One-step rapid preparation of CL-20/TNT co-crystal assembly and spheroidized coating based on droplet microfluidic technology
2023-10-09JihuiShiBidongWuJinqingZhouDweiRenDongxuZhngChongweiAnJingyuWng
Jihui Shi ,Bidong Wu ,* ,Jinqing Zhou ,b ,Dwei Ren ,Dongxu Zhng ,Chongwei An ,Jingyu Wng
a School of Environment and Safety Engineering,North University of China,Taiyuan,030051,China
b School of Mechatronical Engineering,Beijing Institute of Technology,Beijing,100081,China
Keywords:Droplet microfluidics CL-20 TNT Co-crystal Spherical coating
ABSTRACT Energetic materials pose challenges in preparation and handling due to their contradictory properties of high-energy and low-sensitivity.The emergence of co-crystal explosives is a new opportunity to change this situation.If the co-crystal explosive is coated into spherical particles with uniform particle size distribution,this contradiction can be further reduced.Therefore,binder-coated hexanitrohexaazaisowurtzitane/2,4,6-trinitrotoluene (CL-20/TNT) co-crystal microspheres were prepared by droplet microfluidic technology in this work.The coating effects of different binder formulations of nitrocellulose(NC) and NC/fluorine rubber (F2604) on the co-crystal spheres were studied.The scanning electron microscopy (SEM) results showed that the use of droplet microfluidic technology with the above binders can provide co-crystal microspheres with regular spherical morphology,uniform particle size distribution and good dispersion.X-ray diffraction(XRD),fourier-transform infrared(FT-IR),differential scanning calorimetry (DSC) and thermo-gravimetric (TG) methods were employed to compare the properties of the co-crystal microspheres,raw material and pure co-crystal.The formation of CL-20/TNT co-crystal in the microspheres was confirmed,and the co-crystal microspheres exhibited better thermal stability than the raw material and pure co-crystal.In addition,the mechanical sensitivity and combustion performance of the co-crystal microspheres were further studied.The results showed that the co-crystal microspheres were more insensitive than CL-20 and pure co-crystal,and displayed excellent self-sustained combustion performance and theoretical detonation performance.This study provides a new method for the fast,simple and one-step preparation of CL-20/TNT co-crystal microspheres,with binder coating,uniform particle size distribution,and excellent performance level.
1.Introduction
At present,high-energy explosives are widely used in modern civil and military applications.However,there exists a contradiction between the high-energy release of high-energy explosives and their mechanical sensitivity.The greater the energy release,the higher the mechanical sensitivity of the explosive.This leads to a potential safety hazard in the production,transportation,storage and use of explosives[1-3].In recent years,researchers have been pursuing explosives with higher energy release and lower mechanical sensitivity[4-6].Many technical means have emerged to achieve this goal.Among them,the explosive co-crystal technology is one of the most representative methods[7,8].Co-crystals are generally multi-component molecular crystals in which two or more molecules form hydrogen bonds or other non-covalent bonds and the structural units are arranged in order[9].The combination of different explosive molecules forms a co-crystal,which can effectively improve the sensitivity and oxygen balance of the target product,and also enhance the explosion heat,thermal stability,flammability and safety of the components [10-13].Co-crystals have better performance than the corresponding raw materials and mixtures[14,15].The explosive co-crystal preparation method is flexible,which has gradually become a new opportunity to improve the performance level of existing energetic materials.
Hexanitrohexaazaisowurtzitane(CL-20 or HNIW)is a new type of energetic compound with high energy density,good oxygen balance,and excellent detonation performance.However,its wide application is severely limited due to its high sensitivity [16,17].2,4,6-Trinitrotoluene (TNT) is an economical insensitive explosive with good thermal stability,but it has the drawbacks of low energy density,poor oxygen balance,and unremarkable detonation properties [18].Bolton used the solvent evaporation method to prepare CL-20/TNT co-crystal explosive for the first time.This cocrystal explosive showed excellent detonation performance and low sensitivity,and its density was lower than that of CL-20,but much higher than that of TNT [19].Subsequently,many scholars have conducted in-depth research on the preparation method,crystal structure,thermal behavior and detonation performance of CL-20/TNT co-crystals [20,21].Jia et al.used a solvent-nonsolvent method to prepare a CL-20/TNT co-crystal with better thermal stability in a molar ratio of 1:1[22].Hu et al.used mechanical ball milling to co-grind CL-20 and TNT to prepare nano-CL-20/TNT cocrystals,which showed higher thermal stability and safety[23].Liu et al.used mechanical ball milling to grind and dissolve CL-20 and TNT in ethanol,and then volatilized the solvent to obtain CL-20/TNT co-crystal in a green and environmentally friendly way [24].However,the current methods used to prepare CL-20/TNT cocrystals are time-consuming and labor-intensive,and difficult to scale up in industrialized production.Moreover,the morphology and size of the co-crystal products are different,and the particle size distribution is not uniform,which can restrict their practical application.
Droplet microfluidics is a recently emerged method that is effective for preparing explosives with high sphericity [25].Compared with other methods,microfluidic technology has the advantages of miniaturization,low reagent consumption,high precision,easy control,and facile scale-up production[26,27].Han et al.used droplet microfluidic technology to prepare hexanitrostilbene (HNS) energetic microspheres with high sphericity using nitrocellulose(NC)as a binder,which enhanced the fluidity and bulk density of HNS[28].Yang et al.used the microfluidic method to accurately prepare 90% loaded CL-20 microspheres with uniform particle size and unique hierarchical structure,which improved the self-sustained combustion of CL-20 [29].Our research group used the droplet microfluidic platform to prepare high-quality octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazine/1,3,5-triamino-2,4,6-trinitrobenzene(HMX/TATB) microspheres.The sample had narrow particle size distribution,high sphericity,and increased the bulk density of explosives [30].The above studies show that the microfluidic droplet technology is suitable for the preparation of energetic material microspheres.Furthermore,the preparation process parameters are easy to control at the microscale,which can help optimize the crystallization environment of explosives and improve their physical and chemical properties.
Therefore,this work used a combination of spheroidized coating,which is a beneficial way to modify explosives,and droplet microfluidic technology to easily and quickly realize the integrated preparation of CL-20/TNT co-crystal assembly and spheroidized coating,in order to improve the application potential of CL-20/TNT co-crystal.Then,coated CL-20/TNT co-crystal microspheres with uniform particle size distribution and excellent performance were obtained.The assembly process of the co-crystal microspheres was studied.The pure CL-20/TNT co-crystal was also prepared by the solvent evaporation method (details given in Supporting Materials),in order to compare with the various characteristics of the prepared CL-20/TNT co-crystal microspheres.The samples were fully characterized by XRD,FTIR,DSC and TG methods,demonstrating the formation of CL-20/TNT co-crystal in the microsphere.In addition,the morphology,flowability,thermal stability,mechanical sensitivity and combustion effect of the CL-20/TNT cocrystal microspheres with different binder coatings were also investigated.This work demonstrates that droplet microfluidic technology provides is an effective method for the integrated preparation of explosives co-crystal assembly and spheroidized coating.
