高脂诱导下雄激素缺乏小型猪肾脏脂质沉积的关键基因表达分析*
2023-03-10范莹盈任裕杰凌云吕东颖蔡兆伟
范莹盈, 任裕杰, 凌云, 吕东颖, 蔡兆伟,△
高脂诱导下雄激素缺乏小型猪肾脏脂质沉积的关键基因表达分析*
范莹盈1, 任裕杰1, 凌云2, 吕东颖2, 蔡兆伟1,2△
(1浙江中医药大学药学院,浙江 杭州 310053;2浙江中医药大学中医药科学院动物实验研究中心,浙江 杭州 310053)
探讨高脂诱导条件下雄激素缺乏小型猪肾脏脂质沉积及关键基因表达的变化。将雄性五指山小型猪随机分为3组,即不去势(IM)组、去势(CM)组和去势+睾酮(CMT)组,每组6只动物,均饲喂高脂饮食。12周后检测血清肾功能指标,测定肾脏甘油三酯(TG)和总胆固醇(TC)含量;进行肾脏苏木精-伊红(H&E)和油红O染色,观察其脂质沉积和组织病理学变化。利用转录组测序分析肾脏组织表达谱差异,并用RT-qPCR和Western blot方法验证参与TG合成、胆汁酸代谢和雌激素合成相关差异表达基因。CM小型猪肾脏重量明显低于IM和CMT小型猪(0.05);与IM组和CMT组小型猪相比,CM小型猪血尿素氮含量显著升高(0.05),但血清肌酐和总蛋白水平没有显著变化;CM组小型猪肾脏内出现大量脂滴,且TG含量显著高于IM和CMT小型猪(0.05);与IM组和CMT组小型猪相比,CM组小型猪肾脏TG合成基因包括固醇调节元件结合转录因子1()、糖类应答元件结合蛋白(/)和硬脂酰辅酶A去饱和酶()等表达升高,而胆汁酸代谢和雌激素合成基因包括法尼酯X受体(/)和雌激素受体1()表达下调;睾酮处理能够逆转去势小型猪肾脏内脂质沉积及相关基因表达变化。和可能通过影响SREBF1脂质合成途径参与高脂诱导的雄激素缺乏小型猪肾脏脂质沉积过程。这为老年男性慢性肾脏疾病防治提供了新思路。
肾脏;脂质沉积;雄激素缺乏;小型猪;高脂饮食
近年来,随着我国人口老龄化及人们生活方式的改变,慢性肾脏病的发病率逐年增加,已成为威胁人类特别是中老年身体健康的重要疾病。随着年龄的增长,中老年男性体内的雄激素水平会逐渐降低,易患肥胖、高胆固醇血症、糖尿病和动脉粥样硬化等疾病,这些均是导致肾脏脂质沉积并造成肾脏损害的独立风险因素[1]。尽管最近国内外有许多研究证实,雄激素水平降低可能是导致慢性肾病的主要内分泌紊乱[2-3],但雄激素缺乏及其诱导的脂代谢紊乱对肾脏脂质沉积和功能的影响迄今仍未完全清楚。
