外泌体中非编码RNA在类风湿关节炎中作用的研究进展*
2023-03-10刘翠杜小正刘莉梅
刘翠, 杜小正, 刘莉梅
外泌体中非编码RNA在类风湿关节炎中作用的研究进展*
刘翠, 杜小正△, 刘莉梅
(甘肃中医药大学针灸推拿学院,甘肃 兰州 730000)
外泌体;非编码RNA;类风湿关节炎
类风湿关节炎(rheumatoid arthritis, RA)是一种全身性慢性自身免疫性疾病,其病理特征以滑膜炎、血管翳生成以及关节软骨破坏为主[1]。RA发病中,免疫细胞分泌的细胞因子通过特定受体介导细胞间通讯以促进RA的发生发展[2]。近年来外泌体已被证实为细胞间信息传递的重要介质,其主要将母细胞中的脂质、蛋白质以及核酸转运到受体细胞而发挥通讯作用[3]。研究表明,外泌体(exosomes)中的非编码RNA(noncoding RNA, ncRNA)虽不能直接编码蛋白质,但可通过调控相关基因、转录因子及信号通路而抑制滑膜炎症与软骨破坏,参与RA的病理过程,已成为当前研究的热点[4-5]。故本文对外泌体ncRNA在RA中的作用研究进行了综述,为RA的诊治提供参考资料。
1 外泌体ncRNA的生物学功能
外泌体是由细胞内多泡体(multivesicular body)通过质膜出芽释放或与膜泡融合释放而形成的直径约为40~160 nm的细胞外囊泡。脂质双层膜结构可以保护膜内富含的脂质、蛋白质和核酸等内容物免受溶酶体降解,进而介导细胞间通讯和信息传递[6]。ncRNA主要包括微小RNA(microRNA, miRNA)、长链非编码RNA(long noncoding RNA, lncRNA)和环状RNA(circular RNA, circRNA),在介导免疫应答方面具有重要的调控作用[7]。其中miRNA可靶向mRNA而发挥生物学功能[8],而lncRNA和circRNA作为竞争性内源RNA(competing endogenous RNA, ceRNA),海绵吸附miRNA而影响mRNA的表达,从而参与免疫应答过程。研究表明circ-013043可直接结合miR-130a-3p,进而介导Kruppel样因子9(Kruppel-like factor 9, KLF9)而抑制关节炎成纤维细胞MH7A的增殖、迁移及侵袭[9];lncRNA HIX003209作为ceRNA,通过海绵化miR-6089而激活巨噬细胞中的Toll样受体4(Toll-like receptor 4, TLR4)/核因子κB(nuclear factor-κB, NF-κB)通路[10];lncRNA MINCR靶向上调miR-584-3p而抑制RA滑膜成纤维细胞的增殖、迁移和侵袭[11]。
在外泌体ncRNA生物学发生过程中,外泌体中与mRNA相互作用的蛋白质和短核苷酸序列可以诱导ncRNA分选后进入外泌体,如存在于外泌体中的异质性细胞核核糖蛋白B1(heterogeneous nuclear ribonucleoprotein A2/B1, hnRNPA2/B1)可直接通过相关的特殊基序与miRNA和lncRNA特异性结合,并控制其装载到外泌体中[12-13]。此外,circRNA广泛分布于除线粒体以外的亚细胞器中,对RNA结合蛋白(RNA binding proteins, RBPs)具有特殊识别作用,可以选择性的包装富含嘌呤5'-GMWGVWGRAG-3'基序的环状RNA[14]。包装完成后,外泌体可将其携带的ncRNA从母细胞转移至远处的受体细胞,进而在免疫反应、肿瘤进展和神经退行性等疾病中发挥重要作用[15-16]。总之,外泌体ncRNA可作为细胞间的通讯因子,通过调控受体细胞的基因、蛋白以及信号通路而参与细胞信号转导。如图1所示。

Figure 1. Biological occurrence process of exosome-encapsulted noncoding RNA (ncRNA). lncRNA: long noncoding RNA; circRNA: circular RNA; miRNA: microRNA.