2.Experiment
2.1.Material
CL-20 and TNT were provided by Modern Chemistry Research Institute of China.NC (12% N,industrial grade) and F2604were purchased from Foshan Junyuan Chemical Co,Ltd.Ethyl acetate was supplied by Tianjin Beichen Founder Chemical Co.,Ltd.Sodium dodecyl benzene sulfonate(SDBS)was provided by Tianjin Kaitong Chemical Reagent Co,Ltd.
2.2.Construction of droplet microfluidic platform
As shown in Fig.1,The droplet microfluidic platform consists of fluid driving unit(Fig.1(a)),droplet generating unit(Fig.1(b)),microsphere collecting unit(Fig.1(c)) and connecting parts.The fluid driving unit includes two syringe pumps to drive the flow of water/oil two-phase liquid.The droplet generating unit is composed of a fluid-focusing micro-droplet chip made of hydrophilic glass,in which the width and depth of the oil-phase channel are 350 μm and 200 μm,and the width and depth of the waterphase channel and the cross-exit channel are 500 μm and 200 μm,respectively.The microsphere collection unit is composed of a constant temperature magnetic stirrer and a collection beaker.Each unit is connected by a 1 mm×1.6 mm polytetrafluoroethylene(PTFE) pipe.When the fluid feed starts,the water and oil phases converge into the micro-droplet chip through the PTFE tube,at the cross passage due to the shear force of the water relative to the oil phase,water-in-oil droplets are formed in the micro-channel,the droplets consist of ethyl acetate solution containing the raw material and binder.The droplets flow in the direction of the microfluid and during the flow the droplets gradually solidify into spheres.A magnetic stirrer in the collection unit was set at the right temperature and speed to collected the microspheres,allowing them to further crystallize and solidify in the aqueous solution in the collection beaker.The constructed platform has the advantages of simple structure,convenient operation,high preparation efficiency,and easy scale-up production.In addition,the instrument miniaturization of the platform and the good heat transfer efficiency of the microchannel improve the intrinsic safety during the preparation of energetic materials.Hence,this method has good economic benefits and application prospects.
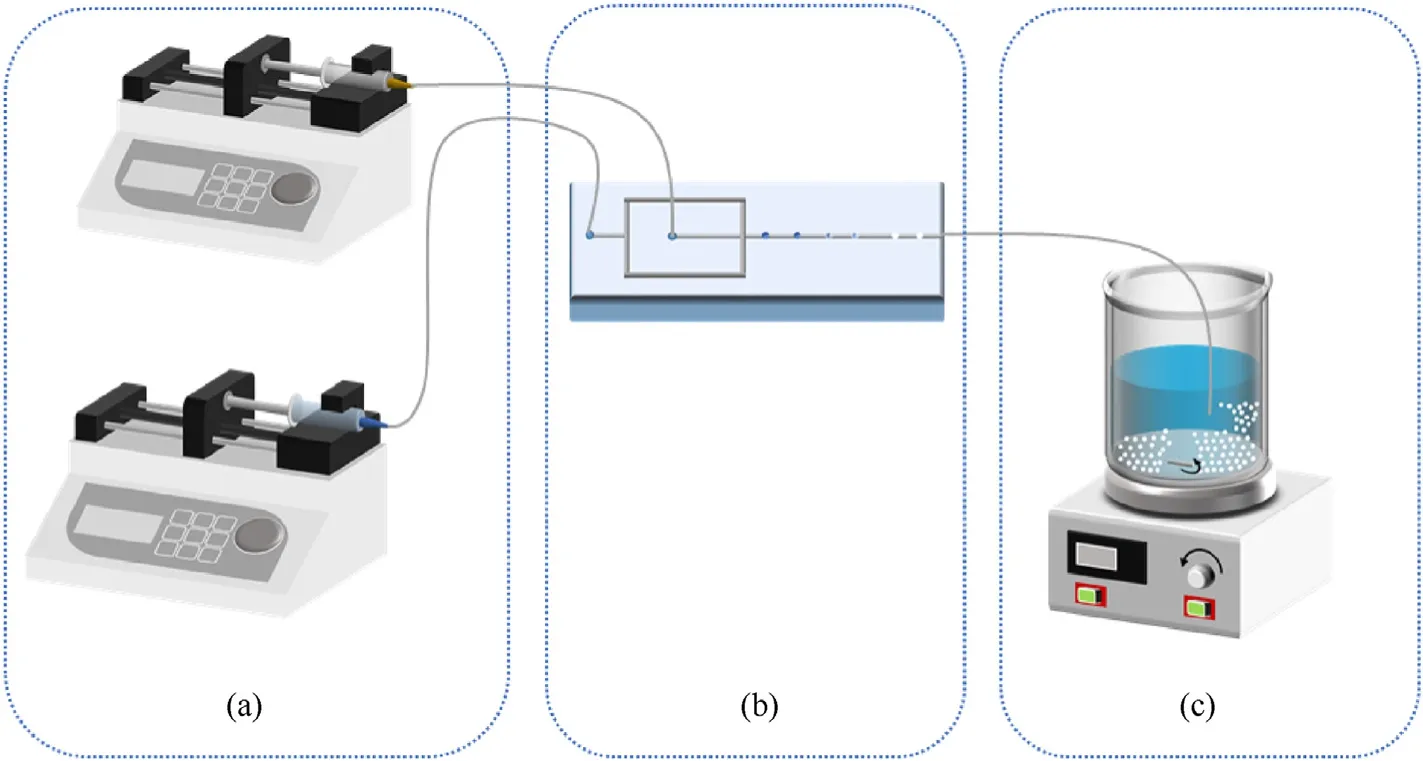
Fig.1.Droplet microfluidics platform.
2.3.Preparation of CL-20/TNT co-crystal microspheres
Configuration of the water phase system: SDBS (0.2 g) was weighed and dissolved in 100 mL deionized water to obtain the aqueous system with 0.2 wt% surfactant content;Configuration of the oil phase system: Two parts of CL-20 (0.438 g) and TNT(0.227 g) with a molar ratio of 1:1 were weighed out into two beakers;the explosive components accounted for 95 wt% of the total material.Then,the two binder systems of NC (0.035 g) and NC(0.021 g)/F2604(0.014 g) were weighed out and placed in the above two beakers,respectively.The binder component accounted for 5 wt% of the total material.Then,5 mL of ethyl acetate was added to the two beakers respectively and sonicated until the materials were completely dissolved.The configured water phase system and oil phase system were extracted into two separate syringes.The water bath was turned on and heated to 40 ℃.The flow rate of the water phase was set to 2.4 mL/min and the flow rate of the oil phase to 0.15 mL/min.The infusion was started,and the obtained microspheres were collected after entering the liquid.The microspheres were filtered,washed and dried.The CL-20/TNT cocrystal microsphere with binder component of NC was labelled as sample 1 (S-1) and that with binder component of NC/F2604compound system was labelled as sample 2 (S-2).