目前研究认为,脂质合成增加在肾脏脂质沉积过程中起重要作用[4]。固醇调节元件结合蛋白1c(sterol regulatory element binding protein-1c, SREBP-1c)是调节脂质代谢的关键核转录因子,参与调节甘油三酯和脂肪酸合成基因的表达,继而影响脂质代谢。有研究发现,高脂饮食能够上调C57BL/6J小鼠肾脏SREBP-1c及其下游脂肪酸合成酶(fatty acid synthase, FASN)、硬脂酰辅酶A去饱和酶(stearoyl-coenzyme A desaturase, SCD)和乙酰辅酶A羧化酶(acetyl-coenzyme A carboxylase, ACC)的表达,导致肾脏脂质沉积和肾小球硬化。进一步研究发现,敲除小鼠能够抵抗高脂诱导的肾脏脂质沉积[5]。衰老过程中,肾脏脂质沉积也和SREBP-1c表达升高有关[6]。国内学者研究发现,SREBP-1c激活介导了糖尿病和高脂诱导的肾脏脂质沉积[7-9]。最近发现,脂肪酸氧化缺陷可能是造成老年小鼠肾脏脂质过度积聚的原因[10]。然而,雄激素缺乏诱导的肾脏脂质沉积相关基因表达变化却不清楚。
本研究拟利用前期建立的雄激素缺乏调控高脂饮食诱导的小型猪模型,在此基础上检测血清肾功能指标、肾脏脂质含量以及组织病理学改变,同时进一步采用转录组测序(RNA-Seq)技术分析筛选雄激素缺乏诱导肾脏脂质沉积的关键基因和途径,旨在从整体水平明确雄激素缺乏对高脂诱导的肾脏脂质沉积和功能的影响,为今后防治中老年男性慢性肾脏疾病提供新的靶点和治疗思路。
材料和方法
1 实验动物与分组
雄性五指山小型猪18只,体重(10.00±1.63) kg,由广东大华农动物保健品股份有限公司提供[SCXK(粤)2013-0022],待适应性饲养3~4周后,将动物随机分为3组:不去势(intact male pigs, IM)组、去势(castrated male pigs, CM)组和去势+睾酮(castrated male pigs with testosterone replacement, CMT)组,每组6只。手术去势是在无菌和麻醉环境下将小型猪阴囊处切口后去除睾丸,不去势组小型猪进行伪手术处理,睾酮处理是在去势小型猪后腿外侧肌肉注射丙酸睾酮注射液(10 mg/kg,每周1次),具体方案见前期发表文献[11-12]。三组动物均饲喂高脂高胆固醇饲料,整个实验持续12周,结束后处死动物,迅速采集肾脏组织样本,置入液氮中速冻,随后保存在-80 ℃备用。
