小鼠囊胚胞外囊泡对子宫内膜上皮细胞E-cadherin和vimentin表达的影响
2023-03-10刘颖段乳侠张浩栋方廖琼
刘颖, 段乳侠, 张浩栋, 方廖琼
小鼠囊胚胞外囊泡对子宫内膜上皮细胞E-cadherin和vimentin表达的影响
刘颖, 段乳侠, 张浩栋, 方廖琼△
(西南大学蚕桑纺织与生物质科学学院,重庆 400716)
探讨小鼠囊胚细胞外囊泡(EVs)对小鼠子宫内膜上皮细胞(mEECs)上皮钙黏素(E-cadherin)和波形蛋白(vimentin)表达的影响。以孕4.5 d的BABL/c小鼠囊胚为材料,利用超高速离心分离纯化小鼠囊胚EVs,粒度仪分析粒径,透射电子显微镜观察形态,Western blot分析Tsg101和CD63的表达;酶消化结合差速贴壁筛选法制备原代mEECs,流式细胞术分析细胞角蛋白8(CK8)的阳性率鉴定mEECs纯度。建立囊胚EVs与mEECs共培养模型,共聚焦显微镜观察PKH26标记的囊胚EVs进入mEECs的形态过程,Western blot和细胞免疫化学法分析共培养中mEECs中E-cadherin和vimentin表达的变化。(1) 小鼠囊胚EVs平均粒径(226.6±3.8) nm,表现囊泡超微形态,表达Tsg101和CD63蛋白标志物。(2) 原代mEECs形态均匀,CK8阳性细胞率80%以上。(3) 与0 h相比,mEECs与囊胚EVs共培养48和96 h时E-cadherin表达水平显著降低(<0.01),vimentin表达水平显著升高(<0.01)。小鼠囊胚EVs可下调E-cadherin表达,上调vimentin表达,诱导mEECs发生上皮-间充质转化。
囊胚;细胞外囊泡;子宫内膜上皮细胞;上皮-间充质转化
上皮-间充质转化(epithelial-mesenchymal transition, EMT)是上皮细胞失去其特性和形态,并获得侵袭性间充质表型的复杂生物学过程[1]。在此过程中,上皮细胞黏附性下降,获得迁移和侵袭的能力,转变为间充质细胞。EMT过程中细胞主要表现为上皮钙黏素(E-cadherin)、细胞角蛋白(cytokeratin, CK)等上皮细胞标志物的下调,以及神经钙黏素(N-cadherin)、波形蛋白(vimentin)等间充质细胞标志物的表达上调[1-3]。
EMT参与胚胎植入和植入后复杂胚胎结构的形成等早期胚胎发育过程中[4]。受精卵发育至囊胚阶段后,必须植入子宫内膜才能在体内继续发育[5]。而胚胎的成功植入取决于可行的胚胎和子宫内膜容受的同步性[6],这种同步性是在胚胎与母体子宫内膜的“crosstalk”中实现的。通过母-胎交互,母体子宫内膜上皮细胞发生EMT,上皮细胞由原来的紧密连接转变为松散的连接,子宫内膜表现出对胚胎的容受性状态,提供胚胎植入的窗口[7]。
细胞外囊泡(extracellular vesicles, EVs)是一种细胞间通讯的重要媒介。源细胞释放这种包含蛋白质、DNA、mRNA、miRNA、lncRNA、细胞因子等多种生物活性物质、直径约100~1 000 nm的脂质双分子膜囊泡[8],可与靶细胞结合[9],参与靶细胞增殖、迁移、免疫调节等多种生物学过程[10]。有研究表明,来自高转移肿瘤细胞[11]和子宫内膜异位症组织[12]的EVs可以介导受体细胞的子宫内膜上皮细胞发生EMT,并观察到不同物种的植入前胚胎分泌EVs且具有调节子宫内膜局部免疫的能力[13-14]。
