Experimental part
2.1.Safety precaution
In the work,all newly prepared energetic compounds are a class of explosive materials with high energy.So,necessary safety precautions should be noted.The experiment ought to be handled in the fume hood during the whole experiments.Additionally,a protective helmet,gloves,and explosion-proof glass ought to be equipped all the time.The small scale trials are necessary.Last,the mechanical force such as impact,friction and spark should be avoided.
2.2.Synthesis
2.2.1.Synthesis of imidazo[4,5-e][1,3]diazepine-4,6,8-triamine (1)
1was synthesized according to the previous Ref.[25].
2.2.2.Synthesis of 4,6,8-triaminoimidazo[4,5-e][1,3]diazepin-1-ium(1a)
The mixture of1(0.354 g,2.0 mmol) in 10 mL distilled water was heated in an oil bath at 60 ℃.The strong acid HClO4was added to the mixture dropwise under constant stirring until the emergence of a clear solution,and then cooled down the reaction.The white crystal was precipitated after standing for half an hour.The filtration was performed to obtain1a(0.487 g,90%).1H NMR chemical shifts (δ,ppm;500 MHz,DMSO-d6):8.50 (s,2H),8.32(s,2H),8.21 (s,2H),7.89 (s,2H);13C NMR chemical shifts (δ,ppm;125 MHz,DMSO-d6):163.80,155.90,140.50,131.20.IR(KBr):3240,2900,1896,1769,1552,1494,1448,1323,1272,1209,1006,962,929,830,775,696,583,551 cm-1.Element analysis (%): C6H9N7O4Cl(MW: 278.63),calculated result: C 25.86,H 3.26,N 35.19;found: C 25.91,H 3.31,N 35.04.
2.2.3.Synthesis of 5-(N′-nitrocarbamimidoyl)-1H-imidazole-4-carboxamide (1b)
5 mL 100% HNO3was added into the 25 mL flask under the lowtemperature bath of -10 ℃.Compound1(0.5 g,2.5 mmol) was patted into the cold flask in small amounts under constant stirring.After completing the feeding,the mixture was stirred for another 3 h at 0 ℃.Finally,the reaction system was quenched by 8 g crushed ice to precipitate a white solid.The product was collected after filtration,washed with water (3 × 3 mL),and dried in air to afford powder (0.25 g,44.7%).1H NMR chemical shifts (δ,ppm;500 MHz,DMSO-d6):8.29(s,2H),8.05(s,H),6.91(s,2H);13C NMR chemical shifts (δ,ppm;125 MHz,DMSO-d6): 163.38,162.73,157.42,144.21,133.03.IR (KBr): 3437,3307,1671,1499,1351,1160,848,657,483 cm-1.Element analysis (%): C5H6N6O3(MW: 198.14),calculated result:C 30.31,H 3.05,N 42.42;found:C 30.20,H 3.31,N
42.44.
2.2.4.Synthesis of imidazo[4,5-e][1,3]diazepine-2,4,6,8-tetraamine(2)
Guanidine:The solid of sodium methoxide(1.080 g,20.0 mmol)was added to 25 mL MeOH and stirred until a clear solution appeared,then guanidine hydrochloride (1.910 g,20.0 mmol) was mixed into the solution.The precipitates emerged and were filtered off after the neutralization reaction for 1 h.The filtrate was collected for the next step.
2-amino-1H-imidazole-4,5-dicarbonitrile (1.330 g,10.0 mmol)was suspended in the above fresh filtrate,then the reaction mixture was warmed slowly in an oil bath until reflux for 20 h.A plentiful of white deposits appeared at the termination of the reaction.The solids were filtrated,washed by MeOH(4×5 mL),and dried in the air to yield a pure yellow product2(1.576 g,82%).1H NMR chemical shifts(δ,ppm;500 MHz,DMSO-d6):7.31(s,2H),7.01(s,2H),6.55(s,2H),5.06 (s,2H);13C NMR chemical shifts (δ,ppm;125 MHz,DMSO-d6):164.21,159.11,156.95,135.96.IR(KBr):3465,3019,1609,1489,1386,1076,730,571,482 cm-1.Element analysis(%):C6H8N8(MW: 192.19),calculated result: C 37.50,H 4.20,N 58.31;found: C 37.31,H 4.31,N 58.45.