2.4.Characterization methods
The surface morphology was characterized by scanning electron microscopy (SEM,SU-8020,Hitachi,Japan) working at an acceleration voltage of 5 kV.Samples were characterized after being coated through gold sputtering.The particle size distribution statistics were carried out according to method 403.2 of GJB770B-2005,where the maximum and minimum size of each particle was measured under a microscope to find the particle size and the particle size statistics were analysed.Fourier transform infrared spectroscopy (FTIR,VERTEX 70,Bruker,Germany) was used to determine the compositional information of samples.The FTIR spectrum was recorded over the range of 4000-400 cm-1.The crystal structures were determined by powder X-ray diffraction(XRD,DX-2700,Dandong Haoyuan Corporation,Liaoning,China).The analysis parameters were as follows: 2θ test angle 5-55°,voltage 40 kV,current 30 mA,and Cu-Kα radiation.Thermal analysis was performed on a differential scanning calorimeter(DSC-131,Setaram,France,Shanghai,China),and the heating rate was 10 ℃/min.The thermal behavior of the sample was characterized by thermal gravimetric analysis (TGA METTLER TOLEDO).The sample(1-3 mg) was placed in a 70 μL ceramic crucible at a constant heating rate of 10 ℃/min from 50 to 400 ℃.The impact sensitivity characterization was based on the EN13631-4:2002 standard,using the BAM impact sensitivity tester to measure the impact sensitivity.Two samples were tested and the minimum ignition energy was taken as the recorded value.The weight of the drop weight was 5 kg,and the volume of each sample was 10 mm3.The friction sensitivity was tested on the WM friction sensitivity meter according to the standard method 602.1 of GJB-772A-97.The test conditions were as follows: the weight of the pendulum was 1.50 kg,the mass of the sample was 20 mg,the relative pressure was 4.9 MPa,and the swing angle was 90°.The friction susceptibility of each test sample was represented by the probability of explosion,and 30 tests were carried out consecutively to characterize the friction susceptibility and probability of explosion of the samples.
3.Results and discussion
3.1.Assembly strategy of co-crystal microspheres
In order to prepare the binder-coated CL-20/TNT co-crystal microspheres,the binder must be compatible with the CL-20/TNT co-crystal.Nitrocellulose (NC) is a commonly used energetic binder with low sensitivity and good compatibility.It can form a unique three-dimensional network structure to coat explosive particles.Fluorine rubber (F2604),a fluorine-containing polymer,is the most commonly used material for coating nitramine explosives due to its excellent heat resistance,good physical and mechanical properties,and suitable compatibility.NC and NC/F2604composite system binders were used herein to build a three-dimensional porous framework structure in the microspheres.As a result,CL-20 and TNT molecules were evenly distributed in the porous framework to achieve co-crystallization,thus completing preparation of the CL-20/TNT co-crystal microspheres.The characterization results showed that the three-dimensional porous framework was successfully constructed using the two binder systems.In the droplet microfluidic system,the fluid flow is mainly affected by viscous force,inertial force,interfacial tension,shear stress,etc.The Reynolds number (Re) is an important dimensionless parameter in fluid mechanics,which represents the ratio of inertial force to viscous force.Its equation is as follows:
whereprepresents the density of the fluid,vrepresents the flow velocity of the fluid in the microchannel,dis the diameter of the microchannel,andurepresents the viscosity of the fluid.In microchannel,the Reynolds number is usually very small,and the inertial force has negligible influence on the liquid flow.The viscous force plays a dominant role,and the liquid flows in the microchannel as a stable laminar flow[31].This stable laminar flow can provide a suitable environment for CL-20/TNT co-crystal formation.In addition,between the two-phase intersecting channels,the oilphase fluid forms highly periodic and ordered oil in water droplets under the action of the water-phase shear force;then,oil in water droplets flow together with water phase in microchannel.Macroscopically,the formed oil phase droplets move forward under the action of the continuous flow of the water phase.During this process,the solvent ethyl acetate (EA) in the droplet exchanges with the external environment;this can be regarded as a convection process.Microscopically,EA in the oil phase droplets undergoes a diffusion process from high chemical potential region to low chemical potential region.That is,the migration of EA in droplets to the water phase is affected by both convection and diffusion.For the time scale of convection,the ratio of physical characteristic scale and flow velocity can be approximated,given by Eq.(2).The time scale of diffusion can be approximated by the ratio of physical characteristic scale and kinematic viscosity,as shown in Eq.(3).
whereLis the width of the microchannel,Vis the flow rate of the fluid,anduis the viscosity of the fluid.Due to the micro-scale size of the channel,the time scale of convection and diffusion is shortened sharply,and the solidification process of droplets in the microfluidic system is a relatively fast process.This process can be described by the schematic diagram in Fig.2.The oil phase fluid forms tiny droplets(Fig.2(a))after being squeezed and sheared by the water phase.After the droplet are fully exposed to the water phase,the water-oil interface maintains constant solvent exchange.As EA in the droplet continues to migrate and diffuse into the water phase,the droplet continues to shrink.The dissolved polymer binder in the oil phase system begins to aggregate and cross-link to create a binder-formed polymer region with higher concentration(Fig.2(b))[32,33].With the progress of solvent diffusion,the threedimensional porous skeleton constructed by the binder gradually forms,which becomes the main structural support inside the droplet.CL-20 and TNT molecules are uniformly distributed in countless small spaces generated in the three-dimensional porous framework.At this time,due to the continuous diffusion of the solvent and the narrower space in the skeleton,the readily soluble CL-20 and TNT in EA rapidly reach local supersaturation and begin to co-crystallize.They are,attached to the three-dimensional skeleton structure formed by the binder (Fig.2(c)) [34].In addition,because the droplets are not disturbed by large external forces,they provide a good environment for the formation of spherical skeleton and co-crystal.Finally,the droplets rapidly compress and solidify into monodispersed CL-20/TNT co-crystal microspheres which are deposited in the receiving solution (Fig.2(d)).
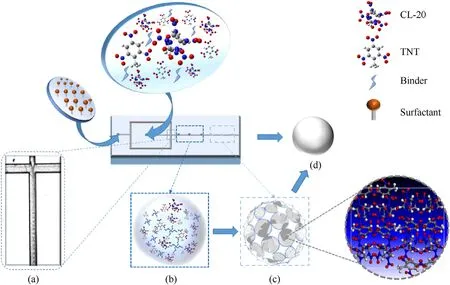
Fig.2.Assembly process of CL-20/TNT co-crystal microspheres.