现已证实,人绒毛膜促性腺激素(human chorionic gonadotropin, hCG)[15]、多种细胞因子、生长因子[16]及Wnt信号通路[17]等参与了母-胎交互过程,但母体与胚胎交流的具体机制仍未完全阐明。因此我们推测囊胚EVs可能在胚胎着床过程的“母胎对话”中发挥胚胎信使的作用,影响子宫内膜上皮细胞的生物学行为,以利于胚胎植入。本研究提取原代小鼠子宫内膜上皮细胞(mouse endometrial epithelial cells, mEECs)和囊胚EVs,通过对共培养体系中E-cadherin和vimentin的检测,验证小鼠囊胚EVs诱导mEECs发生EMT的可能性。
材料和方法
1 动物
SPF级BALB/c小鼠,10~12周龄,体重21 g左右,共300只(雌鼠200只,雄鼠100只),在独立通气笼具系统条件下喂养,由重庆医科大学动物中心提供,许可证号:SYXK(渝)2018-0003。腹腔注射孕马血清促性腺激素(pregnant mare serum gonadotropin, PMSG;每只10 U)45 h后继续注射hCG(每只10 U),雌鼠与雄鼠按2∶1分别配对过夜;检测阴栓为怀孕,此时记为D1,继续培养至D4.5,用于后续提取囊胚EVs的实验。
2 主要试剂及仪器
分散酶II和胰蛋白酶购自Roche;子宫内膜上皮细胞专用培养基购自Gbico;PMSG和hCG购自宁波三生药业有限公司;胎牛血清(fetal bovine serum, FBS)和0.25%胰蛋白酶购自HyClone;双抗、FITC标记的山羊抗兔Ⅱ抗、HRP标记的山羊抗兔Ⅱ抗、Hanks平衡盐溶液(Hanks' balanced salt solution, HBSS)、BCA蛋白浓度测定试剂盒和SDS-PAGE凝胶快速配置试剂盒购自上海碧云天生物技术有限公司;兔抗小鼠E-cadherin、vimentin、CK8、肿瘤易感基因101(tumor susceptibility gene 101, Tsg101)、CD63和GAPDH单克隆抗体购自Abcam。
荧光倒置显微镜购自Olympus;透射电子显微镜购自Hitachi;激光共聚焦显微镜购自Leica;粒度分析仪购自Malvern;流式细胞分析仪购自Beckman Coulter;CO2培养箱购自Shel-Lab。
3 主要方法
3.1原代mEECs分离纯化将10~12周龄的健康BABL/c雌鼠的子宫内膜剪成约2 cm长的片段,加入消化液(4 mL HBSS+0.5 mL 50 g/L分散酶II+0.5 mL 60 g/L胰蛋白酶)4 ℃消化90 min,室温消化30 min,加入2 mL完全培养液(含10% FBS和1%双抗的DMEM/F12培养液)终止消化;子宫内膜片段转移至新的含5 mL HBSS的培养皿中涮洗20 s;加0.5 mL 5% BSA,静置5 min;取上清,继续静置7 min;弃上清,加入2 mL HBSS静置7 min;弃上清,加入完全培养液将细胞悬液在CO2培养箱中培养;30 min后将细胞培养悬液转移至新的培养皿中培养,所得即是mEECs。
3.2细胞免疫荧光染色检测CK8、E-cadherin和vimentin表达制作原代mEECs爬片,PBS清洗3次,4%多聚甲醛固定10 min;PBS清洗2次,加入Triton X-100通透液通透10 min;PBS清洗3次后,5% BSA室温封闭1 h;加入兔抗小鼠CK8单克隆抗体4 ℃孵育过夜,然后Ⅱ抗37 ℃避光孵肓1 h;DAPI染色15 min,PBS清洗3次后加入抗荧光淬灭剂封片观察。在48孔板中提前放入细胞爬片,接种mEECs(1×109cells/cm2),细胞贴壁后加入30 μL囊胚EVs悬液;细胞免疫荧光检测共培养0、48和96 h时E-cadherin和vimentin的表达,步骤同上。
