Effect of inorganic salt on the thermal degradation of nitrocellulose and reaction mechanism of its mixture
2023-10-09GuozhongXuXuGaoMiLiZhongxuanHanLinJiang
Guo-zhong Xu,Xu Gao,Mi Li,Zhong-xuan Han,Lin Jiang
School of Mechanical Engineering,Nanjing University of Science and Technology,China
Keywords:Thermal analysis Thermal decomposition mechanism Malek method Nitrocellulose Inorganic salt
ABSTRACT In this study,to better understand the reaction mechanism between inorganic salts and nitrocellulose,CaCO3 and Li2CO3 were evaluated with respect to their effects on the thermal degradation of NC in nitrogen atmosphere using TG/DSC at three different heating rates (2,5,10 K/min).The numerical relationship between activation energy (E)and conversion rate was obtained by FWO and KAS method,and it was discovered that CaCO3 could improve the thermal stability of NC.Activation energy values were calculated by Kissinger method,and it was found that NC that contain Li2CO3 had the highest activation energy while NC containing CaCO3 had the lowest E value.By combining the thermal analysis data with Malek method,the most probable mechanism model of thermal degradation is obtained as esták-Berggren model,which expression is f(α)=αm(1-α)n.As a result of this study,there are certain guiding principles that can be applied to the pyrolysis reaction model and to the actual production process of nitrocellulose.
1.Introduction
Nitrocellulose (NC) is a typical flammable and explosive substance,which is not only an important component of propellants and high explosive,but also widely used as additives in paints and varnishes [1].In the military and civil industries,spontaneous combustion is one of the problems faced because of NC’s flammable and explosive properties.
Studies have been conducted on the mechanism of NC spontaneous combustion.Historically,nitrogen oxides,primarily NO2,have been known to reduce the induction period of NC,and the presence of oxygen increases the heat flow during NC pyrolysis.In the previous studies,it had been pointed out that the stability of NC was affected by nitrogen dioxide,therefore,the initial stage of thermal degradation of NC was affected by nitrogen dioxide [2].Based on this finding,the commonly stabilizers of NC,terephthalic(TPA),N,N′-Dimethyl-N,N′-diphenylurea(C2),Akardite-II(AKII),andN-Methyl-4-nitroaniline(MNA),can capture the nitrogen oxides released during NC pyrolysis to improve thermal stability[3].For these typical stabilizers,scholars have recently conducted the following studies.Wei et al.studied the effect of plasticizer DPA on the thermal decomposition of NC and it was found that at lower temperatures,DPA improved the thermal stability of NC,but no improvement was observed at higher temperature [4].Zhao Yang et al.[5] investigated the effects of four typical stabilizers (DPA,AKII,MNA,C2) on the thermal decomposition of NC by Adiabatic acceleration calorimeter (ARC) apparatus,and found that the addition of stabilizers would change the autocatalytic reaction mechanism of NC,and the reaction mechanism of NC might become biased from Bnaautocatalytic reaction mechanism to nlevel reaction mechanism;MNA had the most significant effect on the autocatalytic reaction characteristics of NC.Cieslak et al.[6]investigated the effect of stabilizers (C1,C2,AKII and TPA) on the thermal stability of nitrocellulose with different nitrogen contents and found that TPA had the best stabilization effect and prolonged the induction period of the autocatalytic reaction compared to other stabilizers.In addition,scholars have also devoted themselves to exploring new,environmentally friendly stabilizers [7-9] and studying the effect of two-component stabilizers on the thermal degradation of NC[10-12].
In 2010,due to the fact that industrial water would be used at the factory to clean NC,and industrial water contains a wide range of inorganic salts.Thus Katoh et al.studied and found that induction periods for NC containing 2% CaCO3under isothermal storage at 120 ℃ were about 15 h longer than those for NC containing CaSO4,CaCl2,ZnSO4,and CuCl2were about 4-5 h shorter than those for pure NC under the condition of isothermal storage at 120 ℃.Therefore,it was considered that when components of industrial water were accumulated in NC,the stability and instability of inorganic salts cancelled each other[13].Katoh et al.selected 17 kinds of weakly basic salts and hydroxides and studied their effects on the thermal stability of NC.It was pointed out that the effect of inorganic salts on the thermal stability of NC could be divided by its acidity and alkalinity:strong alkaline solution(pH>13)contracted the induction period,alkaline saturated solution (pH=10-12)prolonged the induction period,and neutral solution accelerated the hydrolysis of NC [14].It was found that the effect of Li2CO3on the thermal stability of NC was about the same as that of conventional stabilizers,and the thermal stability increased with the increase of the amount of inorganic salt under the condition of 120 ℃ isothermal experimental [15].In 2018,Wei et al.analyzed the thermal behavior of NC with different aging periods.Results showed that NC had the worst thermal stability when they were stored at 90 ℃ for 24 days,and the thermal stability of NC could significantly increase when the storage time exceed 32 days [16].
Most of the aforementioned work focused on the effect of additive on NC and it was found that the alkaline environment of NC could inhabit the occurrence of thermal spontaneous combustion.In practice,inorganic salts are usually added to NC-humectant systems,and the reactions between NC and humectant [17-20]have been studied deeply,but the reactions between NC and inorganic salts are less studied and not mathematically expressed.The focus of this paper is to study the reaction mechanism of the mixture,NC contains Li2CO3or CaCO3,which was analyzed using a Thermogravimetry/Differential Scanning Calorimetry (TG/DSC)analyzer to obtain the TG-DTG-DSC curves and thus calculate the kinetic parameters.