3.2.Morphology characterization
Fig.3 shows the electron microscopy image,SEM image and particle size distribution of S-1 and S-2 prepared by droplet microfluidics.Fig.3(a1)and Fig.3(a2)show microscopic images of the samples The CL-20/TNT co-crystal was coated by the binder in the spheres,showing white colour and regular spherical shape,flat inter-particle arrangement,no obvious particle aggregation,no significant difference between samples S-1 and S-2.Fig.3(b1) and Fig.3(b2)are the SEM images of the microspheres,Samples S-1 and S-2 both showing a high degree of spheroidization.Micropores and regular strip-shaped aggregated scratches can be seen on the surface of the microspheres (Fig.3 (c1),Fig.3(c2)).This was because the diffusion of the solvent inside the microspheres during the curing process formed pores on the surface.In addition,the rotating flow of the microspheres in the microchannel rubbed against the channel wall to form strip-shaped scratches.The surface morphology of sample S-1 was more regular than that of S-2,and no co-crystal particles were found to be attached to the surface of S-1,and the coating effect was better than that of S-2,indicating that the addition of F2604to S-2 would have a negative effect on the surface morphology of the microspheres and on the coating of the co-crystal.Fig.3(d1) and Fig.3(d2) show that the interior of the microspheres was mainly composed of submicron CL-20/TNT cocrystal particles and three-dimensional framework composed of binder (as indicated by the red dotted line).This result confirmed the successful construction of the three-dimensional framework of the binder in the microspheres.The orange dotted line indicates that the formed CL-20/TNT co-crystal was distributed on the threedimensional framework.Compared to S-1,the interior of S-2 exhibits a more dense and complex three-dimensional skeletal structure,suggesting that the addition of F2604was more conducive to the establishment of the three-dimensional skeletal structure.The co-crystal particles in samples S-1 and S-2 were sub-micron lumpy particles.Fig.3(e1) and Fig.3(e2) show the 3D scanning image of the surface of the CL-20/TNT co-crystal microspheres.Samples with both binders showed capillary roughness on the surface of the microspheres.This was because the microspheres shrank and solidified during the solvent diffusion process,thereby roughening the surface.Fig.3(f)and Fig.3(j)show the particle size distributions of S-1 and S-2,respectively.The average particle size of the microspheres prepared with NC binder was 87 μm,and that of microspheres prepared with NC/F2604binder was 93 μm.The size distribution ranges from 70-105 μm for S-1 and 70-115 μm for S-2,indicating that S-1 has a smaller particle size and a narrower size distribution than S-2.Under the same experimental conditions,the addition of F2604resulted in co-crystal microspheres with a larger particle size.Both microspheres exhibited uniform particle size distribution,which would be beneficial to ensure consistent performance of the CL-20/TNT co-crystal microspheres [30].
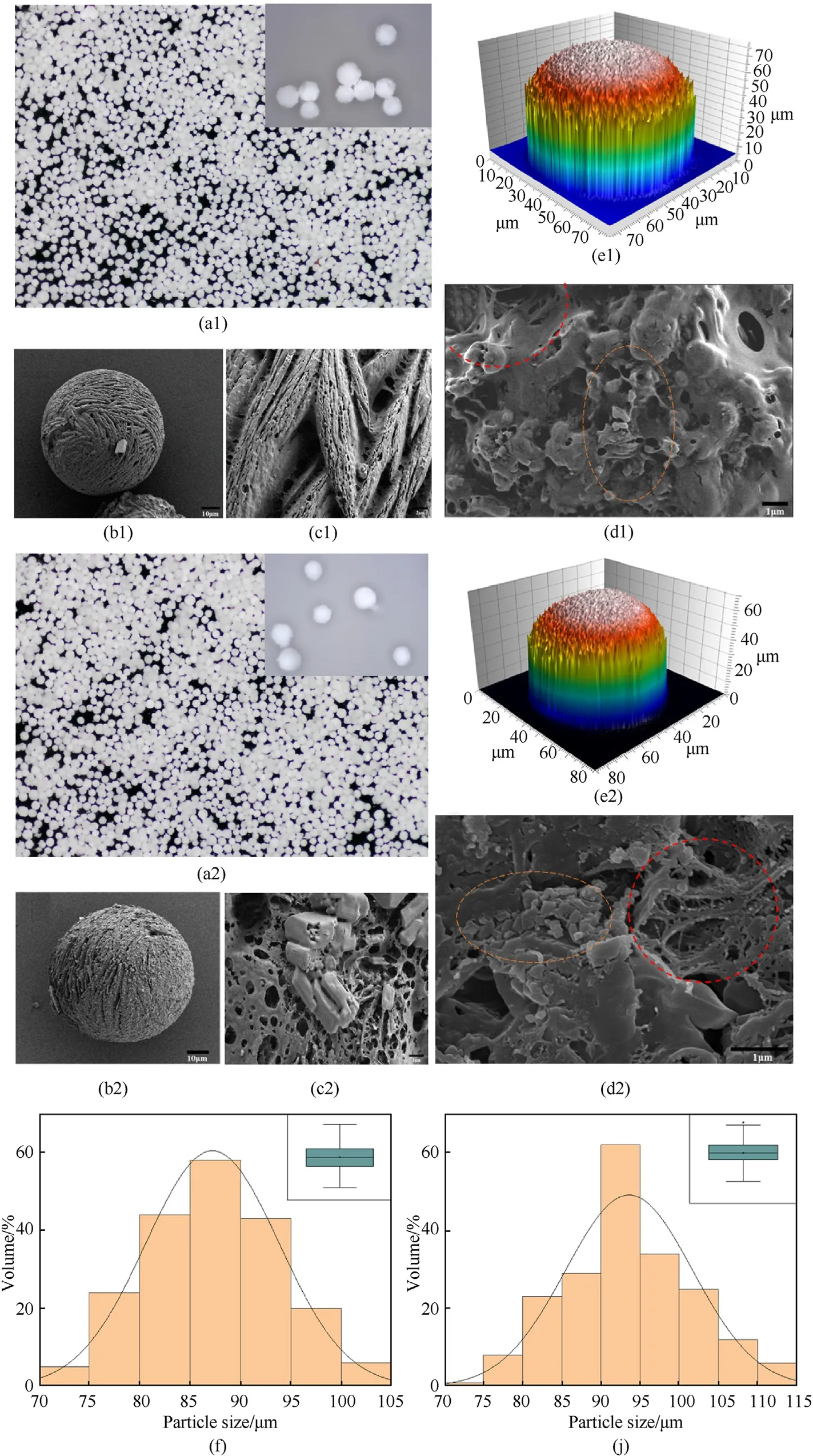
Fig.3.(a1) Microscope images,(b1,c1,d1) SEM images,(e1) 3D scanning image and (f) particle size distribution of S-1.(a2) Microscope images,(b2,c2,d2) SEM images,(e2) 3D scanning image and (j) particle size distribution of S-2.