3.3流式细胞术检测CK8阳性率用胰酶消化mEECs,用完全培养液配成1×106mL-1的细胞悬液,4%多聚甲醛室温固定30 min;300×离心5 min,沉淀用PBS清洗2次;300×离心5 min,弃上清加入兔抗小鼠CK8单克隆抗体4 ℃孵育过夜;300×离心5 min,沉淀用PBS清洗2次;300×离心5 min,弃上清加入Ⅱ抗室温避光孵育20 min;PBS清洗细胞2次,300×离心5 min;PBS重悬细胞后流式细胞仪检测。
3.4囊胚EVs制备与鉴定收集200个D4.5的BABL/c小鼠囊胚,红细胞裂解液室温裂解15 min,PBS洗3次后加入pH 2.5的HCl,透明带消失后加入2 mL胰酶,37 ℃恒温摇床消化30 min;10% FBS(去囊泡)中止消化;4 ℃、300×离心15 min,取上清;4 ℃、2 000×离心15 mim,上清过滤后4 ℃、50 000×离心70 min,收集沉淀1;上清继续4 ℃、100 000×离心70 min,收集沉淀2;PBS混合沉淀1、2,得到囊胚EVs。
PBS稀释囊胚EVs为1×1010mL-1,取10 µL包埋铜网,过夜晾干,透射电镜观察囊胚EVs形态,参考吴伟东等[18]的操作方法。PBS稀释囊胚EVs为1×1012mL-1,比色皿内加入1 mL,Malvern粒度仪检测囊胚EVs的粒径分布。提取囊胚EVs蛋白,Western blot检测Tsg101和CD63蛋白表达,步骤见3.6。
3.5囊胚EVs与mEECs共培养及形态观察对囊胚EVs进行PKH26染色,7 min后加入FBS终止染色,4 ℃、100 000×离心70 min,收集被染色的囊胚EVs沉淀,30 μL PBS重悬,与第2代的mEECs共培养(1×109/cm2)。在0和96 h时,加入DAPI染细胞核,激光共聚焦显微镜下观察囊胚EVs进入mEECs的情况。
3.6Western blot检测E-cadherin和vimentin表达30 μL EVs悬液和1×109cells/cm2的mEECs共培养0、48和96 h后,分别加入含罗氏蛋白酶抑制剂的RIPA裂解液,提取总蛋白,BCA法测定蛋白浓度;变性后取5 µg总蛋白样品进行SDS-PAGE;120 V,转膜60 min,TBST缓冲液清洗PVDF膜3次,5% BSA封闭,加入兔抗小鼠E-cadherin和vimentin单克隆抗体,4 ℃孵育过夜;加入HRP标记的Ⅱ抗,孵育2 h。清洗后ECL法显色,以GAPDH为内参照,采用ImageJ软件进行灰度分析。
4 统计学处理
用GraphPad Prism 5.0软件进行统计学分析。数据均采用均数±标准差(mean±SD)表示。组间均数比较采用单因素方差分析。以<0.05为差异有统计学意义。实验均重复3次。
结果
1 mEEC分离纯化结果
分散酶II与胰蛋白酶消化后差速贴壁得到的细胞悬液先培养30 min,此时贴壁的为杂细胞,而mEECs未贴壁(图1A);继续将未贴壁细胞转移至新的培养皿中培养3 h,有形状椭圆如鹅卵石的上皮细胞贴壁,杂细胞较少(图1B)。培养2 d后,mEECs长满培养皿的90%。
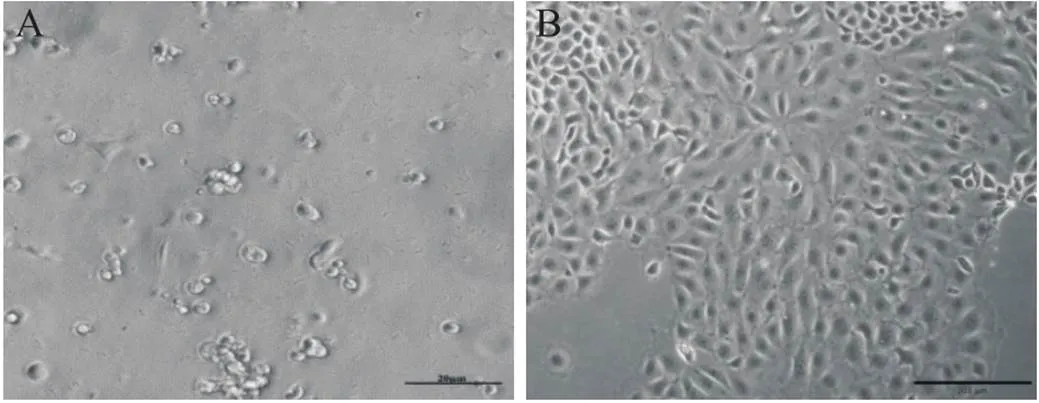
Figure 1. Morphological changes of isolated and purified mEECs after 30 min (A; scale bar=20 µm) and 3 h (B; scale bar=200 µm) of incubation.