2.2.5.Synthesis of 2,6,8-triamino-4-oxo-4,5-dihydroimidazo[4,5-e][1,3]diazepine-1,7-diium (2a)
The related synthetic method of2ais similar to that of compound1a.The white filter cake was confirmed to be2a(0.600 g,76%).1H NMR chemical shifts(δ,ppm;500 MHz,DMSO-d6):9.20(s,2H),8.80(s,2H),8.20(s,2H),7.06(s,2H);13C NMR chemical shifts(δ,ppm;125 MHz,DMSO-d6):163.38,162.73,157.42,144.21,133.03.IR(KBr):3323,3148,2803,1598,1328,738,581,464 cm-1.Element analysis (%):C6H9N7O9Cl2(MW:394.08),calculated result:C 18.29,H 2.03,N 24.88;found: C 18.03,H 2.10,N 25.04.
2.2.6.Synthesis of 2-nitro-1H-imidazole-4,5-dicarbonitrile (3)
3was synthesized according to the previous Ref.[26].
2.2.7.Synthesis of 2-nitroimidazo[4,5-e][1,3]diazepine-4,6,8-triamine (4)
2-nitro-1H-imidazole-4,5-dicarbonitrile (1.631 g,10.0 mmol)was suspended in a methanol solution of guanidine.The reaction was kept at 70 ℃ in an oil bath for 20 h.A great many yellow precipitates were formed and filtered out in the end.The filter cakes were collected,washed by MeOH(4×5 mL),and air dried to obtain a yellow product4(1.667 g,80%).1H NMR chemical shifts(δ,ppm;500 MHz,DMSO-d6): 8.04 (s,4H),7.54 (s,2H);13C NMR chemical shifts (δ,ppm;125 MHz,DMSO-d6): 164.04,158.74,156.96,135.80.IR(KBr):3438,2617,1713,1584,857,802,537 cm-1.Element analysis (%): C6H6N8O2(MW: 222.17),calculated result: C 32.44,H 2.72,N 50.44;found: C 32.47,H 2.80,N 50.60.
3.Results and discussion
3.1.Synthesis
In this study,the synthetic routes of all prepared compounds were presented in Fig.2.Compound1was prepared according to the reported literature.Then,various nitration conditions such as fuming HNO3,100% HNO3,H2SO4/98% HNO3and KNO3/H2SO4were tried for the nitration of1,but none of the nitramino groups were introduced successfully.Unexpectedly,a ring-opening product1bwas obtained accidently by treating1with 100% HNO3.To improve the density and detonation performances of1,perchlorate1awas synthesized by using cheap and oxygen-rich perchloric acid [27].Since high nitrogen content can generally lead to an increase in enthalpy of formation [28],the introduction of an amino group at the unmodified position ofp1was tried to gain an optimized substratep2.Hence,compound2was synthesized by the cyclization of two cyano-groups attached top2in the presence of guanidine and methanol.Considering that the nitro group is a favorable energetic substituent in realizing high energy density materials[29],we tried multiple oxidation systems (H2SO4/NaNO2,Oxone and H2O2/H2SO4) to oxidize the amino groups of2,but the long trials ended in failure.Therefore,based on the successful case of1a,perchlorate2awas obtained by using perchloric acid as the neutralization reagent to improve its detonation performance.Interestingly,one of the amino groups in the seven-membered ring of2was converted to a carbonyl group,which might be due to the strong oxidability of perchloric acid.Significantly,another strategy was proposed to introduce the nitro group in the imidazole ring based on optimization of the reaction substrate[30].First,2-nitro-1H-imidazole-4,5-dicarbonitrile (3) was synthesized by oxidating the amino group in substratep2,and then the optimized substrate with a nitro group was participated in the heterocyclization reaction to obtain compound4.All newly prepared compounds were characterized by NMR spectra (Nuclear Magnetic Resonance:1H and13C),EA (Element Analysis) and IR (Infrared Spectroscopy).