2 外泌体ncRNA在RA中的作用
RA的发病中,外泌体ncRNA将母细胞中的脂质、蛋白质以及核酸转运到受体细胞,通过调控相关基因、转录因子及信号通路而调节T淋巴细胞增殖分化,减轻细胞因子的分泌,抑制成纤维细胞样滑膜细胞(fibroblast-like synoviocytes, FLS)增殖、迁移和侵袭,改善软骨破坏等。如图2所示。
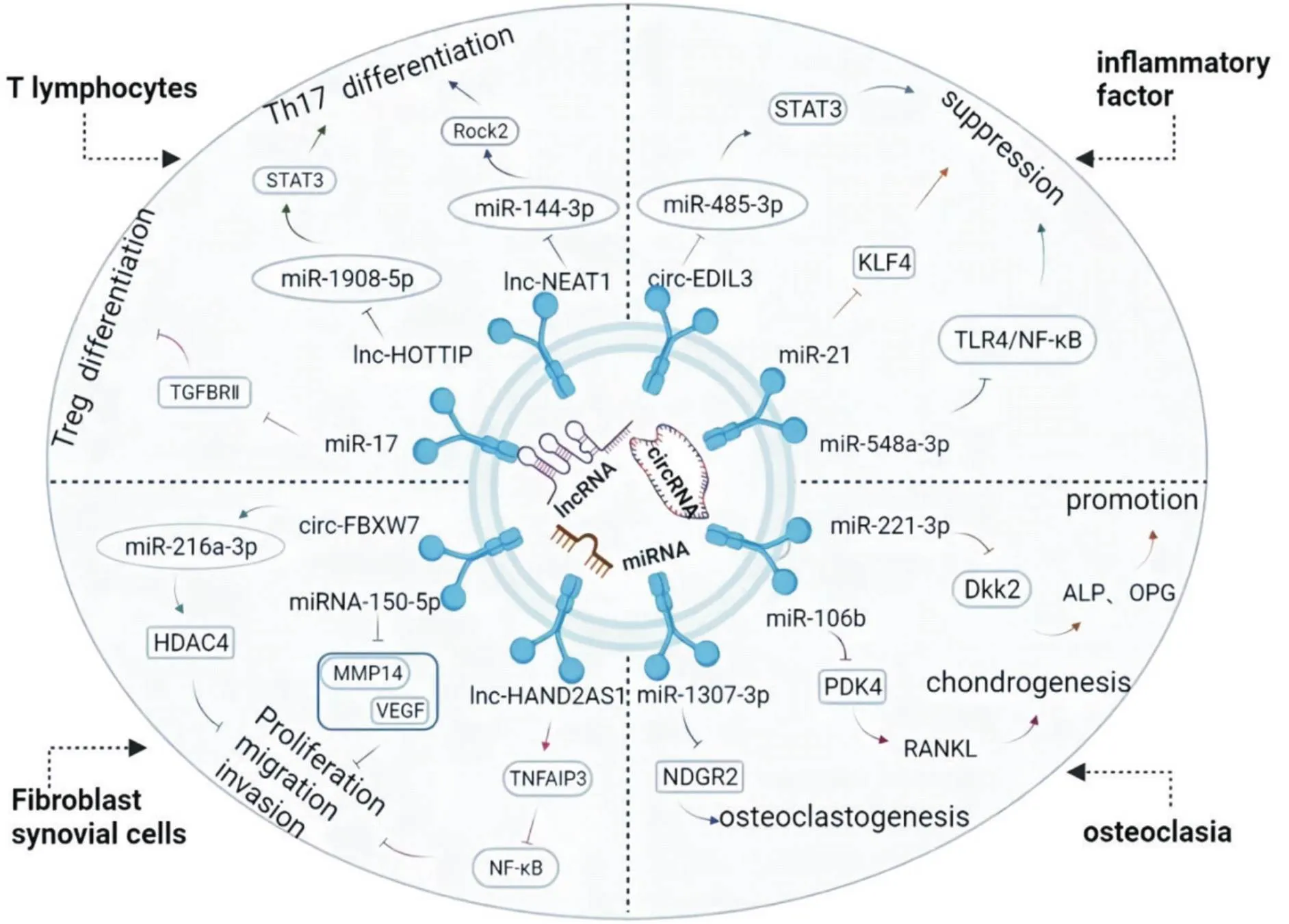
Figure 2. The mechanism of exosome-encapsulted noncoding RNA in rheumatoid arthritis. Th17: T helper cell 17; Treg: regulatory T cells; STAT3: signal transducer and activator of transcription 3; TGFBRII: TGF beta receptor type II; Rock2: Rho-associated protein kinase 2; lnc-HOTTIP: long noncoding RNA-HOXA transcript at the distal tip; lncRNA NEAT1: long noncoding RNA nuclear-enriched abundant transcript 1; KLF4: Kruppel-like factor 4; TLR4: Toll-like receptor 4; NF-κB: nuclear factor-κB; circ-EDIL3: circular RNA EDIL3; HDAC4: histone deacetylase 4; MMP14: matrix metalloproteinase 14; VEGF: vascular endothlial growth factor; TNFAIP3 : tumor necrosis factor alpha-induced protein 3; lnc-HAND2AS1: long noncoding RNA heart and neural crest derivatives expressed 2-antisense RNA 1; circ-FBXW7: circular RNA FBXW7; NDRG2: N-myc downstream-regulated gene 2; PDK4: pyruvate dehydrogenase kinase 4; RANKL: receptor activator of nuclear factor-κB ligand; Dkk2: dickkopf 2; ALP: alkaline phosphatase; OPG: osteoprotegerin.