接着对分离纯化得到的mEECs进行细胞免疫荧光染色和流式细胞术,检测上皮细胞标志物CK8的表达。DAPI染胞核,呈蓝色荧光;胞质内的CK8蛋白呈绿色荧光;绿色荧光包裹蓝色荧光的细胞被认为是mEECs,只有蓝色荧光的是杂细胞。如图2所示,mEECs的胞核被绿色荧光包围,纯度很高,杂细胞很少,且形态均匀。流式细胞术检测CK8的阳性率可达80%左右(图3)。这说明通过分散酶II与胰蛋白酶消化后差速贴壁的方法得到了纯度高、形态均匀且可用于后续实验的原代mEECs。

Figure 2. The expression of CK8 detected by cellular immunofluorescence staining (scale bar=20 µm).

Figure 3. The positive rate of CK8 detected by flow cytometry.
2 囊胚EVs的鉴定结果
光学显微镜观察植入前的囊胚形态,可见明显囊胚腔及其内细胞团,外层为滋养层细胞(图4A)。透射电镜显示小鼠囊胚EVs呈典型的圆球状,可见脂质双分子层(图4B)。EVs表达CD63和Tsg101这2种标志蛋白(图4C)。EVs的粒径大部分在115~255 nm之间,主要集中在112和185 nm处,平均粒径为226.6 nm(图4D)。

Figure 4. Identification results of blastocyst EVs. A: morphological changes of mouse blastocyst observed by microscopy (scale bar=25 µm); B: transmission electron microscopy showed typical round spherical shape of mouse blastocyst EVs (scale bar=2 µm); C: protein expression of Tsg101 and CD63 (markers of EVs) detected by Western blot; D: nanoparticle size detection of blastocyst EVs.
3 mEEC摄入囊胚EVs
激光共聚焦显微镜观察共培养体系中mEECs摄取囊胚EVs的过程,0 h时只可见蓝色荧光,囊泡还未进入mEECs中聚集;96 h后红色荧光周围包裹着蓝色荧光,说明mEECs胞核被囊胚EVs包围,见图5。

Figure 5. Fluorescence staining results of mEECs co-cultured with PKH26-prestained blastocyst EVs for 0 and 96 h(scale bar=20 μm).
4 囊胚EVs降低mEECs中E-cadherin表达,诱导vimentin表达
将囊胚EVs与mEECs共培养,分别收集培养了0、48和96 h的mEECs,提取总蛋白,进行Western blot检测;同时制作共培养体系中的mEECs爬片,细胞免疫荧光染色进行检测。Western blot(图6A、7A)和细胞免疫荧光染色(图6B、7B)结果均显示,随着培养时间的增加,E-cadherin的表达显著下降,vimentin的表达显著上升(与0 h相比,均0.01)。

Figure 6. Results of E-cadherin expression by mEECs in co-culture system. A: Western blot; B: cellular immunofluorescence staining (scale bar=5 μm). Mean±SD. n=6. **P<0.01 vs 0 h.

Figure 7. Results of vimentin expression by mEECs in co-culture system. A: Western blot; B: cellular immunofluorescence staining (scale bar=5 μm). Mean±SD. n=6. **P<0.01 vs 0 h.
讨论
EVs是细胞膜向外出芽形成的小囊泡[19]。Tsg101是囊泡发生相关蛋白[20];CD63属于四跨膜蛋白超家族,是EVs中最重要的进化保守蛋白[21]。对EVs的鉴定主要包括大小、形态、特异性蛋白、浓度等。但纳米粒子跟踪分析法结合透射电子显微镜仍然是鉴定EVs的主流方法[22]。我们将从D4.5孕鼠子宫腔中得到的囊胚,经红细胞裂解液洗涤、稀盐酸酸解、胰酶消化后,采用超高速差速离心制备出平均粒径为(226.6±3.8) nm的微粒,透射电镜观察微粒呈脂质双分子膜圆球状,直径集中分布在112和185 nm处并且表达囊泡特异性蛋白CD63和Tsg101。这些结果从形态、超微结构及特异性蛋白表达,均显示小鼠囊胚EVs的存在,且与牛[23]、猪[24]、鼠[14]及人[25]等不同物种的胚胎囊泡表现出结构的相似性,而这些囊泡在妊娠早期胚胎与母体的相互作用中发挥重要作用[26]。