3.3.Flowability characterization
The spherical morphology and surface roughness of the microspheres have a significant effect on the fluidity of the samples.The flow ability of raw materials and samples was analysed by measuring the angle of repose [35,36].An angle of repose below 30°indicates good flow,angle of repose between 30-45°indicates somewhat viscous flow,and angle of repose between 45-55°indicates viscous flow[37].The angles of repose of raw material CL-20,raw material TNT,Pure CL-20/TNT co-crystal,S-1 and S-2 were found to be 42.1°,41.9°,40.3°,28.2 and 30.1°,respectively,as shown in Fig.4.It can be seen that the co-crystal microspheres exhibited better fluidity than the raw materials and Pure CL-20/TNT co-crystal,and the dispersion effect was obviously better than that of the raw materials and Pure CL-20/TNT co-crystal.Furthermore,the co-crystal microspheres prepared by using NC binder had better fluidity than those prepared with NC/F2604composite binder.

Fig.4.Angle of repose results for raw materials,pure co-crystal and co-crystal microspheres.
3.4.Crystalline structure and chemical structure analysis
XRD analysis can yield information on co-crystal formation[38].The crystal structures of CL-20,TNT,pure CL-20/TNT co-crystal and as-prepared S-1 and S-2 were analysed by XRD,as shown in Fig.5,the red bar on the coordinate axis was the standard characteristic peak of CL-20,and the gray bar was the standard characteristic peak of TNT.It was found that S-1 and S-2 had the same characteristic peaks in the XRD pattern,indicating that the added F2604binder had no effect on the crystal structure of the co-crystal microspheres.Furthermore,the XRD pattern of the co-crystal microspheres was not a simple superposition of the raw material spectra,and had obvious differences with the raw material spectra.The characteristic diffraction peaks appearing at 10.9°,12.7°,16.5°,25.9°,and 30.5°in CL-20 did not appear in the XRD pattern of cocrystal microspheres.Similarly,the characteristic diffraction peaks appearing at 12.7°,17.8°,25.2°,27.3°,and 32.7°in TNT also did not appear in the XRD pattern of co-crystal microspheres.In addition,some new diffraction peaks appeared for the co-crystal microspheres,at 12.1°,13.8°,27.6°,etc.The disappearance of the diffraction peaks of the raw materials and the appearance of new diffraction peaks of the co-crystal microspheres indicated that the two CL-20/TNT co-crystal microspheres were not a simple mixture of the raw materials,but formed a co-crystal structure.Moreover,by comparing the diffraction peaks of the prepared pure CL-20/TNT co-crystal,it was observed that the co-crystal microspheres had the same diffraction peaks at 9.0°,12.5°,14.7°,20.2°,21.7°,29.7°,etc.However,there were offsets and merges between some of the characteristic peaks of pure CL-20/TNT co-crystal and CL-20/TNT co-crystal microspheres,resulting in inconsistencies in some of the characteristic peaks.This was probably due to the cocrystallization of CL-20/TNT within the three-dimensional pores formed by the binder.During the crystalline growth of the cocrystal the restriction of the pores leads to the limitation of the crystal growth space,while the increase in supersaturation keeps the crystallization going,at which point the binding force of the pores interacts with the expansion force of the crystal growth until the crystal stops growing.In this process,the crystal growth and expansion forces were greater than the binding force of the binder pores,and the continuous growth and expansion of the crystal will dope the binder,resulting in the doping of heteroatoms in the lattice and a large angular shift of the diffraction peaks[39].On the other hand,there can be stress-induced lattice distortions due to the binding force on the expansion force,resulting in a shift of the diffraction peak towards a small angle [40].At the same time,the constraining effect of the pores causes incomplete growth of the corresponding crystalline surface between the two nearby characteristic peaks,resulting in the phenomenon of merging peaks[41].The above results strongly demonstrate the successful formation of CL-20/TNT co-crystals in the prepared microspheres.
FT-IR spectroscopy can be used to study interactions between molecules and also to characterize co-crystals.Fig.6 shows the FTIR spectra of CL-20,TNT,pure CL-20/TNT co-crystal,S-1 and S-2.It can be seen that the use of different binders led to a slight shift in the individual absorption peaks of the CL-20/TNT co-crystal microspheres S-1 and S-2,but the shape of the absorption peaks remained consistent.The binder material coated with co-crystal microspheres caused a shift in the absorption peak position relative to the pure CL-20/TNT co-crystal,but the number of main absorption peaks did not change.This indicated that the molecular vibrations in the as-prepared co-crystal microspheres were consistent with those of pure CL-20/TNT co-crystal.Compared with raw materials,the spectrum of a co-crystal may correspond to a mixture of both single component spectra with slight shift in peak position [42].In addition,some of the characteristic infrared absorption peaks of the co-crystal microspheres also changed significantly.For example,the C-H stretching vibration moved from 3091 cm-1and 3039 cm-1for TNT and CL-20,to 3099 cm-1and 3027 cm-1for the co-crystal microspheres S-1 and S-2,respectively.The antisymmetric stretching vibration of -NO2in TNT and CL-20 shifted from 1541 cm-1and 1600 cm-1to 1554 cm-1and 1608 cm-1in the co-crystal microspheres S-1 and S-2,respectively.These changes indicated that the original structures of CL-20 and TNT underwent great changes in the co-crystal microspheres,due to the formation of co-crystal between CL-20 and TNT molecules.Due to the 5% of the total mass of the NC component in S-1,the 3% and 2% of the total mass of the NC and F2604components in S-2 respectively,the relatively low content of the binder,as well as the overlap of the F2604characteristic peak 885 cm-1,the NC characteristic peaks 1274 cm-1and 825 cm-1,and the raw material CL-20 characteristic peak (as shown in Fig.S2),resulted in the absence of the infrared characteristic peak of the binder in the graph [43,44].