2.1外泌体ncRNA调节T淋巴细胞的增殖分化CD4+T淋巴细胞是RA免疫反应的主要参与者,Th17细胞的活化增殖和调节性T细胞(regulatory T cell,Treg)数量与功能的异常改变是RA发病的重要机制[17]。外泌体通过抑制CD4+T亚群Th17细胞的增殖、诱导Treg细胞分化而发挥免疫调控作用[18]。远端HOXA转录本(HOXA transcriptat the distal tip, HOTTIP)是一类能够抑制细胞增殖分化和促进细胞凋亡的lncRNA,在免疫炎症疾病中的作用已得到证实[19]。研究显示,外泌体lncRNA HOTTIP在RA-FLS中高表达,其可负性调控miR-1908-5p,增加信号转导及转录激活因子3(signal transducer and activator of transcription 3, STAT3)表达,经其处理后的胶原诱导关节炎(collagen-induced arthritis, CIA)小鼠IL-17和视黄酸受体相关孤儿受体γt的表达显著上调,Th17细胞比例从1.58%上升到3.3%,而Treg细胞表面标志物叉头框蛋白P3(forkhead box P3, FOXP3)的表达下调,Treg细胞比例从14.85%下降到7.76%。此研究表明,lncRNA HOTTIP通过影响Th17/Treg比例失衡而参与到RA的发生发展过程[20]。此外,lncRNA核富集转录本1(nuclear-enriched abundant transcript 1, NEAT1)为类风湿关节炎病程进展中的重要调节分子。研究显示,lncRNA NEAT1可下调RA中miR-144-3p的表达而间接激活Rho相关蛋白激酶2及Wnt/β-catenin信号通路,促进RA中CD4+T细胞向Th17的分化,抑制CD4+T细胞凋亡而加重RA病情进展[21]。
除lncRNA外,miR-17、miR-19B及miR-121在RA患者血清外泌体的表达升高,且与Treg细胞的表达呈负相关,其中miR-17阻止Treg细胞分化可能与抑制转化生长因子β受体Ⅱ有关[22]。而滑膜组织衍生的外泌体miR-424协同FOXP3可抑制Treg细胞分化[23]。此外,有研究显示RA患者外周血中hsa-circ-0089172 (circNUP214)表达显著增加,circNUP214可通过抑制miR-125a-3p表达,增强IL-17A转录活性,导致Th17细胞增加[24]。以上研究表明,lncRNA及circRNA可促进Th17细胞分化,而miRNA可诱导具有抑炎效应的Treg细胞增殖。因此,外泌体ncRNA靶向恢复Th17细胞与Treg细胞比率失衡,将成为治疗RA的潜在靶点。
2.2外泌体ncRNA抑制细胞因子的分泌和表达RA的炎症进程中,TNF-α、IL-1β和IL-6等细胞因子发挥着重要作用[25],NF-κB、TLR和STAT3等信号通路亦广泛参与其中。而外泌体ncRNA在抑制细胞因子分泌及调控上述信号通路活性,减轻RA滑膜炎症方面饰演了重要角色[26]。lncRNA NEAT1可激活FLS增殖活性、磷酸化p65及促进TNF-α、IL-1β和IL-6等细胞因子的分泌,而沉默lncRNA NEAT1后,外泌体miR-23α、miR-129和miR-204的表达水平升高,丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)/细胞外信号调节激酶(extracellular signal-regulated kinase, ERK)信号通路被抑制,FLS活力减弱,细胞因子释放减少[27]。除了吸附miRNA,lncRNA NEAT1亦可直接调控NF-κB p65表达,上调多效性赖氨酸乙酰转移酶p300及其同源物CBP而提高IL-18的转录水平,加重RA炎症反应[28]。另有研究显示,lncRNA HOTTIP通过募集DNA甲基转移酶3b,抑制分泌型卷曲相关蛋白1甲基化,激活Wnt信号通路而增强RA滑膜成纤维细胞的增殖和迁徙能力,诱导炎症因子的分泌[29]。
研究显示RA患者血清外泌体miR-548a-3p的表达显著降低,其靶向TLR4/NF-κB信号通路可抑制巨噬细胞样THP-1细胞增殖活化及细胞因子TNF-α和IL-6的产生[30];同样,血清外泌体miR-6089可通过靶向TLR4信号通路,抑制脂多糖诱导的THP-1细胞增殖活化,并减少促炎因子的表达[31]。另外,有研究显示,上调骨髓间充质干细胞(bone marrow mesenchymal stem cell, BMSC)衍生的外泌体miR-192-5p表达可减轻RA大鼠地诺前列酮、TNF-α和IL-1β等细胞因子的分泌[32];亦有研究证实BMSC衍生的miR-21通过Tet甲基胞嘧啶双加氧酶1去甲基化抑制Kruppel样因子的表达,下调CIA小鼠血清和滑膜组织中炎性细胞因子的分泌[33];BMSC来源的外泌体miR-223可抑制巨噬细胞的增殖及核苷酸结合寡聚化结构域样受体蛋白3(nucleotide-binding oligomerization domain-like receptor protein 3, NLRP3)的激活,从而减轻细胞因子的释放[34]。同样,BMSC分泌的外泌体miR-205-5p通过鼠双微体基因2抑制MAPK和NF-κB活性,从而减轻CIA小鼠滑膜炎症反应[35]。