CK8常表达于单纯上皮细胞,并被用于子宫内膜上皮细胞的鉴定。目前还缺乏理想的子宫内膜上皮细胞模型,原代提取的方法也不够成熟和统一。子宫内膜上皮细胞的分离纯化,一般采用组织块分离法和酶消化法从子宫内膜获得。组织块分离法所需时间长,不易形成单层贴壁细胞,难得到纯度高的子宫内膜上皮细胞[27]。而仅通过一种酶进行消化,由于组织来源不同,难以控制消化时间,杂细胞较多[28]。本研究采用分散酶II与胰蛋白酶共同作用并且控制时间消化子宫内膜上皮细胞,因为子宫内膜上皮细胞与基质细胞对胰蛋白酶的耐受性不同[29],胰蛋白酶短时间作用时基质细胞会先消化下来。后续纯化过程,则是利用基质细胞会比上皮细胞先贴壁生长的特点,重复几次贴壁后取细胞悬液培养。细胞免疫荧光染色直观显示CK8的表达,结合流式细胞术检测CK8阳性率达到80%以上,并且细胞形态均匀呈椭圆形,贴壁生长状态良好。所以,分散酶II与胰蛋白酶消化后差速贴壁是一种良好的分离纯化原代子宫内膜上皮细胞的方法。
E-cadherin属于Ⅰ类经典钙黏素,是跨膜糖蛋白的一种,介导上皮细胞之间的黏附,对维持细胞连接有重要作用[30]。EMT往往表现为E-cadherin表达的缺失[31-32]。vimentin是间充质细胞标志蛋白,是中间纤维蛋白家族的一员,具有维持细胞骨架的作用[33],细胞vimentin表达的上调也被认为是EMT的分子标志[34]。我们检测到,随着与小鼠囊胚EVs共培养时间的增加,mEECs表达E-cadherin水平显著下降(0.01),表达vimentin能力显著上升(0.01),提示mEECs发生EMT。子宫内膜上皮细胞的间充质转化是胚胎植入过程中的重要事件,子宫内膜上皮细胞的细胞骨架发生改变,细胞间连接松散,有利于囊胚的滋养层细胞的侵入和黏附[35]。
本项工作采用分散酶II与胰蛋白酶消化、差速贴壁筛选,获得纯化的原代mEECs;观察到植入前小鼠囊胚EVs的存在;在与小鼠囊胚EVs共培养体系中,mEECs表达E-cadherin能力下降,表达vimentin能力增加,以利于胚胎植入。我们推测,囊胚EVs能够促进子宫内膜上皮细胞发生EMT,这或许是胚胎植入过程中胚胎与母体子宫上皮交互“对话”的结果。
[1] Georgakopoulos-Soares I, Chartoumpekis DV, Kyriazopoulou V, et al. EMT factors and metabolic pathways in cancer[J]. Front Oncol, 2020, 10:499.
[2] Son HJ, Moon A. Epithelial-mesenchymal transition and cell invasion[J]. Toxicol Res, 2010, 26(4):245-252.
[3] Pearson GW. Control of invasion by epithelial-to-mesenchymal transition programs during metastasis[J]. J Clin Med, 2019, 8(5):646-660.
[4] Liu H, Zhang X, Li J, et al. The biological and clinical importance of epithelial-mesenchymal transition in circulating tumor cells[J]. J Cancer Res Clin Oncol, 2015, 141(2):189-201.
[5]张颖, 吴金香, 王海滨. 胚胎植入的研究进展:从基础到临床[J]. 中国实用妇科与产科杂志, 2017, 33(11):1117-1121.
Zhang Y, Wu JX, Wang HB. Research progress of embryo implantation: from basic research to clinical application[J]. Chin J Pract Gynecol Obstet, 2017, 33(11):1117-1121.
[6] Altmäe S, Koel M, Võsa U, et al. Meta-signature of human endometrial receptivity: a meta-analysis and validation study of transcriptomic biomarkers[J]. Sci Rep, 2017, 7(1):10077-10092.
[7] Chavatte-Palmer P, Guillomot M. Comparative implantation and placentation[J]. Gynecol Obstet Invest, 2007, 64(3):166-174.
[8] Zhu S, Li S, Yi M, et al. Roles of microvesicles in tumor progression and clinical applications[J]. Int J Nanomed, 2021, 16:7071-7090.
[9]王飞虾, 李蒙蒙, 王玲, 等. 细胞外囊泡在慢性肝病中作用的研究进展[J]. 中国病理生理杂志, 2018, 34(2):358-363.
Wang FX, Li MM, Wang L, et al. Advances on role of extracellular vesicles in chronic liver diseases[J]. Chin J Pathophysiol, 2018, 34(2):358-363.