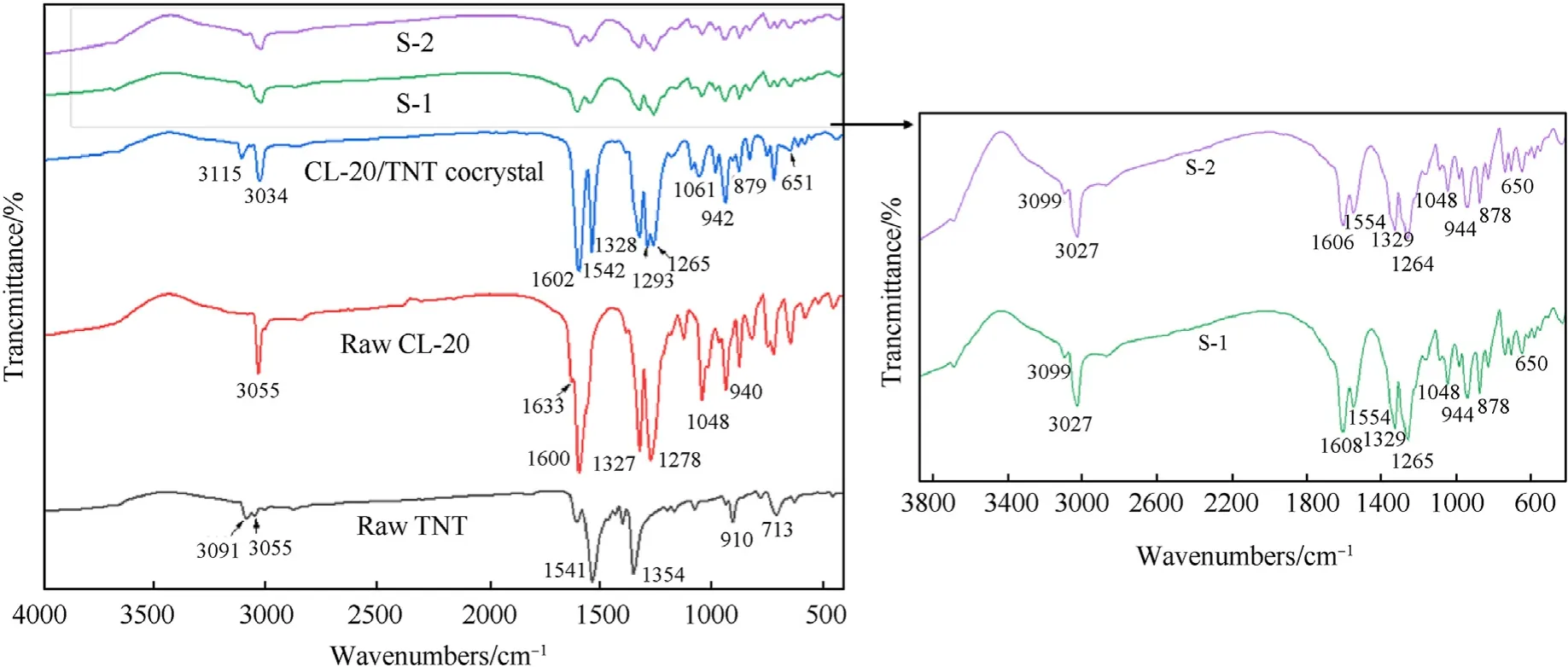
Fig.6.FTIR spectra of raw materials and samples.
3.5.Thermal behavior
Differential scanning calorimetry (DSC) was used to study the thermal properties of the raw materials,pure CL-20/TNT co-crystal,S-1and S-2,as shown in Fig.7.It can be clearly seen from the DSC diagram that the prepared co-crystal microspheres and the pure co-crystal had the same endothermic peak shape,which was clearly different from the thermal decomposition curve of the raw material.The thermal decomposition process of co-crystal microspheres included two processes: endothermic melting and exothermic decomposition.S-1 showed a melting endothermic peak at 135.91 ℃,and S-2 had a melting endothermic peak at 135.72 ℃.Compared with the endothermic peak(80.69 ℃)of TNT,the endothermic peaks of S-1 and S-2 were obviously delayed.The uniform particle size distribution of co-crystal microspheres S-1 and S-2 was more conducive to heat transfer,and their endothermic peaks were 4.77℃ and 4.96 ℃ earlier than that of pure cocrystal (140.68 ℃).Compared with the exothermic peak temperatures of CL-20 and TNT (245.51 ℃ and 225.88 ℃),the exothermic peaks of S-1 and S-2 lagged significantly and had a wider exothermic temperature range.This implied that the prepared co-crystal microspheres were not a simple mixture of raw materials,and possessed better thermal stability and exothermic properties than the raw materials.These results all indicated the successful preparation of co-crystal microspheres,further confirming the FTIR and XRD results.On the other hand,compared with the exothermic peak temperature of pure co-crystal(251.56 ℃),it was found that the exothermic peak temperatures of S-1 and S-2 were delayed by 2.09 ℃ and 3.23 ℃,respectively.This showed that the thermal stability of co-crystal microspheres can be further improved by coating with binder.Furthermore,the addition of binder F2604in S-2 (254.79 ℃) resulted in higher thermal stability of the co-crystal microspheres compared to the exothermic peak of S-1 (253.65 ℃).This method of rapidly obtaining CL-20/TNT co-crystal microspheres using droplet microfluidics provides an effective way to obtain co-crystal explosives with excellent thermal properties and thermal stability.

Fig.7.DSC curves of raw materials and co-crystal microspheres.
Fig.8 shows the TG loss curves of the raw material,pure cocrystal and microsphere samples.Consistent with the results of pure co-crystal,the co-crystal microspheres S-1 and S-2 showed three-stage thermos-gravimetric behavior,indicating a heterogeneous decomposition process,which was different from the homogeneous thermos-gravimetric process of the raw materials.In the first two stages of weight loss,between 100 ℃ and 200 ℃ and between 200 ℃ and 240 ℃,the co-crystal microspheres underwent a relatively slow thermal weight loss process,which may be caused by the evaporation loss of the co-crystal[42].As marked in the figure,during the first stage of weight loss,the evaporation loss of the co-crystal microspheres(S-1:24.30%;S-2:21.00%)was lower than that of the pure co-crystal (31.97%),due to the protection of the co-crystal by the coating layer in the co-crystal microspheres.This indicated that the coating layer slowed down the thermal weight loss of the co-crystal microspheres.The 3.3% lower evaporation loss of co-crystal in S-2 compared to S-1 suggests that the addition of F2604was more effective in preventing evaporation of CL-20/TNT co-crystal in the first stage of weight loss and provides better protection than the NC.In addition,since the spherical structure of co-crystal microspheres with uniform size distribution had better heat transfer ability,it entered the second-stage weight loss process earlier than pure co-crystal.At this time,due to the high temperature,the protection of the co-crystal by the coating layer weakened,resulting in an increase in the evaporation loss of the co-crystal microspheres (S-1: 9.98%;S-2: 11.98%) compared with the pure co-crystal (5.16%).The third stage was the main weight loss process of CL-20/TNT co-crystal.Rapid thermal weight loss was observed for pure co-crystal (240.53-253.83 ℃),S-1(238.16-251.33 ℃),and S-2 (238.16-253.67 ℃).This was because the CL-20/TNT co-crystal underwent a strong thermal decomposition reaction at this time,which corresponded to the thermal decomposition curve of DSC.At 400 ℃,the remaining residual mass of S-2 was the highest,because the thermal decomposition temperature of F2604was not reached,it was not decomposed.