2.Experiment
The experiment used a NC,and CaCO3,Li2CO3were produced by Sinopharm Chemical Reagent Co.,Ltd.and Shanghai Aladdin Biochemical Technology Co.,Ltd.,respectively.The purity of these inorganic salts reached to 99%,and 99.99% respectively.The mixture was prepared according to the principle that the inorganic salt mass was 5% of the total mass.Fig.1 shows that the preparation process of the samples: the inorganic salts were added to the corresponding mass of NC,soaked in anhydrous ethanol,stirred and then baked in an oven at 45℃ for 9 h.The samples were turned and baked in the oven for another 4.5 h.There are 3 kinds of samples,2 of which are the mixture of NC and inorganic salt.NC samples containing CaCO3,Li2CO3were named as NC-C,NC-L,respectively.The 3rd kind of samples is the pure NC (NC-P) that is used for comparison (shown in Table 1).
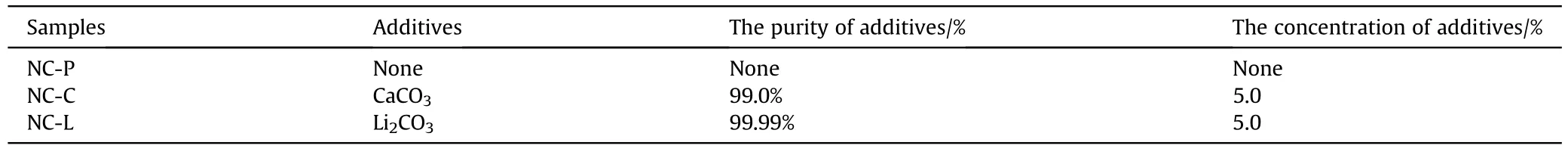
Table 1 Samples nomenclature and inorganic salt content in the samples.
Fig.1.A brief process of samples processing is shown.
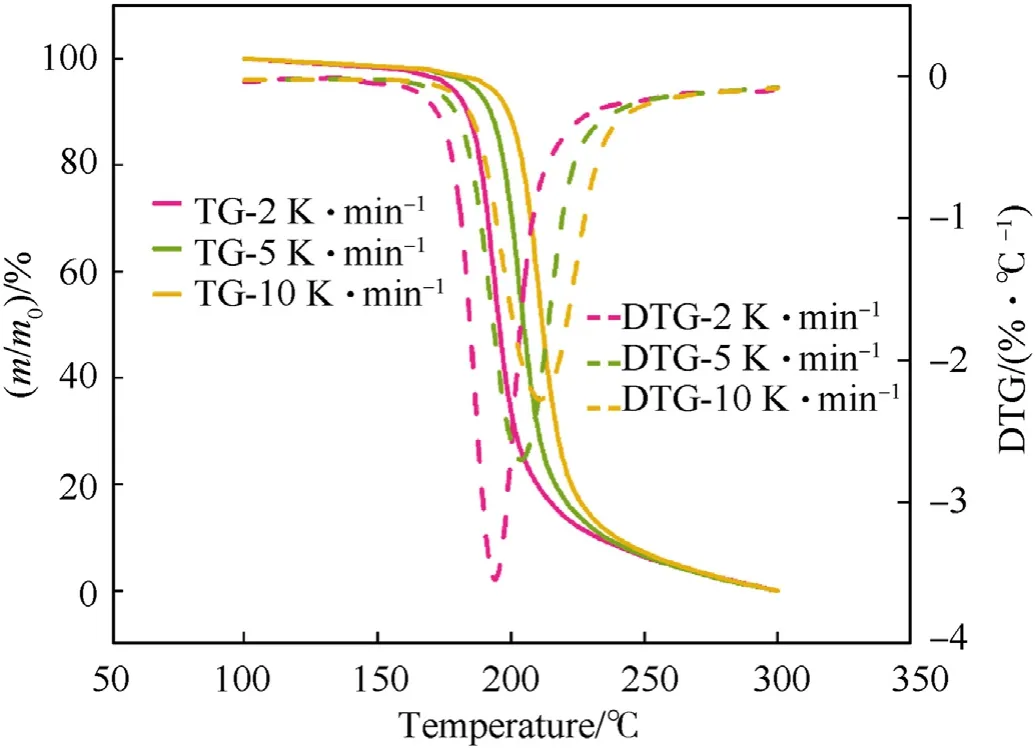
Fig.2.The TG and DTG curves of NC-P at different heating rates were compared.
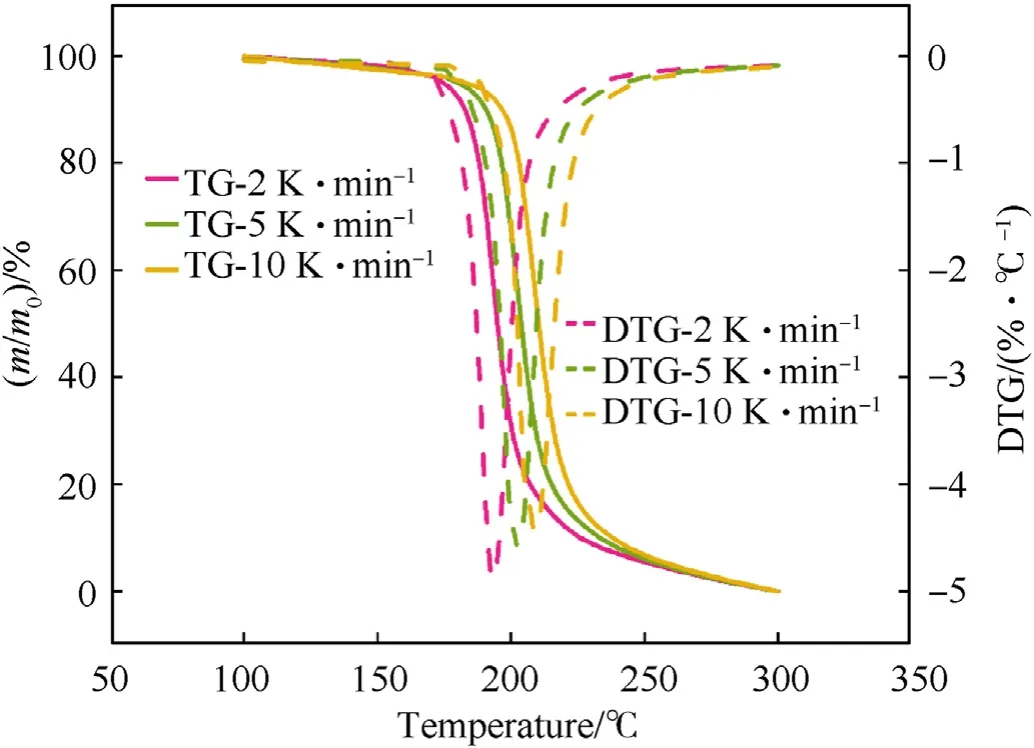
Fig.3.The TG and DTG curves of NC-C at different heating rates were compared.