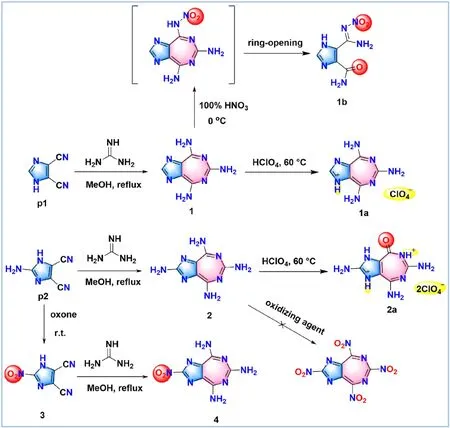
Fig.2.Synthesis of 5,7-fused energetic compounds 1-4 and 1a-2a.
3.2.Single crystal X-ray diffraction
Single crystals of compounds1a,2aand4were cultivated and characterized by single crystal X-ray diffraction to confirm their special structures and further explore their structure-property relationships(see Fig.3).Crystal1a∙H2O and2a∙2H2O were afforded by slow cooling of the reaction system,while crystal4∙DMSO grew from its saturated solution after slow evaporation at atmospheric temperature.
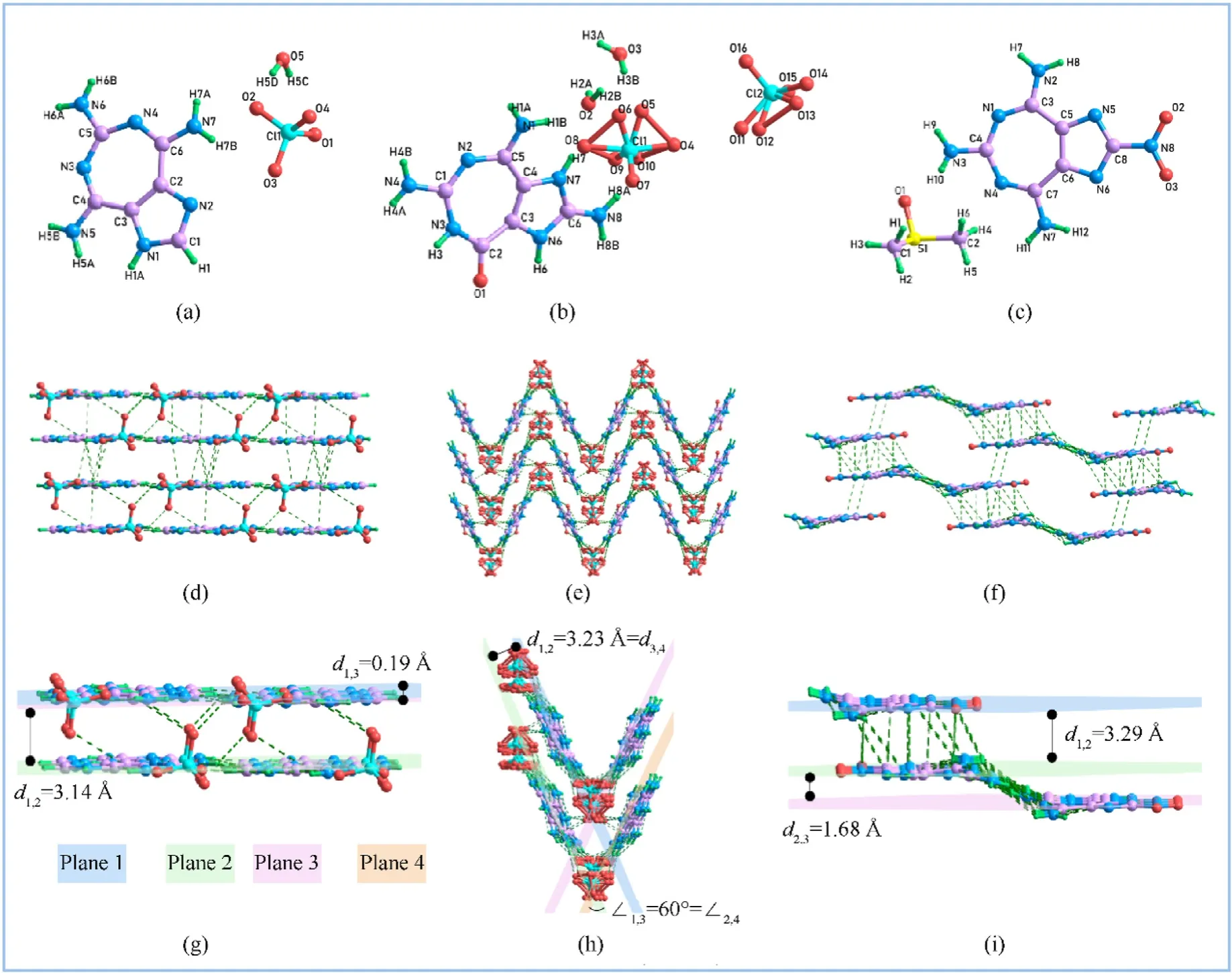
Fig.3.(a)-(c) The X-ray crystal structures for 1a∙H2O,2a∙2H2O,4∙DMSO,respectively;(d)-(f) The packing diagrams and intermolecular hydrogen bonding (H-bonding,green dotted line) of 1a∙H2O,2a∙2H2O,4∙DMSO,respectively (the solvents were removed for clarity);(g)-(h) The enlarged view form packing diagrams of 1a∙H2O,2a∙2H2O,4∙DMSO,respectively.