[10] Sedgwick AE, D'Souza-Schorey C. The biology of extracellular microvesicles[J]. Traffic, 2018, 19(5):319-327.
[11] Yang B, Feng X, Liu H, et al. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma[J]. Oncogene, 2020, 39(42):6529-6543.
[12] Chen D, Qiao H, Wang Y, et al. Adenomyosis-derived extracellular vesicles endow endometrial epithelial cells with an invasive phenotype through epithelial-mesenchymal transition[J]. Genes Dis, 2020, 7(4):636-648.
[13] Simon B, Bolumar D, Amadoz A, et al. Identification and characterization of extracellular vesicles and its DNA cargo secreted during murine embryo development[J]. Genes, 2020, 11(2):203-220.
[14] Kim J, Lee J, Lee TB, et al. Embryotrophic effects of extracellular vesicles derived from outgrowth embryos in pre- and peri-implantation embryonic development in mice[J]. Mol Reprod Dev, 2019, 86(2):187-196.
[15] Makrigiannakis A, Vrekoussis T, Zoumakis E, et al. The role of hCG in implantation: a mini-review of molecular and clinical evidence[J]. Int J Mol Sci, 2017, 18(6):1305.
[16] 王海滨. 胚胎植入研究的进展与展望[J]. 生命科学, 2017, 29(1):31-42.
Wang HB. Embryo implantation: progress and challenge[J]. Chin Bull Life Sci, 2017, 29(1):31-42.
[17] Xie H, Tranguch S, Jia X, et al. Inactivation of nuclear Wnt-β-catenin signaling limits blastocyst competency for implantation[J]. Development, 2008, 135(4):717-727.
[18] 吴伟东, 易永盛, 刘丹, 等. 外泌体对A549细胞增殖、凋亡及迁移功能的影响[J]. 广东医学, 2017, 38(10):1477-1480.
Wu WD, Yi YS, Liu D, et al. Effect of exosomes on the proliferation, apoptosis and migration of A549 cells[J]. Guangdong Med J, 2017, 38(10):1477-1480.
[19] Quesenberry PJ, Goldberg LR, Aliotta JM, et al. Cellular phenotype and extracellular vesicles: basic and clinical considerations[J]. Stem Cells Dev, 2014, 23(13):1429-1436.
[20] Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review[J]. Cancer Metastasis Rev, 2013, 32(3/4):623-642.
[21] Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication[J]. J Proteomics, 2010, 73(10):1907-1920.
[22] 钟宇彤, 黎艳红, 林辉, 等. 微囊泡产生及鉴定体系现状分析[J]. 中国生物医学工程学报, 2020, 39(3):362-366.
Zhong YT, Li YH, Lin H, et al. Analysis of microvesicle generation and identification system[J]. Chin J Biomed Eng, 2020, 39(3):362-366.
[23] Mellisho EA, Velasquez AE, Nunez MJ, et al. Identification and characteristics of extracellular vesicles from bovine blastocysts produced[J]. PLoS One, 2017, 12(5):e0178306.
[24] Saadeldin IM, Kim SJ, Choi YB, et al. Improvement of cloned embryos development by co-culturing with parthenotes: a possible role of exosomes/microvesicles for embryos paracrine communication[J]. Cell Reprogram, 2014, 16(3):223-234.
[25] Giacomini E, Vago R, Sanchez AM, et al. Secretome ofcultured human embryos contains extracellular vesicles that are uptaken by the maternal side[J]. Sci Rep, 2017, 7:5210.
[26] Das M, Kale V. Extracellular vesicles: mediators of embryo-maternal crosstalk during pregnancy and a new weapon to fight against infertility[J]. Eur J Cell Biol, 2020, 99(8):151125-151136.
[27] 赵英博, 张君涛, 贺来增, 等. 胎牛子宫类器官及内膜上皮细胞的分离培养[J]. 畜牧与兽医, 2021, 53(11):54-60.
Zhao YB, Zhang JT, He LZ, et al. lsolation and culture of organs and endometrial epithelial cells of fetal bovine uterine[J]. Anim Husb Vet Med, 2021, 53(11):54-60.
[28] 何志全, 沈文正, 窦忠英. 孕鼠子宫内膜上皮细胞分离、培养及活力检测[J]. 西北农林科技大学学报(自然科学版), 2006, 34(2):12-16.