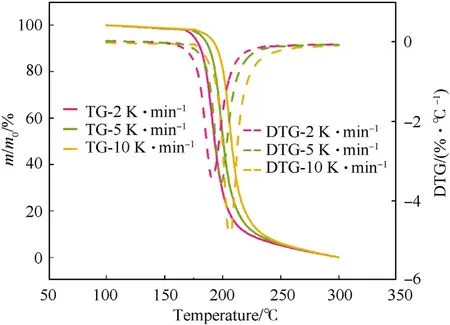
Fig.4.The TG and DTG curves of NC-L at different heating rates were compared.
The instrument type used in this research is METTLER TOLEDO TG/DSC 3+made in Switzerland,which has a heating rate of 0.02-150 K/min,and the sensitivity of balance is 0.1 μg.The heating rates of experiments were 2,5,10 K/min,respectively.Nitrogen was selected as the flowing gas under the flow rate of 30mL/min-1;the sample mass of each experiment was 1 mg and the initial mass error of the sample should not exceed 0.1 mg;the temperature range for all samples was set as 25-450 ℃.
3.Calculation methods
For the decomposition process of NC,the chemical reaction rate can be described by Eq.(1) according to Arrhenius equation
whereTis the temperature in absolute temperature,K;β is the constant heating rate,K/min,and its expression is β=dT/dt;k(T)is the chemical reaction rate constant,s-1;f(α) is the reaction mechanism function,dimensionless;the conversion rate,α,is the ratio of the instant mass loss of the sample to the total mass loss in a certain temperature range.The conversion rate can be calculated using Eq.(2)
Here,mo,mandmfare the initial mass,the mass at timet,and the residual mass of the sample at the end,respectively,all samples in this paper are in milligram.
k(T) can be expressed by Arrhenius equation as follows:
whereAis the pre-exponential factor,min-1;Eis the activation energy,J/mol;Ris the gas constant,and its value equals to 8.314 J/(mol∙K).
Based on the above equation,Eq.(1) can be described by the following equation:
Among the above parameters,the activation energyE,preexponential factorAand reaction mechanism functionf(α) are combined into a definition of kinetic triplet.It can be obtained that the relevant conclusions of the thermal stability and thermal decomposition mechanism of the sample through the calculation and analysis of the kinetic triplet during the pyrolysis of NC and mixture samples.
Activation energyErepresents the minimum energy required for reactant molecules to reach the active molecules.In other words,low activation energy represents a high risk of materials.The TG curves is obtained under non-isothermal conditions,and the activation energy can be calculated by the iso-conversional rate method.The typical iso-conversional rate methods include Kissinger-Akahira-Sunose (KAS) [21,22],Flynn-Wall-Ozawa (FWO)[23,24] and Friedman [25] for calculations.The expressions of the calculation methods are as follows:
whereTα,i,Aα,are the absolute temperature,pre-exponential factor at the extent of conversion α,respectively.βiis the heating rate.G(a)is the integral form of the reaction mechanism function.The subscriptiindicates different heating rate.By plotting lnβi/Tα,i2,lnβi,and ln[βi(dα/dt)α,i] against 1/T for the whole range of conversion(0-1),the activation energy can be obtained according to the corresponding slopes from Eqs.(5)-(7).
Currently,a number of methods exist for determining the reaction mechanism function,including Satva method,Bagchi method,the double extrapolation method,Malek method,Dollimore method,CRTA method,etc.[26-28].It should be noted that the formal assumptions and approximations of the mechanism function leads to systematic errors in all of these methods.Professor J.M.Criado and the International Society of Thermal Analysis have proposed that the Malek method can significantly reduce the range of kinetic mechanism function,because there are no assumptions and approximate conditions in this method,so the accuracy of the mechanism function is effectively improved[28-32].In this paper,the Malek method was employed to determine the thermal decomposition mechanism function of NC and mixture samples.The detail derivation process of Malek method is as follows:
Reaction rate equation and the Coats-Redfern equation are shown in Eqs.(4) and (8)
According to Eqs.(4) and (8),it can be deduced that
when α equals to 0.5,Eq.(9) can be written as
whereT0.5and(dα/dt)0.5are the temperature and reaction rate when α is 0.5,respectively.
Dividing Eq.(9) by Eq.(10) gives the expression fory(α)
Here,y(α) is the definition function.There are 19 commonly used kinetic mechanism functions,corresponding to different mechanism model [33].The mechanism function is shown in Table 2.