2 方法
2.1血清生化指标检测分别在造模前0周和造模后4、8和12周时,取小型猪前腔静脉血5 mL,以3 000 r/min离心15 min,分离血清。采用全自动生化分析仪检测血清肌酐(creatinine, CREA)、血尿素氮(blood urea nitrogen, BUN)、白蛋白(albumin, ALB)和总蛋白(total protein, TP)等指标变化,具体方法按照试剂盒的操作说明使用(南京建成生物工程研究所)。
2.2肾脏指标检测称取500 mg肾脏组织,加入生理盐水制成10%的组织匀浆,室温下振荡,以3 000 r/min离心15 min,取上清测定组织中甘油三酯(triglyceride, TG)和总胆固醇(total cholesterol, TC)含量。
2.3肾脏称重及组织病理学染色动物处死后,取肾脏称重,计算肾脏指数:肾脏重量(g)/体重(kg)。肾脏组织用10%中性甲醛固定,常规脱水、石蜡包埋后,切片进行H&E染色。另取部分肾脏经液氮速冻后保存于冷冻冰箱备用,冰冻切片后用油红O染色,观察组织脂质沉积情况。
2.4转录组测序分析提取各组动物肾脏组织总RNA后,采用安捷伦2100生物分析仪(Agilent)和NanoDrop 2000(Thermo Scientific)检测所提RNA的质量,待样品检测合格后,构建cDNA测序文库,采用Illumina Hiseq 2500测序平台进行测序。获得RNA-Seq的原始数据后,用Tophat软件和Bowtie软件将所有测序读段mapping定位到猪基因组上(http://www.ensembl.org/info/data/ftp/index.html; Sscrofa 10.2)。差异基因筛选和分析参见前期已发表文献[11],具体采用DEGSeq算法筛选差异表达基因(differentially expressed genes, DEGs),使用Cluster 3.0进行聚类分析。通过时间序列的短时间序列表达挖掘器(short time-series expression miner, STEM; http://www.cs.cmu.edu/~jernst/st/)方法的聚类分析确定筛选出来的DEGs的表达谱特征,应用基于基因本体(gene ontology, GO)分类数据库分析具有显著趋势的DEGs参与的生物学功能。