Crystal1a∙H2O belongs to the triclinic crystal system,the P-1 space group,and its crystal density was calculated to be 1.754 g/cm3at 170 K.In Fig.3(d),the packing diagram of the cationic components in1acan be described as face-to-face stacking,and the attached anions are embedded in those layers at regular intervals.To obtain more details about crystal packing arrangements,the enlarged view of packing diagrams is displayed in Fig.3(g).The two adjacent layers are arranged in parallel with a tight distance of 3.14 Å (d1,2).Actually,each horizontal layer consists of a discontinuous tier with a tiny distance of 0.19 Å(d1,3).All atoms of cationic components in crystal1a∙H2O lie in the same plane,which can be confirmed reasonably by the torsion angles of C1-N1-C3-C4 179.1(2)o,C1-N2-C2-C3 0.1(3)o,C6-N4-C5-C3 0.9(5)o(ESI).The crystal system of2a∙2H2O is belong to the monoclinic crystal system,P21/n space group with a calculated density of 1.873 g/cm3at 170 K.The seven-membered ring is slightly distorted due to the difference between the carbonyl and amino groups in polarity(Fig.3(b)).The packing diagram of the cationic components in2acan be regarded as wave-like stacking.Among them,the neighboring fragments are linked head to tail by related anions(Fig.4(e)).In the zoomed-in view of the packing diagram of crystal2a∙2H2O(Fig.3(h)),the distance between the adjacent parallel plane is measured to be 3.23 Å(d1,2=d3,4)and the dihedral angle between two intersecting planes is measured to be 60°.The crystal system of4∙DMSO is the same as that of1a∙H2O,which belongs to the triclinic crystal system,P-1 space group,and its crystal density was calculated to be 1.646 g/cm3at 170 K (Fig.3(c)).The low density may be caused by the presence of DMSO.As shown in the packing diagram(Fig.3(i)),crystal4can be viewed as flat“Z”stacking.The interlayer distance between two horizontally staggered planes is measured to be 3.29 Å(d1,2).Besides,there is a slight slide between two contiguous layers connected by DMSO with a distance of 1.68 Å(d2,3).
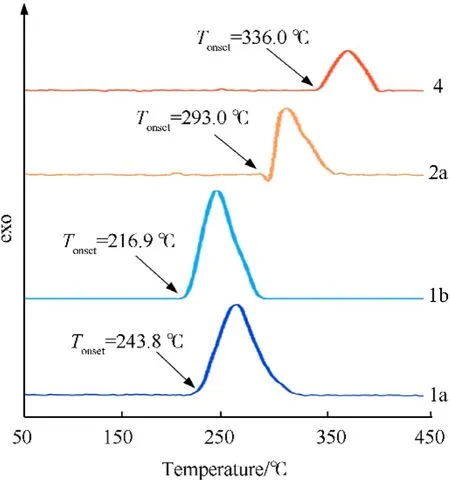
Fig.4.DSC plots of newly developed compounds.