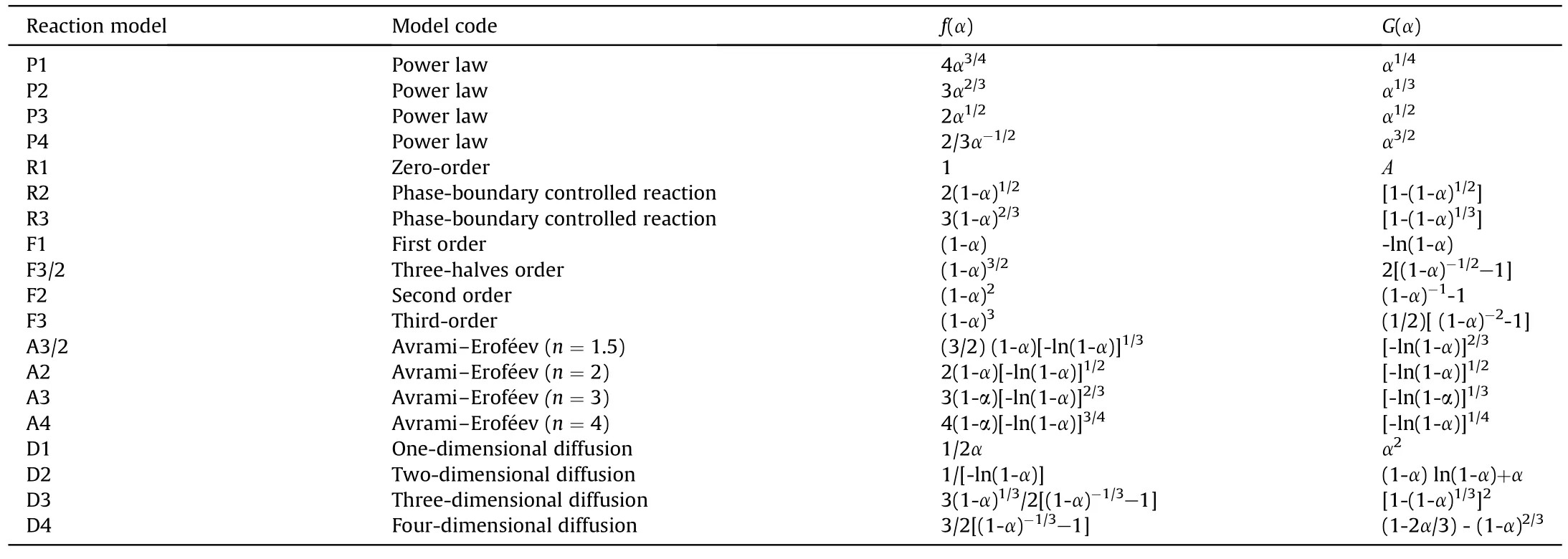
Table 2 Commonly used reaction models describing the pyrolysis process of matters.
3.1.The most probable f(α) using the y(α) - α standard curve
The standard data consisting of αi,f(αi) (i=1,2,3,…,j) and α=0.5,f(0.5) were substituted into Eq.(11).And it was obtained that the relationship curve betweeny(α) against α,which is regarded as the standard curve[33].
The experimental data consisting of αi,Ti,(dα/dt)I(i=1,2,3,…,j)and α0.5,T0.5,(dα/dt)0.5were substituted into Eq.(11).It was gained that the relationship curvey(α)against α,which is regarded as the experimental curve.
3.2.The most probable f(α) using the Z(α) - α standard curve
In non-isothermal experiment,the relationship between sample temperature and experimental time is as follows:
whereT0is the initial temperature of sample,K;tis experimental time,min.
As shown in Eq.(13),E/RT,which is common in Arrhenius equation,has defined asu
The temperature integral equation
According to Eqs.(4) and (12)-(14),it is known that
where π(u) is the rational function in the temperature integral Eq.(14),and its expression is π(u)=1/(u+2).
According to Eq.(16),the expression ofZ(α) is
The standard data that the data are αi,Z(αi) (i=1,2,3,…,j) are substituted into Eq.(17) and the graph is made to obtain in the Z(α)-α relationship curve,which is regarded as the standard curve to obtain the standard profile [33].
The experimental data that the data are β,αi,Ti,(dα/dt)i(i=1,2,3,…,j) and theEvalue that obtained by FWO method are substituted into Eq.(17)for theZ(α)-α relationship curve,which is the experimental curve[33].
4.Results and discussion
4.1.TG/DSC analysis
From Figs.2-4,it is shown that the thermal degradation processes of NC and its mixtures have a main peak,and the temperature of the main peak increases with the increase of heating rate.For example,the temperature corresponding to the maximum exothermic rate of NC-P are 193.98,204.00 and 211.02 ℃,respectively.It can be seen from Fig.5 that the heat flow of NC-P pyrolysis increases with the increase of heating rate,and the values are in turn:2.45,7.09,13.85 W/g.Combining Figs.5 and 6 and Table 3,it is found that with the increasing of heating rate,CaCO3has more and more obvious influence on the thermal degradation of NC.Combined Figs.5-8 and Table 3,it is found that Li2CO3has a certain inhibitory effect on the heat flow during NC pyrolyzes at all heating rates,the heat releases during NC-L thermolysis are 1 time,0.735 times and 0.896 times of those of NC-P,respectively.At the same heating rate,the TG curves of these samples are similar,but the DSC curves of these samples are not similar.When the heating rate is 2 K/min,the peak values of DSC curves of NC-C are much higher than those of NC-P and NC-L,and the curves of the latter two are relatively similar;when the heating rate is increased,the peak values of DSC curve of NC-L are much smaller than those of the other two,and the heat releases during NC-C pyrolyzes are always higher than that of NC-P.To sum up,it can be presumed that in the case of non-isothermal condition,CaCO3can catalyze the exothermic of NC and Li2CO3can hinder the heat release of NC thermolysis.