2.5RT-qPCR验证差异基因表达为了验证转录组测序的可靠性,我们将提取的肾脏RNA样品使用反转录试剂盒反转录成cDNA,用SYBR Green荧光染料检测验证目的基因的表达,所有荧光定量PCR反应均在伯乐IQ5实时荧光定量PCR仪上进行。每个样品PCR反应重复3次,根据溶解曲线判断产物特异性。目的基因检测引物序列信息见表1。

表1 RT-qPCR引物序列
2.6Western blot验证差异基因蛋白表达按照蛋白提取试剂盒说明书提取肾脏组织总蛋白,BCA法测定蛋白浓度。取蛋白样品进行SDS-PAGE,转膜,并用丽春红染液检测转膜效果。将转好的膜置于室温下,用5%的脱脂牛奶封闭1 h,然后加入Ⅰ抗,4 ̊C孵育过夜。PBST洗膜3次,后加入Ⅱ抗进行孵育,室温下30 min。洗膜后,用Odyssey红外荧光扫描成像系统拍照分析。Ⅰ抗[固醇调节元件结合转录因子1(sterol regulatory element binding transcription factor 1, SREBF1)抗体(ab235177)、SCD抗体(ab39969)和β-actin抗体(ab8226)]均购自Abcam。
3 统计学处理
所有数据以平均值±标准差(mean±SD)来表示,统计结果用GraphPad prism 7.0软件进行分析,两组数据比较采用检验,<0.05代表有统计学差异。
结果
1 去势和睾酮处理对高脂诱导小型猪肾脏功能指标的影响
解剖发现,CM组小型猪肾脏体积较IM组和CMT组小型猪明显缩小,重量指数显著低于其他两组动物,见图1A~C。在高脂诱导8周前,去势和睾酮处理对小型猪BUN含量没有影响,但12周时CM组小型猪BUN含量显著高于IM组和CMT组(<0.05),见图1D。8周时,CM组小型猪血清ALB含量显著高于IM组小型猪,但与CMT小型猪相比没有显著差异,见图1G。整个饲喂期间,去势和睾酮处理对小型猪血清CREA和TP水平没有显著影响,见图1E、F。
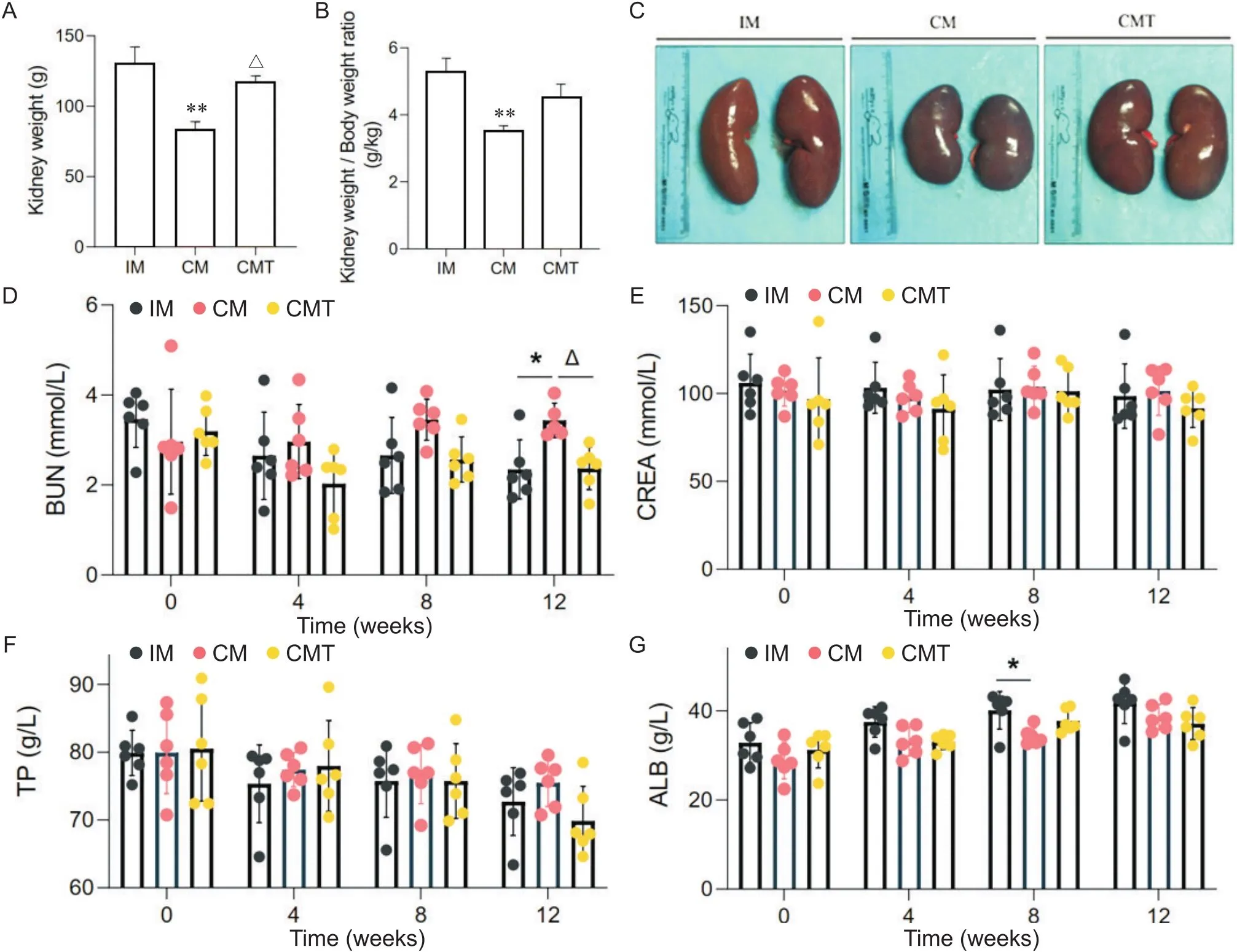