此外,近期研究显示circ-EDIL3在CIA小鼠的滑膜间充质干细胞(mesenchymal stem cell, MSC)中高表达,通过靶向核心分子STAT3/miR-485-3p,血管内皮因子(vascular endothlial growth factor, VEGF)的生成被抑制,CIA小鼠的后爪肿胀减轻,这是迄今为止发现的第一个可同时参与RA炎症和血管生成的circRNA[36]。同时,circRNA_09505可通过激活miR-6089/AKT1/NF-κB信号轴,加重CIA小鼠关节炎症。另有研究显示,除了靶向吸附miRNA外,circRNA 0003353可直接激活JAK2/STAT3信号通路而加重滑膜炎症[37]。因此,3种外泌体ncRNA主要通过调节信号通路或靶向相关基因而调控炎性细胞因子的分泌,抑制RA炎症反应。
2.3外泌体ncRNA调节RA-FLS增殖、迁移和侵袭FLS是RA滑膜组织增生的主要细胞类型,其增殖、迁移和侵蚀能力是RA滑膜炎症、关节软骨的侵蚀破坏的关键病理因素,而外泌体ncRNA通过影响FLS增殖、迁移和侵袭参与RA的发生发展[38]。研究显示MSC衍生的外泌体miRNA-150-5p通过下调CIA小鼠血清基质金属蛋白酶14和VEGF的表达而抑制FLS的迁移、侵袭,缓解CIA小鼠炎症反应[39]。滑膜细胞衍生的外泌体miR-320a通过下调CXC趋化因子配体9,抑制RA患者FLS增殖与侵袭,降低CIA小鼠血清中IL-1β、IL-6和IL-8等炎症因子的表达[40]。有研究将MSC来源的外泌体miR-124a与MH7A细胞共同孵育,结果显示miR-124a可以将MH7A细胞阻滞在G0/G1期,减少细胞划痕闭合和细胞迁移,增加相关凋亡蛋白的表达,表明来自MSC的外泌体是传递miRNA的载体,为RA的治疗提供了新方向[41]。此外,T细胞外泌体中含有大量miR-204-5p,这些miRNA可以转移到滑膜成纤维细胞中,抑制细胞增殖活化,延缓CIA小鼠疾病进展[42]。
除了miRNA,外泌体包裹的circRNA和lncRNA在抑制FLS增殖、迁移及侵袭方面的作用也逐渐得到证实。MSC来源外泌体中的circ-FBXW7其通过下调miR-216a-3p以促进组蛋白脱乙酰酶4释放,从而抑制FLS的增殖、迁移以及减轻RA大鼠的炎症反应[43]。lncRNA HAND2AS1 (heart and neural crest derivatives expressed 2-antisense RNA 1)是一种公认的肿瘤抑制因子。研究显示,RA-FLS中HAND2-AS1的表达水平与FLS的增殖和侵袭呈负相关,上调HAND2-AS1可正向调控TNF-α诱导蛋白3的表达而降低p65的表达水平,失活NF-κB通路,抑制FLS的增殖、迁移和炎症[44]。此外,HAND2AS1还可抑制FLS中miR-143-3p的表达,进而诱导滑膜细胞的凋亡[45]。lncRNA LINC01419在抑制胃癌、肺腺癌等细胞生长及转移方面有重要调节作用[46]。抑制LINC01419的表达可通过靶向上调miR-320a而减轻RA患者FLS增殖、迁移和侵袭[47]。此外,lncRNA THRIL亦可直接激活PI3K/AKT信号通路而调节FLS的增殖与侵袭[48]。这些研究皆证实了外泌体ncRNA可通过介导FLS的增殖、侵袭和迁移而参与RA的调控,有望成为RA治疗的潜在策略。
2.4外泌体ncRNA调节RA骨破坏骨破坏是RA发病后期出现的一个核心特征,多因成骨细胞的形成不及与破骨细胞的吸收太过所致[49-50]。有研究应用基因阵列法分析RA模型小鼠滑膜组织外泌体miRNA的表达差异,结果显示miR-221-3p和miR-224-5p等基因上调,miR-486-5p和miR-133a-5p等基因下调,上调基因组鉴定的通路中有核因子κB配体受体激活因子(receptor activator of nuclear factor-κB ligand, RANKL)/骨钙素(osteoprotegerin, OPG)等骨破坏及炎症相关通路的富集,而下调基因组通过骨形态发生蛋白(bone morphogenetic protein, BMP)/Wnt信号通路间接调控了骨破坏;此研究还显示在炎症环境下,FLS来源的外泌体miR-221-3p表达增多,通过负向调控Dickkopf 2 ()基因,降低RA中碱性磷酸酶(alkaline phosphatase, ALP)和OPG表达[51]。此外,人脐带MSC来源的外泌体miR-140-3p可沉默血清及糖皮质激素诱导蛋白激酶1的表达而减轻RA大鼠的骨破坏及关节损伤[52]。亦有研究证实,来源于滑膜成纤维细胞的miR-106b通过外泌体从滑膜成纤维细胞传递至软骨细胞,抑制软骨细胞的增殖和迁移,促进细胞凋亡,且可通过抑制丙酮酸脱氢酶激酶4而调控NF-κB配体系统RANKL以参与骨调节[53]。