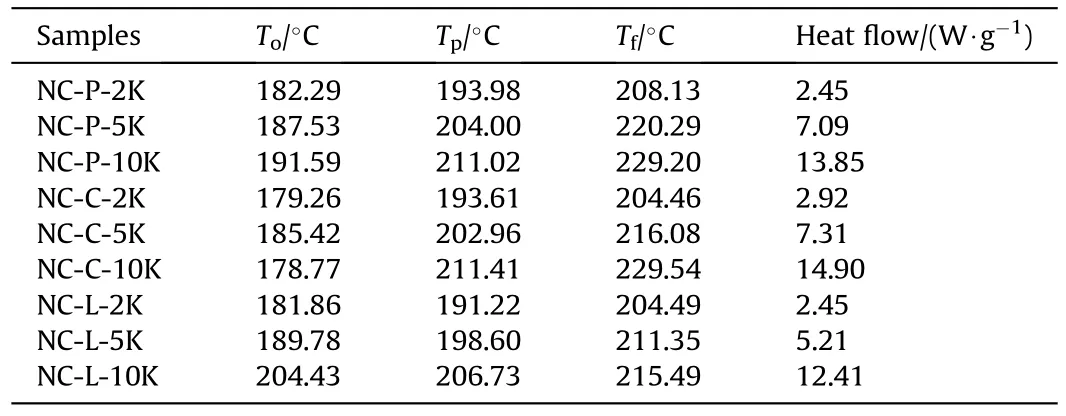
Table 3 The characteristic temperatures and heat flow of the decomposition process obtained at different heating rates.
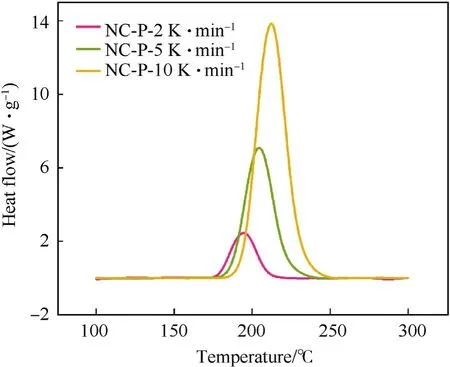
Fig.5.The DSC curves of NC-P at different heating rates were compared.
It can be found from Table 3 that the presence of inorganic salts can change the onset temperature of the thermal degradation process of NC,such as the presence of CaCO3makes the onset degradation temperature of NC decreases at all heating rates (see Fig.6).On the other hand,Li2CO3makes that the onset degradation temperature of NC descends at a lower heating rate;when β=5 or 10 K/min,the onset degradation temperature is increased,which contributed to the thermal stability of NC.This is because Li2CO3can effectively inhibit NOxreleased from NC by neutralizing the acids that accelerates the thermal degradation of O-NO2bond (as shown in R1-R3)[15].It's important to point out that the amount of NOxreleased from NC is independent of the thermal behavior of NC.
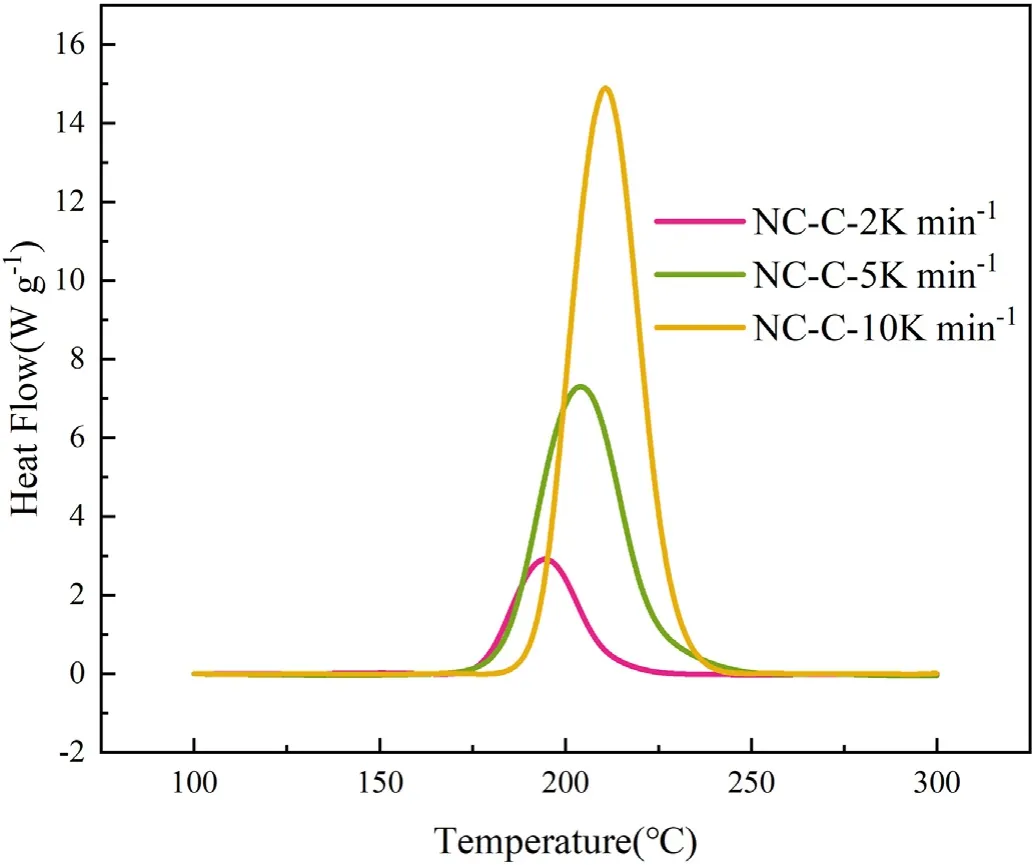
Fig.6.The DSC curves of NC-C at different heating rates were compared.