He ZQ, Shen WZ, Dou ZY. Isolation, culture and vitality examination of endometrium epidermal cells from pregnant mouse[J]. J Northwest Agric For Univ (Nat Sci Ed), 2006, 34(2):12-16.
[29] 凌芳, 郝科兴, 陈慧慧, 等. 绵羊子宫内膜上皮细胞和基质细胞的分离与鉴定[J]. 畜牧与兽医, 2021, 53(7):64-68.
Ling F, Hao KX, Chen HH, et al. lsolation and identification of endometrial epithelial cells and stromal cells in sheep[J]. Anim Husb Vet Med, 2021, 53(7):64-68.
[30] 赵慧晨, 王赛琪, 陈贝贝, 等. E-钙黏蛋白在胃癌中的研究进展及临床应用[J]. 肿瘤学杂志, 2021, 27(10):813-817.
Zhao HC, Wang SQ, Chen BB, et al. The research progress and clinical application of E-cadherin in gastric cancer[J]. J Chin Oncol, 2021, 27(10):813-817.
[31] Chen T, You Y, Jiang H, et al. Epithelial-mesenchymal transition (EMT): a biological process in the development, stem cell differentiation and tumorigenesis[J]. J Cell Physiol, 2017, 232(12):3261-3272.
[32] Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis[J]. Dev Cell, 2019, 49(3):361-374.
[33] 付祎婷, 程爱兰. 波形蛋白诱导EMT在肿瘤侵袭转移中的研究进展[J]. 中南医学科学杂志, 2019, 47(1):89-94.
Fu YT, Cheng AL. Vimentin-induced EMT: the movers in tumor invasion and metastasis[J]. Med Sci J Cent South China, 2019, 47(1):89-94.
[34] 朱军辉, 金明, 邱浩, 等. Calreticulin通过诱导细胞EMT促进鼻咽癌迁移和侵袭[J]. 中国病理生理杂志, 2018, 34(5):925-929.
Zhu JH, Jin M, Qiu H, et al. Calreticulin promotes migration and invasion of nasopharyngeal carcinoma cells by inducing cell EMT[J]. Chin J Pathophysiol, 2018, 34(5):925-929.
[35] 施爽. 胎盘滋养层细胞外泌体miRNA-1290靶向LHX6调控子宫内膜容受态的机制[D]. 杭州: 浙江大学, 2021.
Shi S. Placental trophoblast cells-derived exosomal microRNA-1290 regulates the endometrial receptivity by targeting LHX6[D]. Hangzhou: Zhejiang University, 2021.
Mouse blastocyst-derived extracellular vesicles regulate expression of E-cadherin and vimentin in endometrial epithelial cells
LIU Ying, DUAN Ruxia, ZHANG Haodong, FANG Liaoqiong△
(,,,400716,)
To find out whether the extracellular vesicles (EVs) secreted by mouse blastocysts affect the expression of E-cadherin and vimentin in mouse endometrial epithelial cells (mEECs).A female mouse uterus was utilized to gather mouse blastocysts on day 4.5 of pregnancy. Transmission electron microscopy, Western blot, and nanoparticle tracking were applied to describe the EVs. Enzyme digestion and differential adhesion screening were applied to obtain primary mEECs. Flow cytometry was used to quantify the proportion of mEECs that were positive for cytokeratin 8 (CK8). The morphological processes of EVs labeled by PKH26 into mEECs were observed by fluorescence confocal microscopy. Western blot and cellular immunofluorescence were utilized to evaluate the expression of E-cadherin and vimentin of mEECs in co-culture system.The mean particle size of EVs was (226.6±3.8) nm and characterized with Tsg101 and CD63 proteins. The primary mEECs labeled with CK8 were enriched, with a positive rate of more than 80%. After 48 and 96 h of co-culture with EVs, mEECs expressed significantly lower levels of E-cadherin and significantly higher levels of vimentin (<0.01).Mouse blastocyst EVs may induce epithelial-mesenchymal transition of mEECs by decreasing E-cadherin expression and increasing vimentin expression.
blastocyst; extracellular vesicles; endometrial epithelial cells; epithelial-mesenchymal transition
R329.2+8; R363.2
A
10.3969/j.issn.1000-4718.2023.02.017
1000-4718(2023)02-0345-07
2022-09-02
2022-12-03
Tel: 023-68250171; E-mail: lqfang06@163.com
(责任编辑:卢萍,罗森)