To further explore the structure-property relationship of these compounds,the intermolecular interaction and stacking distances of crystals1a,2aand4were analyzed to elucidate their effects on the energetic properties [31].The hydrogen-bonding interaction(green dotted line) between N and O atoms in molecules can be observed in Fig.3(d)-(f).Among them,2ahas the largest number of hydrogen bonds,resulting in its highest density.Furthermore,the interlayer distances of these packing molecules are all within the scope of the interaction distance of π-π stacking(Dd<4 Å)[32].The strong hydrogen bond interaction and π-π stacking interaction in molecules contribute to the thermostabilities and insensitivities of compounds,thus the desirable thermostability and the low sensitivities of compounds 1a,2a and 4 can be explained.
3.3.Detonation property and mechanical sensitivity
A series of physicochemical properties of the developed compounds were displayed in Table 1.All synthetic products were fully dried at the standard condition to remove solvents before performance tests.
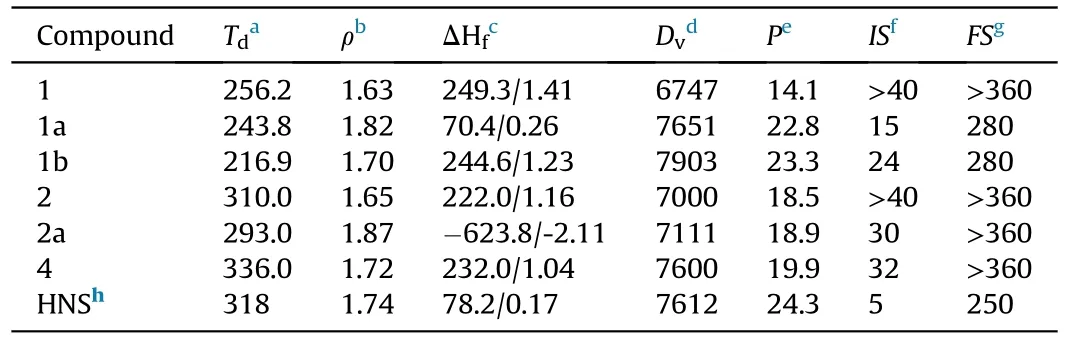
Table 1 The energetic and sensitive properties of 1-4 and 1a-2a.
Thermal stability is one of the important parameters that should be concerned in practical applications [33].Generally,the decomposition temperature of contemporary EMs is required more than 180 ℃ [34-36],because the low thermostability may limit their practical application in transportation,storage and use safety[37].In this work,decomposition temperatures of all developed compounds were measured depending on DSC (Differential Scanning Calorimetry) device in a nitrogen atmosphere at a heating rate of 5oC/min.All compounds showed good thermostability and their onset decomposition temperatures were in the range of 243.8-336 ℃.Among them,neutral compound4displays the highest onset decomposition temperature of 336 ℃,which is superior to that of the typical heat-resistant energetic material HNS(Td=318 ℃).In term of energetic salts,the decomposition temperature of perchlorate2a(Td=293.0 ℃) shows an increase of 49.2 ℃ compared with perchlorate1a(Td=243.8 ℃)(Fig.4).In addition to thermal stability,mechanical sensitivities are another factor that needs to be considered at the security level,including impact sensitivity (IS) and friction sensitivity (FS) [38].The mechanical sensitivities of these developed compounds were put to the test through the standard BAM method.All compounds showed low mechanical sensitivities (IS: >15 J,FS: >280 N),which are all superior to that of the classical heat-resistant energetic material HNS (IS: 5 J,FS: >250 N).
To get a more profound understanding of the high heat resistance and the low mechanical sensitivity of these compounds,the analysis of Hirshfeld surfaces and 2D fingerprint plots for compounds1a,2aand4were employed respectively (the solvent molecules of the three compounds were not selected in the formation process of Hirshfeld surfaces and fingerprint plots)[39].The weak intermolecular interactions are visualized graphically in Fig.5(a)-Fig.5(c),the prominent red spots can be found on the side edges of the plates,representing that there are hydrogen bonding interactions in the corresponding regions [40],and more detailed information about the weak intermolecular interaction is presented in Fig.5(d)-Fig.5(g).As can be seen in the 2D fingerprint plots,several needle-like shapes could be observed in the bottom lefthand corner of the images[41],which are formed by the N…H and O…H interactions between molecules.Further combined with the data statistics from Fig.5(g),the hydrogen bonding interactions of the three compounds are dominant in the weak interactions and the percentage of 1a and 2a account for well over half of the total.Overall,the extensive hydrogen bonding interactions can not only stabilize the stacking system to improve the heat resistance performance,but also short the interlayer distance of molecular packing in crystal to decrease the mechanical sensitivity [42].Therefore,the high heat resistant and the low mechanical sensitivities of compounds 1a,2a,and 4 can be reasonably explained.