Fig.7.The DSC curves of NC-L at different heating rates were compared.
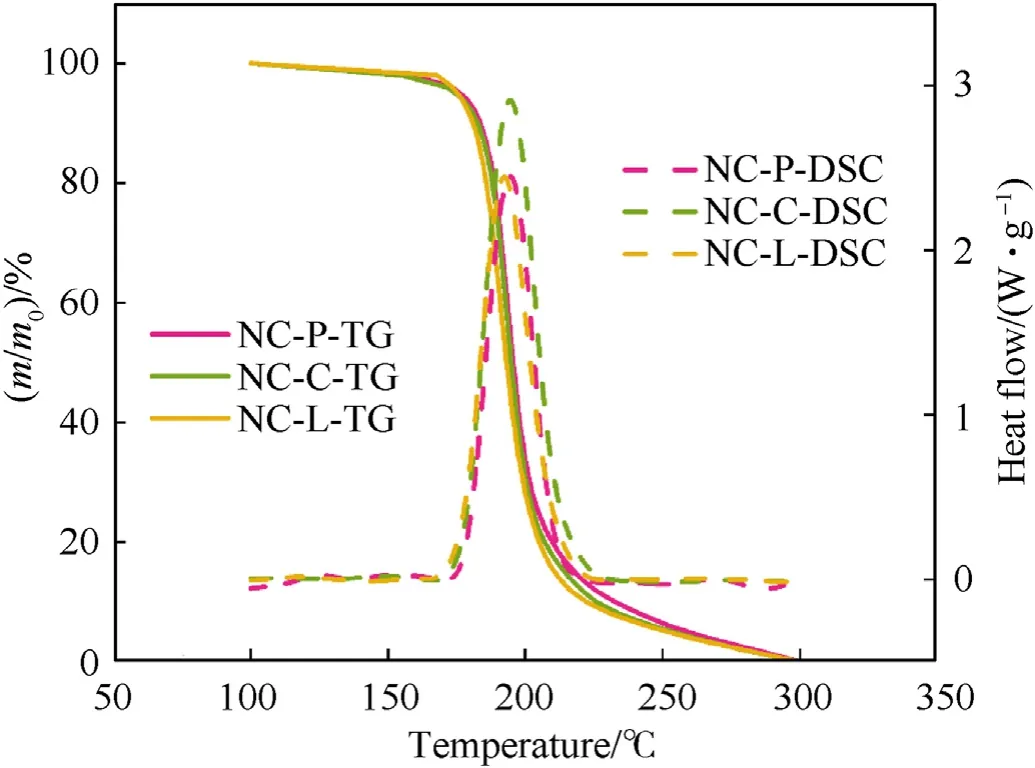
Fig.8.When β=2K/min,the TG and DSC curves of samples were compared.
Two iso-conversional methods,including KAS,FWO methods,were applied to calculate the thermal kinetics of sample pyrolysis.The relationship between the activation energy (E) and the conversion rate (α) can be obtained by applying aforementioned kinetic methods.It can be seen from Fig.9 that the activation energy of NC-P increases with the growth of conversion,and the activation energy of NC-C decreases initially,and then remains roughly constant;and when α <0.68,the activation energy of NC-C is higher than that of NC-P,it is caused by that CaCO3stabilized by neutralizing acids.As compared to the previous samples,NC-L shows different patterns ofE-α relationship: with the growth of conversion,the activation energy of NC-L increases firstly,and then decreases;when α ≤0.22,the values are smaller than that of NC-P;when α >0.24,the activation energy value of NC-L is significantly larger than that of NC-P.It indicates that Li2CO3can reduce the thermal stability of NC.
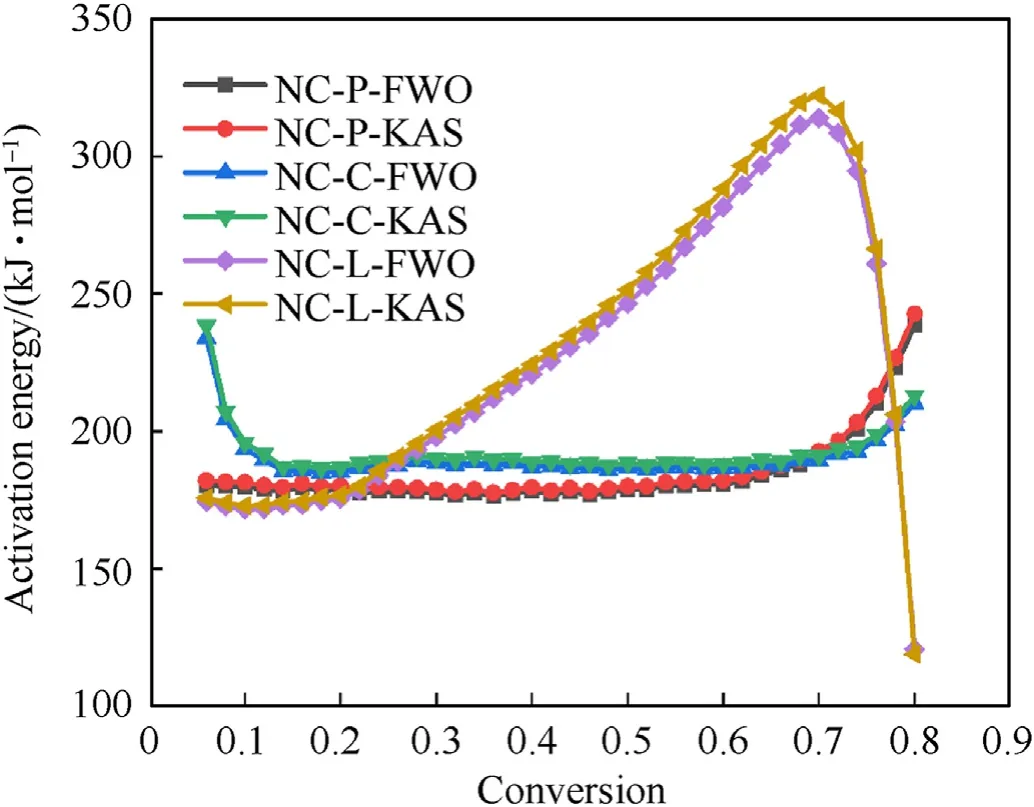
Fig.9.The E-α relation of each sample was calculated by FWO and KAS method.