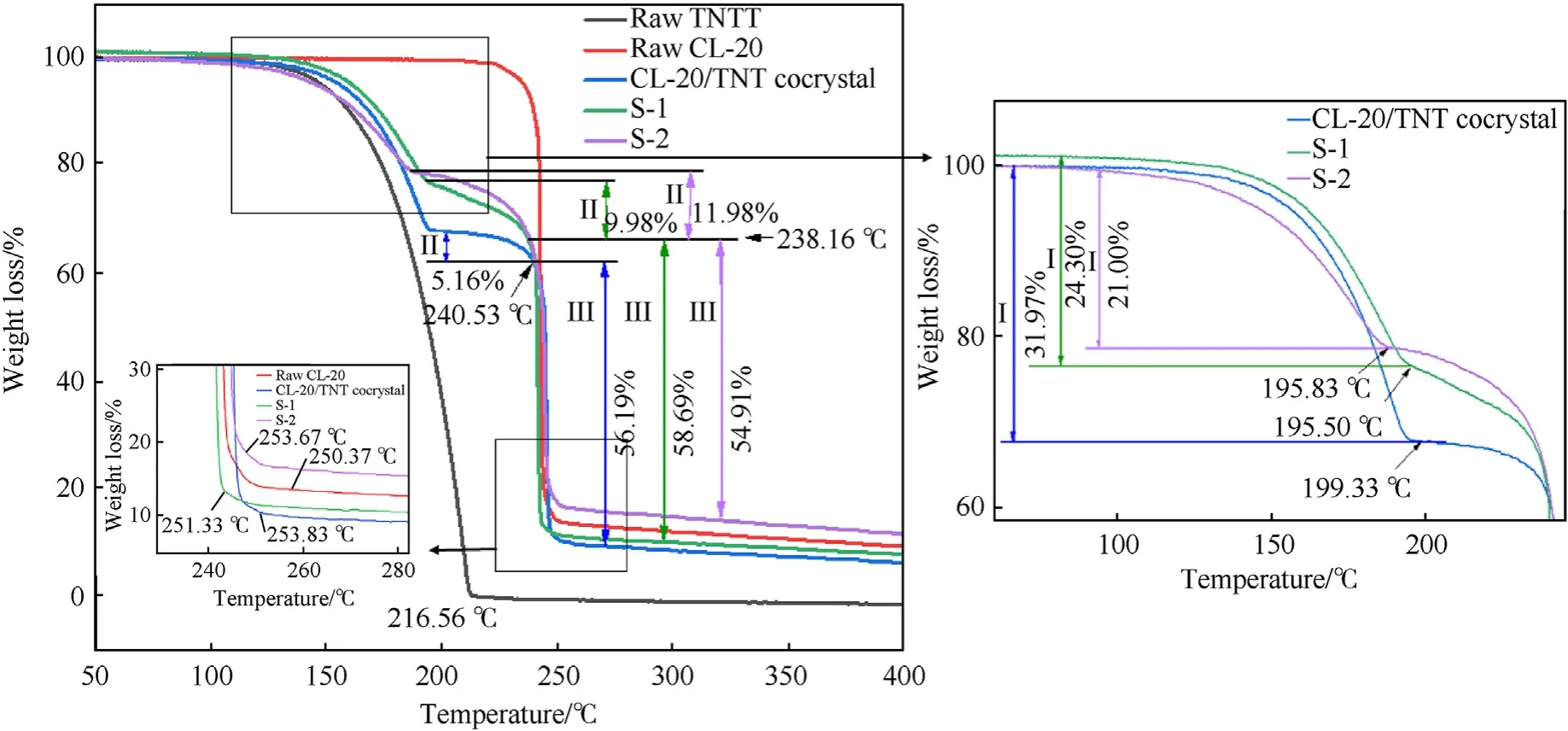
Fig.8.TG curves of raw materials and co-crystal microspheres.
3.6.Mechanical sensitivity
The safety of the prepared co-crystal microspheres was evaluated by testing the impact sensitivity and friction sensitivity of the raw materials,pure co-crystal and microsphere S-1,S-2.The results are shown in Table 1.Compared CL-20(3 J),the impact sensitivity of the co-crystal microspheres S-1 and S-2 increased by 7.5 J and 8.5 J,respectively,and increased by 1.5 J and 2.5 J respectively compared with the pure co-crystal (9 J).The explosion probability also showed a significant reduction under friction stimulation.As can be seen in the SEM images,the coated co-crystal in the samples of S-1 and S-2 were in the form of sub-micron strips with no obvious sharp edges.This allows the crystals to reduce the probability of inter-particle hot spot formation when subjected to relative sliding by external mechanical action[45].In addition,the particle size of the co-crystal particles to reduce the specific surface area increases,the particle surface atomic distribution increases,so that the cocrystal particles to enhance the ability to transfer heat,hot spots generated when the heat will be able to disperse out in a timely manner [46].Further,the CL-20 and TNT molecules were tightly and uniformly packed during co-crystallization through intermolecular interactions such as hydrogen bonding and other directional interactions [47].This packing stabilized the structure and effectively reduced the volume and number of voids in the crystal structure,thereby reducing the likelihood of hot spots forming under shock and frictional stimuli[48,49].At the same time,the cocrystal particles are coated to modify the surface of the particles to make them more regular,so that the co-crystal microspheres can be cushioned from external mechanical stimuli and dissipate external forces[50].The coating material provides a lubricating and heat-absorbing effect,these can improve the safety of S-1 and S-2.The comparison between S-1 and S-2 shows that S-2 has a lower mechanical susceptibility than S-1.This was because the F2604incorporated in S-2 was a polymer elastomer with better mechanical properties than NC,which can more effectively buffer the tendency of stress between explosive crystals when subjected to external mechanical action,further reducing the of the sensitivity explosive [51].Then,due to the uniform spherical structure of the co-crystal microspheres,the spheres were broken when the external force acted.This offset part of the force and inhibited the formation of hot spots,thereby further reducing the sensitivity of the CL-20/TNT co-crystal.These results indicated that the CL-20/TNT co-crystal microspheres prepared by droplet microfluidic technology possessed an excellent level of mechanical sensitivity.
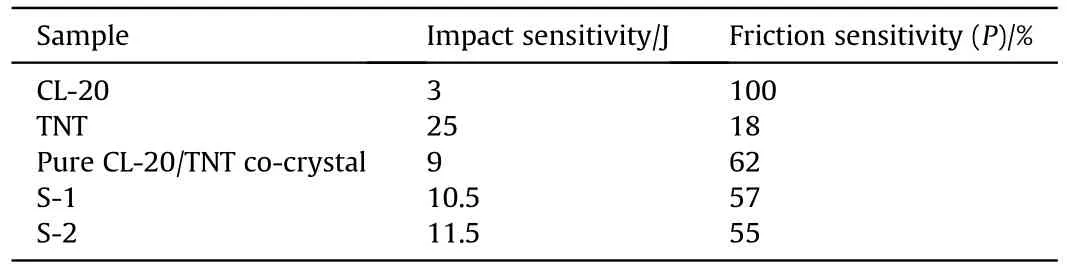
Table 1 Impact and friction sensitivity test results.