RNA测序显示,在TNF-α诱导的MH7A细胞中,miR-155-5p、miR-146a-5p、miR-323a-5p及miR-1307-3p在细胞外泌体中显著上调,而miR-1307-3p可靶向N-Myc下游调控基因2来抑制单核细胞向破骨细胞分化[54]。miR-486-5p亦是来源于滑膜成纤维细胞的外泌体,其靶向抗增殖蛋白家族成员Tob1而负调控BMP/Smad信号通路,进而增强了成骨细胞的细胞活力、ALP活性,以及osterix蛋白和(distal-less homeobox 2)基因的表达;体内实验也证实了经miR-486-5p处理的CIA小鼠的软骨损伤和滑膜炎症均得到了显著减轻[55]。以上研究提示调节RA骨破坏的外泌体ncRNA主要为miRNA,其可通过调控相关基因蛋白以增强成骨细胞的活力而抑制RA的骨破坏。
3 结语和展望
综上所述,外泌体ncRNA可通过调节T淋巴细胞增殖分化,限制细胞因子的合成与分泌,抑制FLS迁移、增殖而延缓RA病情进展,而少数外泌体ncRNA亦可促进RA的发生发展。如miR-103a通过抑制肝细胞核因子4A表达,激活JAK/STAT3通路而加重滑膜炎症;miR-223及miR-221可介导NLRP3及TLR4/MyD88通路,促进炎性因子的分泌[56]。因此,靶向与此相关外泌体ncRNA为RA的治疗提供新途径和方法。如一线抗风湿药甲氨蝶呤可通过抑制RA患者血清HOTAIR的表达,缓解TNF-α诱导的滑膜炎症[57];雷公藤内酯醇通过下调miR-221及miR-223而抑制炎性因子的分泌[58]。而沉默lncRNA NEAT1可抑制MAPK/ERK信号通路,减轻FLS侵袭与增殖活性[27],与此相关的靶点将成为RA药物研发的新方向。
目前,虽外泌体ncRNA对RA的作用研究有一定的进展,但仍存在局限性:(1)外泌体携带的大多数ncRNA抑制RA的进展,而关于加重RA病情发展的ncRNA研究较少;(2)lncRNA与circRNA除了通过吸附miRNA发挥功能,亦可通过结合RBS和翻译短肽发挥功能[14, 59-60],但在RA研究中尚未见相关报道;(3)中医药在RA的治疗中发挥着重要的作用,但目前基于外泌体ncRNA生物学功能研究中医药防治RA机制的相关报道亦较少。因此,今后在以上方面需要更加深入的研究,为RA的诊治提供更可靠的理论基础。
[1] Cush JJ. Rheumatoid arthritis: early diagnosis and treatment[J]. Med Clin North Am, 2021, 105(2):355-365.
[2] Zhao J, Guo S, Schrodi SJ, et al. Molecular and cellular heterogeneity in rheumatoid arthritis: mechanisms and clinical implications[J]. Front Immunol, 2021, 12:790122.
[3] Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes[J]. Science, 2020, 367(6478):eaau6977.
[4] Rodríguez-MS, Altuna-CA, Castro-OS, et al. A serum biomarker panel of exomiR-451a, exomiR-25-3p and soluble TWEAK for early diagnosis of rheumatoid arthritis[J]. Front Immunol, 2021, 12:790880.
[5] Liao TL, Hsieh SL, Chen YM, et al. Rituximab may cause increased hepatitis c virus viremia in rheumatoid arthritis patients through declining exosomal microRNA-155[J]. Arthritis Rheumatol, 2018, 70(8):1209-1219.
[6] Chan BD, Wong WY, Lee MM, et al. Exosomes in inflammation and inflammatory disease[J]. Proteomics, 2019, 19(8):e1800149.
[7] Cable J, Heard E, Hirose T, et al. Noncoding RNAs: biology and applications: a keystone symposia report[J]. Ann N Y Acad Sci, 2021, 1506(1):118-141.
[8] Saquib M, Agnihotri P, Monu, et al. Exogenous miRNA: a perspective role as therapeutic in rheumatoid arthritis[J]. Curr Rheumatol Rep, 2021, 23(6):43.