As shown in Table 4,activation energy is calculated using the Kissinger method for each sample atTp,the peak temperature of the DTG curves,it is found that NC-L exhibits the highest activation energy,with a value of 261.14 kJ/mol;NC-C exhibits the lowest activation energy,with a value of 162.33 kJ/mol.Despite the fact that the values of activation energy are different from those shown in Fig.9,it can be pointed out that the sample containing Li2CO3has the largest value of activation energy in the whole process.
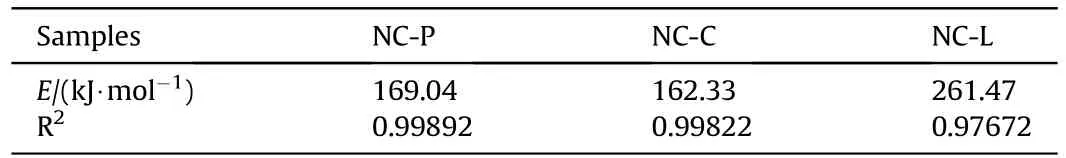
Table 4 Activation energy for each sample was calculated by the Kissinger method [22].
4.2.Determination of mechanism function of thermal decomposition
4.2.1.Determination of the most probable mechanism function of thermal decomposition
The Malek method can be used to determine the category by comparing they(α)-α andZ(α) -α of the experimental data with the standard curve,or they(α)shape and eigenvalues can be used to infer the most probablef(α).In this paper,the latter approach is taken.
The FWO method was applied to calculate theEvalues of each sample.Based on the TG data,the reaction rate dα/dt,y(α)and the corresponding temperatureTwere calculated by Eq.(11) andZ(α)was obtained by Eq.(17).The experimental curves ofy(α)-α andZ(α)-α at various heating rates are shown in Fig.10.
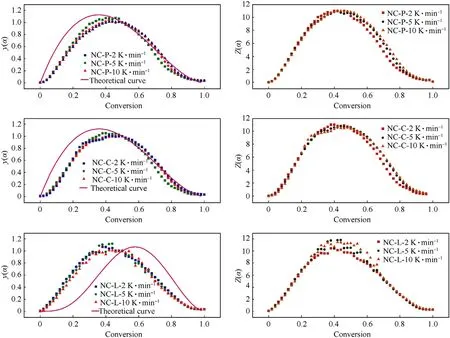
Fig.10.Curves obtained by Malek method of all samples.Note:The figures in the left row show the y(α)-α curves obtained from experimental data and theoretical formulas,and the figures in the right row show the Z(α)-α curves;from top to bottom,the pictures in each row are the experimental curves of NC-P,NC-C,NC-L respectively.
The curves obtained from the experimental data are used to find the eigenvalues αMandboth of which are the corresponding α at the peak of they(α)-α andZ(α)-α curves,respectively,αpis the α at the peak of the DSC curve.The most probable mechanismf(α)can be inferred from these parameters.Table 5 presents the characteristic parameters derived from the fitted curves.
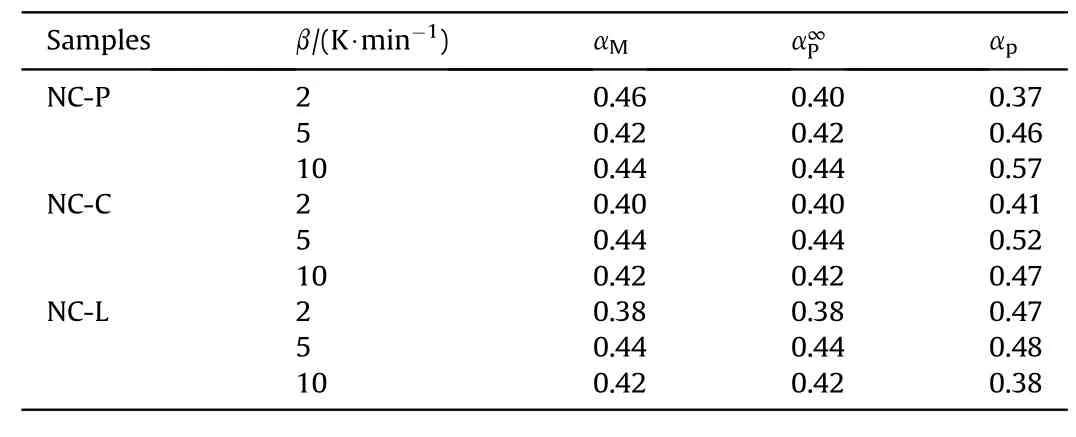
Table 5 Characteristic parameters of each sample.