Figure 1. Effects of castration and testosterone replacement on kidney weight and renal function indexes of miniature pigs fed with high-fat diet. A: kidney weight; B: kidney weight/body weight ratio; C: kidney morphological changes; D~G: blood urea nitrogen (BUN), serum creatinine (CREA), serum total protein (TP) and serum albumin (ALB) levels. Mean±SD. n=6. *P<0.05, **P<0.01 vs IM group;△P<0.05 vs CM group.
2 去势和睾酮处理对高脂诱导小型猪肾脏组织病理学的影响
高脂饲喂后,3组小型猪肾脏组织肾小球细胞系膜细胞呈现不同程度的增生,但CM组小型猪肾小球体积轻微增大,基底膜不规则增厚,部分肾小管上皮细胞出现空泡变性(图2C)。油红O染色结果显示,CM组小型猪肾小管上皮细胞内出现大量脂滴,部分肾小球内也可见脂质积聚,睾酮处理能够改善去势小型猪肾脏内沉积的脂质(图2D)。生化检测结果表明,CM小型猪肾脏甘油三酯含量显著高于IM和CMT小型猪(<0.05),但胆固醇含量没有明显差异,进一步证实了油红O染色的结果(图2A、B)。
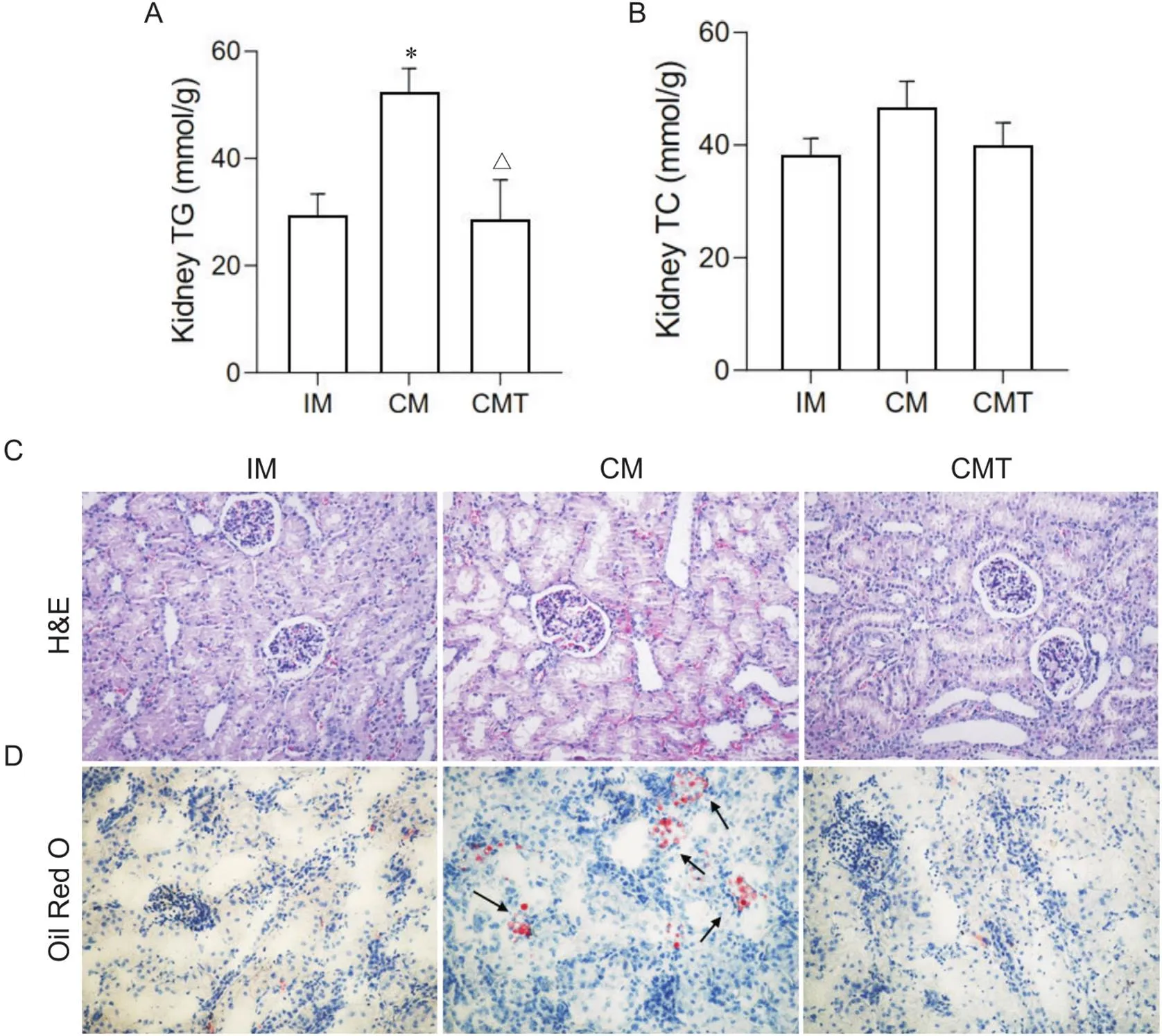
Figure 2. Effects of castration and testosterone replacement on kidney lipid deposition and histopathology in miniature pigs fed with high-fat diet. A: kidney TC content; B: kidney TG content; C: kidney sections stained with H&E (×200); D: oil red O staining of lipid in kidney (×200). Mean±SD. n=6. *P<0.05 vs IM group;△P<0.05 vs CM group.