[9] Li L, Zhan M, Li M. Circular RNA circ_0130438 suppresses TNF-α-induced proliferation, migration, invasion and inflammation in human fibroblast-like MH7A synoviocytes by regulating miR-130a-3p/KLF9 axis[J]. Transpl Immunol, 2022, 72:101588.
[10] Yan S, Wang P, Wang J, et al. Long non-coding RNA HIX003209 promotes inflammation by sponging miR-6089 via TLR4/NF-κB signaling pathway in rheumatoid arthritis[J]. Front Immunol, 2019, 10:2218.
[11] 杨波, 杨洁, 傅自力, 等. 长链非编码RNA MYC诱导的长链非编码RNA靶向miR-584-3p对类风湿关节炎滑膜成纤维细胞增殖侵袭和迁移能力的影响[J]. 中华风湿病学杂志, 2021, 25(10):669-675.
Yang B, Yang J, Fu ZL, et al. Effect of lncRNA MINCR on the proliferation,invasion and migration of rheumatoid arthritis synovial fibroblasts by targeting miR-584-3p[J]. Chin J Rheumatol, 2021, 25(10):669-675.
[12] Zang J, Lu D, Xu A. The interaction of circRNAs and RNA binding proteins: an important part of circRNA maintenance and function[J]. J Neurosci Res, 2020, 98(1):87-97.
[13] Groot M, Lee H. Sorting mechanisms for microRNAs into extracellular vesicles and their associated diseases[J]. Cells, 2020, 9(4):1044.
[14] Zhang J, Zhang X, Li C, et al. Circular RNA profiling provides insights into their subcellular distribution and molecular characteristics in HepG2 cells[J]. RNA Biol, 2019, 16(2):220-232.
[15] Sun L, Su Y, Liu X, et al. Serum and exosome long non coding RNAs as potential biomarkers for hepatocellular carcinoma[J]. J Cancer, 2018, 9(15):2631-2639.
[16] Yue B, Yang H, Wang J, et al. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis[J]. Cell Prolif, 2020, 53(7):e12857.
[17] Koga T, Kawakami A, Tsokos GC. Current insights and future prospects for the pathogenesis and treatment for rheumatoid arthritis[J]. Clin Immunol, 2021, 225:108680.
[18] Cosenza S, Toupet K, Maumus M, et al. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis[J]. Theranostics, 2018, 8(5):1399-1410.
[19] He X, Gao K, Lu S, et al. LncRNA HOTTIP leads to osteoarthritis progression via regulating miR-663a/Fyn-related kinase axis[J]. BMC Musculoskelet Disord, 2021, 22(1): 67.
[20] Yao X, Wang Q, Zeng P, et al. LncRNA HOTTIP from synovial fibroblast-derived exosomes: a novel molecular target for rheumatoid arthritis through the miR-1908-5p/STAT3 axis[J]. Exp Cell Res, 2021, 409(2):112943.
[21] Liu R, Jiang C, Li J, et al. Serum-derived exosomes containing NEAT1 promote the occurrence of rheumatoid arthritis through regulation of miR-144-3p/ROCK2 axis[J]. Ther Adv Chronic Dis, 2021, 12:2040622321991705.
[22] Wang L, Wang C, Jia X, et al. Circulating exosomal miR-17 inhibits the induction of regulatory T cells via suppressing TGFBR II expression in rheumatoid arthritis[J]. Cell Physiol Biochem, 2018, 50(5):1754-1763.
[23] Ding Y, Wang L, Wu H, et al. Exosomes derived from synovial fibroblasts under hypoxia aggravate rheumatoid arthritis by regulating Treg/Th17 balance[J]. Exp Biol Med, 2020, 245(14):1177-1186.
[24] Peng H, Xing J, Wang X, et al. Circular RNA circNUP214 modulates the T helper 17 cell response in patients with rheumatoid arthritis[J]. Front Immunol, 2022, 24(13):885896.
[25] Ridgley LA, Anderson AE, Pratt AG. What are the dominant cytokines in early rheumatoid arthritis?[J]. Curr Opin Rheumatol, 2018, 30(2):207-214.
[26] Liang JJ, Li HR, Chen Y, et al. Diallyl trisulfide can induce fibroblast-like synovial apoptosis and has a therapeutic effect on collagen-induced arthritis in mice via blocking NF-κB and Wnt pathways[J]. Int Immunopharmacol, 2019, 71:132-138.
[27] Chen J, Luo X, Liu M, et al. Silencing long non-coding RNA NEAT1 attenuates rheumatoid arthritis via the MAPK/ERK signalling pathway by downregulating microRNA-129 and microRNA-204[J]. RNA Biol, 2021, 18(5):657-668.
[28] Guo T, Xing Y, Chen Z, et al. Long non-coding RNA NEAT1 knockdown alleviates rheumatoid arthritis by reducing IL-18 through p300/CBP repression[J]. Inflammation, 2022, 45(1):100-115.