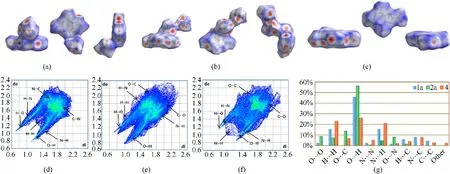
Fig.5.(a),(b)Hirshfeld surfaces for 1a,2a and 4(view from the front,vertical,and side,respectively);(c),(d)2D fingerprint plots for 1a,2a and 4,respectively;(e)The bar graph of individual atomic contacts percentage for 1a,2a and 4.
Detonation velocity (Dv) and detonation pressure (P) are two essential indicators of EMs,because they can give us the basis for evaluating the detonation power of the developed explosives.First of all,density (ρ) and enthalpy of formation (ΔHf) should be available before calculating the detonation performance [43].In this work,the densities of all compounds ranged from 1.63 to 1.87 g/cm,which were tested by a gas pycnometer in a helium atmosphere.Furthermore,all compounds except carbonyl ionic compounds 2a present positive enthalpies of formation.After the two main variables (ρ and ΔHf) were obtained,the detonation properties of all newly synthesized energetic compounds were calculated by the EXPL05 v6.01 program.Among them,the detonation velocities of compounds 1a (Dv=7651 m/s) and 4 (Dv=7600 m/s) are comparable to that of HNS (Dv=7612 m/s).
4.Conclusions
Considering the shortcoming of some reported 5,5-fused and 5,6-fused bicyclic compounds with intricate synthesis and low yield,we designed and developed a class of 5,7-fused bicyclic compounds with high output and convenient synthesis (two-step synthesis).As yet,the energetic skeleton of 5,7-fused bicyclic imidazole-diazepine is rarely reported in the field of EMs.In this study,three neutral 5,7-fused imidazole-diazepine compounds1,2,and4were synthesized by the cyclization of two cyano-groups attached to related imidazole substrates,respectively.Since several nitrification and oxidation reactions had been attempted but failed,perchlorate1aand2awere further synthesized to improve their detonation performances.Furthermore,the physicochemical and energetic properties of the developed compounds were experimentally and theoretically ascertained.Among them,the detonation performances of compounds1a(Dv=7651 m/s)and4 (Dv=7600 m/s) are comparable to that of the classical heatresistant energetic material HNS (Dv=7612 m/s).Moreover,4possesses the high decomposition temperature and low mechanical sensitivities (Td=336 ℃;IS=32 J;FS>353 N),which are superior to that of HNS (Td=318 ℃;IS=5 J;FS=250 N).On the one hand,the large conjugation of the 5,7-fused bicyclic skeleton can enhance the thermal stability and facilitate the intermolecular π-π stacking to reduce their sensitivity.On the other hand,the properties of 5,7-fused bicyclic compounds can be reasonably adjusted due to the multiple modifiable sites of the diazepine ring.In brief,the application of the 5,7-fused bicyclic energetic skeleton can provide a new pioneering guide for the design of novel energetic materials and effectively alleviate the growing demand for heat-resistant energetic materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was completed with support from the National Natural Science Foundation of China (Grant No.22075143,21875110),as well as the Science Challenge Project(Grant No.TZ2018004).H.Yang thanks the Qing Lan Project for the grant.
Appendix B.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2022.09.003.
杂志排行
Defence Technology的其它文章
- Cooperative maneuver decision making for multi-UAV air combat based on incomplete information dynamic game
- Satellite breakup behaviors and model under the hypervelocity impact and explosion: A review
- Recognition model and algorithm of projectiles by combining particle swarm optimization support vector and spatial-temporal constrain
- Time-sequenced damage behavior of reactive projectile impacting double-layer plates
- One-step rapid preparation of CL-20/TNT co-crystal assembly and spheroidized coating based on droplet microfluidic technology
- Mechanical properties and failure behavior of 3D printed thermoplastic composites using continuous basalt fiber under highvolume fraction