Based on the criterion in Ref.[33] combined with the data in Table 5,it can be inferred that the decomposition of these samples conforms to theesták-Berggern mechanism model,which indicates that the decomposition of these samples fits the autocatalytic pyrolysis model.The mechanism function of the S.B.model can be described asf(α)=αm(1-α)n.
4.2.2.Determination of the kinetic parameters m,n
The parameters,mandn,can be determined by the following equation
The result is shown in Table 6.
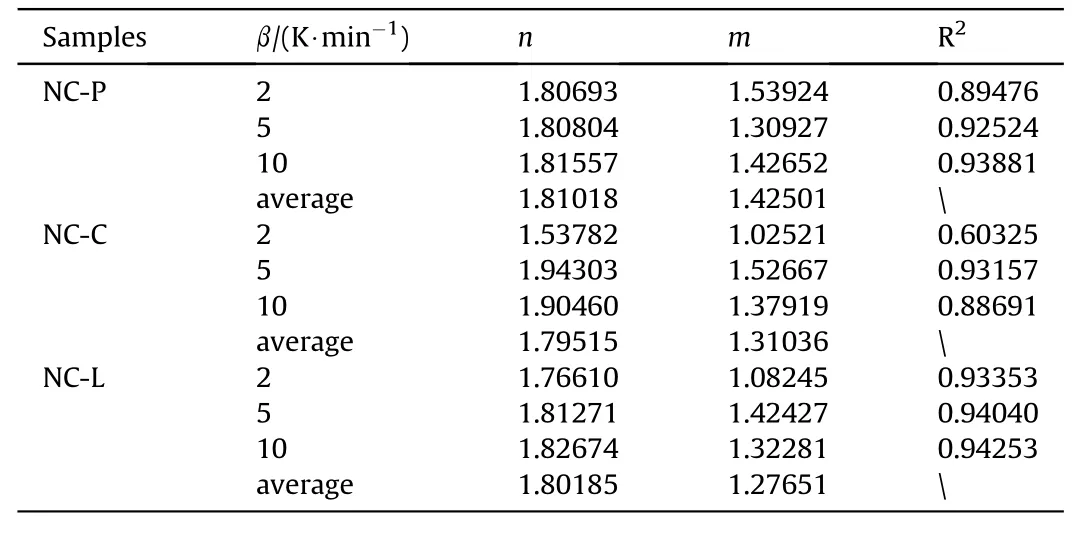
Table 6 Kinetic parameters were calculated at different heating rates.
Therefore,the thermal decomposition mechanism of NC-P,NCC,NC-L are.
Fig.10 illustrates the comparison of they(α)-α curves obtained from Eqs.(20)-(22) with those obtained from the experimental data.
4.3.The limitation of the research
Although the Malek method has the advantages of avoiding the trouble of tryingf(α)one by one and the effect of KCE whenE,Aandf(α)are obtained simultaneously,the limitations of this paper are respectively.
Firstly,in the previous study,the scholars found that the pyrolysis of NC was affected by the n-order model,the autocatalytic reaction model [34-38] and the modulation function [39,40].However,both thenth-order model and the autocatalytic model are not represented in this paper,this may be due to the fact that the addition of inorganic salts may lead to the ratio of nth-order model and the autocatalytic model.This paper assumes in the theoretical part only for the autocatalytic model,which is the main problem.
Secondly,in the actual storage,inorganic salts are usually added to NC-humectants system to achieve a better stable effect.In this paper,the authors only consider the reaction between inorganic salts and NC and ignore the situation in the actual storage.The authors plan to investigate the mechanism and decoupling analysis of inorganic salt in NC-humectants in the next experiment to explore the optimal ratio of inorganic salt in this system.
5.Conclusions
The thermal stability,reaction mechanism of NC with different inorganic salts and pure NC were investigated using a TG/DSC apparatus.The main conclusions from the analysis can be summarized as follows:
(1) The characteristic temperatures of NC and its mixtures indicate that the presence of inorganic salt can have some effect on the thermal degradation of NC.The TG curves of samples heated at different heating rates were analyzed using the FWO and KAS method,and it was found that: the activation energy value of NC-L increased first,followed by a decline,and when α <0.24,the values are less than that of NC-P.In other words,with the presence of Li2CO3,the thermal stability of NC was reduced.The activation energy values of NC-C declined and then enhanced with the growth of α and was higher than that of NC-P when α <0.68.Based on the Kissinger method,it was found that NC-L had the highest activation energy,while NC-C had the lowest.
(2) The kinetics of the exothermic decomposition of NC-P,NC-C,NC-L were studied using DSC and TG data using the Malek method,which revealed theesták-Berggren model to be the most appropriate model for describing the pyrolysis process of these samples,and reaction mechanism equations for each sample are as follows:f(α)=α1.425009(1-α)1.81018,f(α)=α1.31036(1-α)1.79515,f(α)=α1.29633(1-α)1.32289,respectively.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the National Natural Science Foundation of China(NSFC,Grants No.52176114 and 52111530091)and Jiangsu Funding Program for Excellent Postdoctoral Talent.
杂志排行
Defence Technology的其它文章
- Cooperative maneuver decision making for multi-UAV air combat based on incomplete information dynamic game
- Satellite breakup behaviors and model under the hypervelocity impact and explosion: A review
- Recognition model and algorithm of projectiles by combining particle swarm optimization support vector and spatial-temporal constrain
- Time-sequenced damage behavior of reactive projectile impacting double-layer plates
- One-step rapid preparation of CL-20/TNT co-crystal assembly and spheroidized coating based on droplet microfluidic technology
- Mechanical properties and failure behavior of 3D printed thermoplastic composites using continuous basalt fiber under highvolume fraction