[29] Hu X, Tang J, Hu X, et al. Silencing of long non-coding RNA HOTTIP reduces inflammation in rheumatoid arthritis by demethylation of SFRP1[J]. Mol Ther Nucleic Acids, 2020, 6(19):468-481.
[30] Wang Y, Zheng F, Gao G, et al. MiR-548a-3p regulates inflammatory response via TLR4/NF-κB signaling pathway in rheumatoid arthritis[J]. J Cell Biochem, 2019, 120(2):1133-1140.
[31] Xu D, Song M, Chai C, et al. Exosome-encapsulated miR-6089 regulates inflammatory response via targeting TLR4[J]. J Cell Physiol, 2019, 234(2):1502-1511.
[32] Zheng J, Zhu L, Iok In I, et al.Bone marrow-derived mesenchymal stem cells-secreted exosomal microRNA-192-5p delays inflammatory response in rheumatoid arthritis[J]. Int Immunopharmacol, 2020, 78:105985.
[33] Li GQ, Fang YX, Liu Y, et al. MicroRNA-21from bone marrow mesenchymal stem cell-derived extracellular vesicles targets TET1 to suppress KLF4 and alleviate rheumatoid arthritis[J]. Ther Adv Chronic Dis, 2021, 12:20406223211007369.
[34] Huang Y, Lu D, Ma W, et al. MiR-223 in exosomes from bone marrow mesenchymal stem cells ameliorates rheumatoid arthritis via downregulation of NLRP3 expression in macrophages[J]. Mol Immunol, 2022, 143:68-76.
[35] Ma W, Tang F, Xiao L, et al. MiR-205-5p in exosomes divided from chondrogenic mesenchymal stem cells alleviated rheumatoid arthritis via regulating MDM2 in fibroblast-like synoviocytes[J]. J Musculoskelet Neuronal Interact, 2022, 22(1): 132-141.
[36] Zhang J, Zhang Y, Ma Y, et al. Therapeutic potential of exosomal circRNA derived from synovial mesenchymal cells via targeting circEDIL3/miR-485-3p/PIAS3/STAT3/VEGF functional module in rheumatoid arthritis[J]. Int J Nanomedicine, 2021, 16:7977-7994.
[37] Wang J, Liu J, Wen JT, et al. Correlation between circRNA0003353 in peripheral blood mononuclear cells and immune inflammation in rheumatoid arthritis patients with damp heat obstruction syndrome[J]. Sichuan Da Xue Xue Bao Yi Xue Ban, 2022, 53(3):437-443.
[38] 史栋梁, 史桂荣. MicroRNA-16对类风湿关节炎患者滑膜成纤维细胞增殖、侵袭及细胞因子分泌的影响[J]. 中国病理生理杂志, 2014, 30(10):1868-1872.
Shi DL, Shi GR. Effects of microRNA-16 on proliferation,invasion and cytokine secretion of synovialfibroblasts from rheumatoid arthritis patients[J]. Chin J Pathophysiol, 2014, 30(10):1868-1872.
[39] Chen Z, Wang H, Xia Y, et al. Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF[J]. J Immunol, 2018, 201(8):2472-2482.
[40] Meng Q, Qiu B. Exosomal microRNA-320a derived from mesenchymal stem cells regulates rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing CXCL9 expression[J]. Front Physiol, 2020, 11:441.
[41] Meng HY, Chen LQ, Chen LH. The inhibition by human MSCs-derived miRNA-124a overexpression exosomes in the proliferation and migration of rheumatoid arthritis-related fibroblast-like synoviocyte cell[J]. BMC Musculoskelet Disord, 2020, 21(1):150.
[42] Wu LF, Zhang Q, Mo XB, et al. Identification of novel rheumatoid arthritis-associated miRNA-204-5p from plasma exosomes[J]. Exp Mol Med, 2022, 54(3):334-345.
[43] Chang L, Kan L. Mesenchymal stem cell-originated exosomal circular RNA circFBXW7 attenuates cell proliferation, migration and inflammation of fibroblast-like synoviocytes by targeting miR-216a-3p/HDAC4 in rheumatoid arthritis[J]. J Inflamm Res, 2021, 14:6157-6171.
[44] Bi X, Guo XH, Mo BY, et al. LncRNA PICSAR promotes cell proliferation, migration and invasion of fibroblast-like synoviocytes by sponging miRNA-4701-5p in rheumatoid arthritis[J]. EBioMedicine, 2019, 50:408-420.
[45] Su Y, Liu Y, Ma C, et al. Mesenchymal stem cell-originated exosomal lncRNA HAND2-AS1 impairs rheumatoid arthritis fibroblast-like synoviocyte activation through miR-143-3p/TNFAIP3/NF-κB pathway[J]. J Orthop Surg Res, 2021, 16(1):116.
[46] Ye Y, Gao X, Yang N. LncRNA ZFAS1 promotes cell migration and invasion of fibroblast-like synoviocytes by suppression of miR-27a in rheumatoid arthritis[J]. Hum Cell, 2018, 31(1):14-21.