3.7.Combustion behavior
The combustion behavior of pure CL-20/TNT co-crystal,S-1 and S-2 was studied through ignition experiments,and the combustion propagation process was recorded by high-speed photography,as shown in Fig.9.It was found that pure co-crystals could not be successfully ignited.After ignition,the pure co-crystal flame cannot be recorded by high-speed photography and is accompanied only by smoke production,showing a “flameless burning”.This was because of the close arrangement of crystals within the pure cocrystal,the convective heat transfer effect is relatively poor,the heat generated cannot be effectively transferred to the interior of the particles,making the heat distribution between the particle bodies uneven,the heat output was low and cannot trigger the burning of adjacent particles,exhibits poor combustion.The prepared co-crystal microspheres were all successfully ignited and could maintain the self-combustion process,in contrast to the combustion process of pure co-crystal.S-1 co-crystal microspheres(Fig.9(a))ignited rapidly and quickly reached a stable combustion stage after a brief deflagration upon ignition.The flame edge was obvious,the centre was bright,and the edge was slightly oscillated,showing an efficient self-sustained combustion performance.S-2 co-crystal microspheres(Fig.9(b))ignited quickly,and also showed stable combustion after a short deflagration upon ignition.The flame height of S-2 was higher than that of S-1,showing brighter flame brightness and brightness area,and the burning time was shorter than that of S-1.This means that S-2 had higher combustion efficiency and exhibited better combustion performance than S-1.This may be due to the presence of high-energy F groups in it,which can form a higher chemically reactive region,thereby promoting the combustion process.Overall,both samples exhibited excellent self-sustained combustion behavior.Since the main body of combustion involved microspheres with uniform particle size distribution,it can accelerate the heat and mass transfer process of the combustion process,further improve the reaction rate,and promote the spread of combustion.Furthermore,the threedimensional porous structure of its microspheres was more conducive to the convective heat transfer in the combustion process.Thus,the self-sustained combustion performance of the cocrystal microspheres was maintained.These results indicated that the CL-20/TNT co-crystal microspheres prepared by droplet microfluidics possessed stable and excellent self-sustained combustion ability,solved the problem of defective combustion of pure CL-20/TNT co-crystal.

Fig.9.Combustion processes of (a) pure CL-20/TNT co-crystal,(b) S-1;and (c) S-2.
3.8.Detonation performance
Detonation velocity,detonation pressure and detonation heat were the main parameters in the detonation performance of explosives.The theoretical maximum detonation performance of CL-20,TNT,pure CL-20/TNT co-crystal and S-1 and S-2 samples was calculated by the EXPLO5 program.The results are shown in Table 2.It was found that the formation of co-crystal between CL-20 and TNT can improve the oxygen balance of the explosive and substantially increase the TNT detonation parameters.For example,compared to pure CL-20/TNT co-crystal detonation parameters,the CL-20 detonation velocity loss was 11.8% and the TNT detonation velocity was increased by 18.86%.The S-2 has a higher detonation velocity and detonation pressure than the S-1,and the S-1 has a higher detonation heat level than the S-2.Further,compared to pure CL-20/TNT co-crystal,S-1 and S-2 offer better thermal stability,lower mechanical sensitivity and more stable self-sustaining combustion performance with only a small loss of detonation parameters and detonation performance almost equivalent to that of the current military standard explosive RDX.In conclusion,the CL-20/TNT co-crystal composite microspheres prepared by droplet microfluidics not only have a higher level of detonation parameters but also further improve the comprehensive performance of the CL-20/TNT co-crystal.
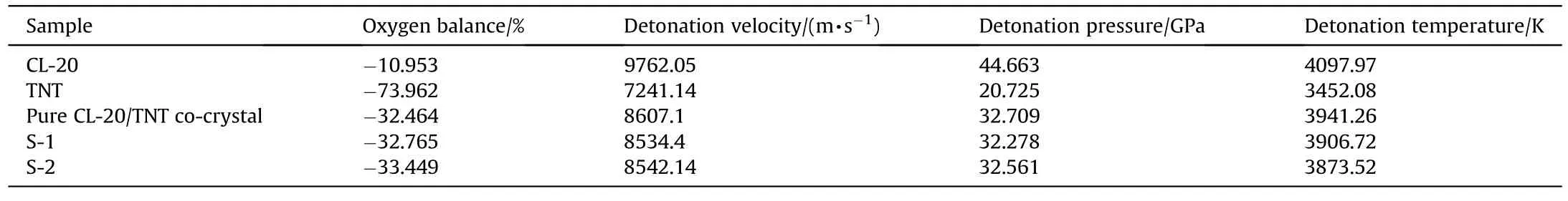
Table 2 Detonation performance of samples.
4.Conclusions
In this study,the integrated preparation of CL-20/TNT co-crystal assembly and spheroidized coating was successfully realized by droplet microfluidic technology.SEM results showed that the use of NC and NC/F2604compound binders had a good coating effect on the co-crystal microspheres,and the obtained microspheres displayed excellent uniformity of size distribution.The angle of repose test showed that the co-crystal microspheres had superior flow properties compared to the raw materials.Comparison of XRD,FTIR,DSC and TG data of the raw materials and pure CL-20/TNT cocrystal verified the formation of CL-20/TNT co-crystal in the microspheres.Moreover,the co-crystal microspheres had higher thermal stability than the raw materials and pure co-crystal;the thermal stability of NC/F2604-coated product was better than that of NC-coated co-crystal microspheres.The mechanical sensitivity test showed that the CL-20/TNT co-crystal microspheres were less sensitive than CL-20,and their sensitivity was further reduced compared to the pure co-crystal due to the coating of the binder.The addition of F2604in the binder can further reduce the sensitivity level of the co-crystal microspheres.In addition,the two prepared co-crystal microspheres exhibited bright combustion flame and long combustion time,as well as excellent self-sustained combustion performance.The co-crystal microspheres showed good theoretical detonation performance.Overall,the proposed novel method can realize the facile and rapid preparation of CL-20/TNT co-crystal microspheres.It also offers both experimental and theoretical guidance for the simple and rapid preparation of similar co-crystal explosives.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge National Natural Science Foundation of China (Grant No.22005275) to provide fund for conducting experiments.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2022.09.013.
杂志排行
Defence Technology的其它文章
- An energetic nano-fiber composite based on polystyrene and 1,3,5-trinitro-1,3,5-triazinane fabricated via electrospinning technique
- Dynamic response of UHMWPE plates under combined shock and fragment loading
- Aerial multi-spectral AI-based detection system for unexploded ordnance
- Model-based deep learning for fiber bundle infrared image restoration
- Robust design and analysis for opto-mechanical two array laser warning system
- Combustion behavior and mechanism of molecular perovskite energetic material DAP-4-based composites with metal fuel Al