[47] 刘延霞, 杨青, 王红怡, 等. lncRNA LINC01419靶向miR-320a对小儿类风湿关节炎滑膜成纤维细胞增殖、迁移和侵袭的影响[J]. 中国免疫学杂志, 2022, 38(4):414-418, 426.
Liu YX, Yang Q, Wang HY, et al. Effects of lncRNA LINC01419 targeting miR-320a on proliferation,migration and invasion of synovialfibroblasts in juvenile rheumatoid arthritis[J]. Chin J Immunol, 2022, 38(4):414-418, 426.
[48] Liang Y, Li H, Gong X, et al. Long Non-coding RNA THRIL mediates cell growth and inflammatory response of fibroblast-like synoviocytes by activating PI3K/AKT signals in rheumatoid arthritis[J]. Inflammation, 2020, 43(3):1044-1053.
[49] Tang M, Lu L, Yu X. Interleukin-17A interweaves the skeletal and immune systems[J]. Front Immunol, 2020, 11:625034.
[50] Maruotti N, Corrado A, Cantatore FP.Osteoblast role in osteoarthritis pathogenesis[J]. J Cell Physiol, 2017, 232(11):2957-2963.
[51] Maeda Y, Farina NH, Matzelle MM, et al. Synovium-derived microRNAs regulate bone pathways in rheumatoid arthritis[J]. J Bone Miner Res, 2017, 32(3):461-472.
[52] Huang Y, Chen L, Chen D, et al. Exosomal microRNA-140-3p from human umbilical cord mesenchymal stem cells attenuates joint injury of rats with rheumatoid arthritis by silencing SGK1[J]. Mol Med, 2022, 28(1):36.
[53] Liu D, Fang Y, Rao Y, et al. Synovial fibroblast-derived exosomal microRNA-106b suppresses chondrocyte proliferation and migration in rheumatoid arthritis via down-regulation of PDK4[J]. J Mol Med, 2020, 98(3):409-423.
[54] Takamura Y, Aoki W, Satomura A, et al. Small RNAs detected in exosomes derived from the MH7A synovial fibroblast cell line with TNF-α stimulation[J]. PLoS One, 2018, 13(8):e0201851.
[55] Chen J, Liu M, Luo X, et al. Exosomal miRNA-486-5p derived from rheumatoid arthritis fibroblast-like synoviocytes induces osteoblast differentiation through the Tob1/BMP/Smad pathway[J]. Biomater Sci, 2020, 8(12):3430-3442.
[56] Huang Y, Lu D, Ma W, et al. MiR-223 in exosomes from bone marrow mesenchymal stem cells ameliorates rheumatoid arthritis via downregulation of NLRP3 expression in macrophages[J]. Mol Immunol, 2022, 143:68-76.
[57] Tan J, Dan J, Liu Y. Clinical efficacy of methotrexate combined with iguratimod on patients with rheumatoid arthritis and its influence on the expression levels of HOTAIR in serum[J]. Biomed Res Int, 2021, 2021:2486617.
[58] Li N, Chen Z, Feng W, et al. Triptolide improves chondrocyte proliferation and secretion via down-regulation of miR-221 in synovial cell exosomes[J]. Phytomedicine, 2022, 29(107):154479.
[59] Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs[J]. Mol Cell, 2018, 71(3):428-442.
[60] Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs[J]. Cell, 2018, 172(3):393-407.
Progress in role of noncoding RNA in exosomes in rheumatoid arthritis
LIU Cui, DU Xiaozheng△, LIU Limei
(,,730000,)
Rheumatoid arthritis (RA) is a systemic chronic autoimmune disease with joint synovitis as the main pathological changes, and its pathogenesis is closely linked to immune dysfunction. Exosomes are cell-derived vesicles that are widely distributed in body fluids such as blood, saliva and synovial fluid. Exosomes play an important role in intercellular communication by transporting lipids, proteins and nucleic acids, and affecting the physiopathological processes of many diseases. Exosome-encapsulated functional noncoding RNA (ncRNA) is critical to regulate the proliferation and differentiation of RA synovial T-lymphocytes. It also inhibits synovial cell proliferation and invasion as well as alleviates cartilage destruction. This article reviews the latest research on ncRNA in exosomes in the pathogenesis and treatment of RA.
exosomes; noncoding RNA; rheumatoid arthritis
R593.22; R363.2
A
10.3969/j.issn.1000-4718.2023.02.019
1000-4718(2023)02-0359-07
2022-08-12
2022-11-01
[基金项目]国家自然科学基金资助项目(No. 82060891);国家中医药管理局甘肃郑氏针法学术流派传承工作室项目(No. 2305135901);甘肃省自然科学基金资助项目(No. 21JR7RA568);甘肃省青年科技基金资助项目(No. 20JR10RA344)
Tel: 15117059960; E-mail: lz-duxiaozheng@163.com
(责任编辑:李淑媛,罗森)