3 肾脏差异基因分析
为了探讨雄激素缺乏诱导小型猪肾脏脂质沉积的机制,我们对3组小型猪肾脏组织进行了RNA-Seq分析。以差异倍数绝对值(| fold change |)≥1.5和<0.05作为标准筛选各组样本之间的DEGs。结果发现,CMIM差异基因1 081个,其中上调基因491个,下调基因590;CMTCM差异基因940个,其中上调基因550个,下调基因390;CMTIM差异基因574个,其中上调基因349个,下调基因225(图3)。
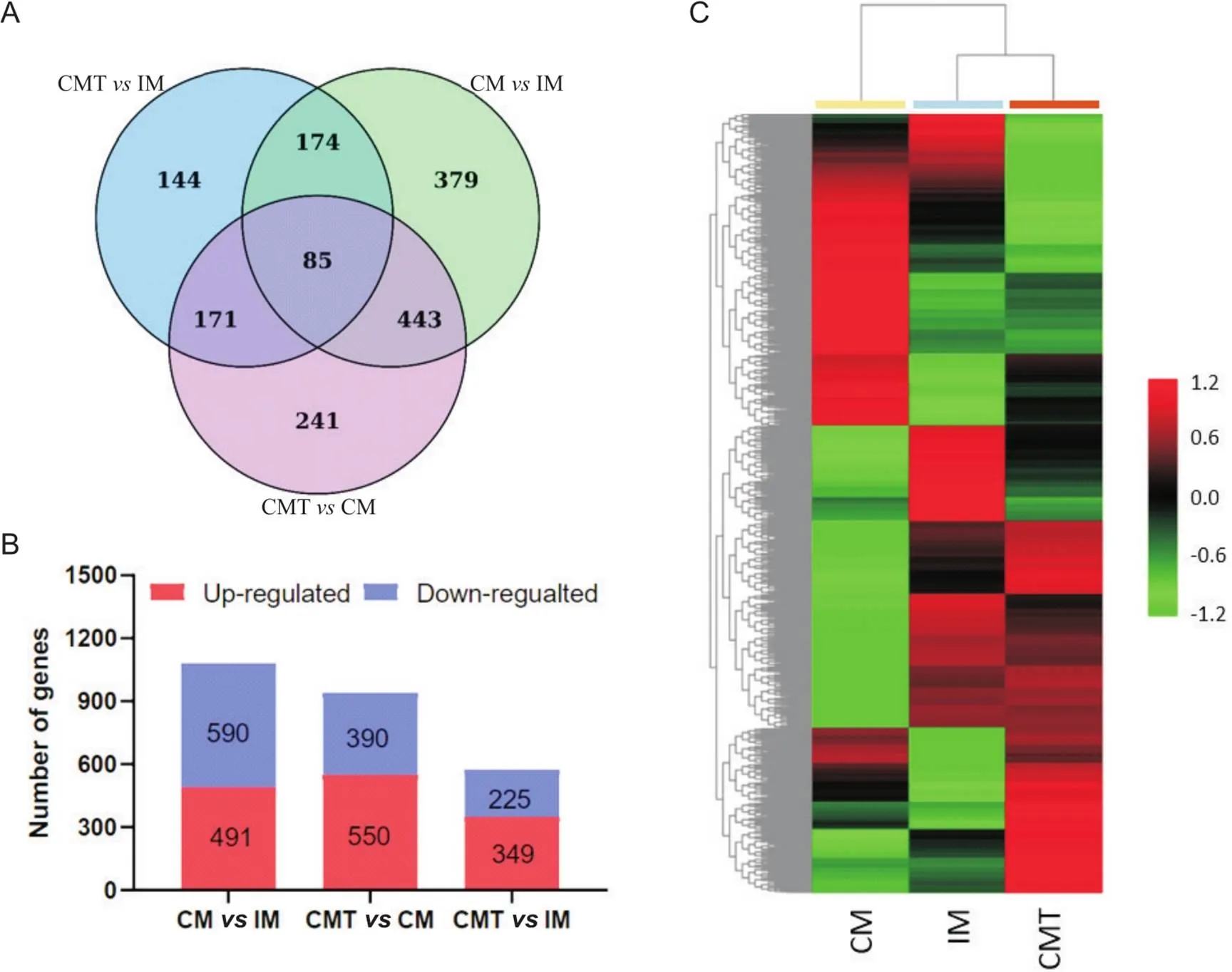
Figure 3. Analysis of differentially expressed genes (DEGs) in kidney of IM, CM, and CMT pigs. A: the numbers of DEGs between groups; B: the numbers of up-regulated and down-regulated DEGs between groups; C: heatmap for hierarchical cluster analysis of DEGs between samples.
4 肾脏差异基因的STEM分析
为更清楚显示筛选获得的差异基因在3组小型猪肾脏中的表达模式,我们采用STEM算法对上述DEGs进行了分析,将它们分成了7个可能的表达模块(Profile)。结果表明,7个模块中只有Profile 2和Profile 5具有统计显著性意义。其中,Profile 2包括396个DEGs,它们在3组样本间(IM→CM→CMT)表达趋势为先下调后上调,而Profile 5包括420个基因,表达趋势则为先上调后下调(图4A)。
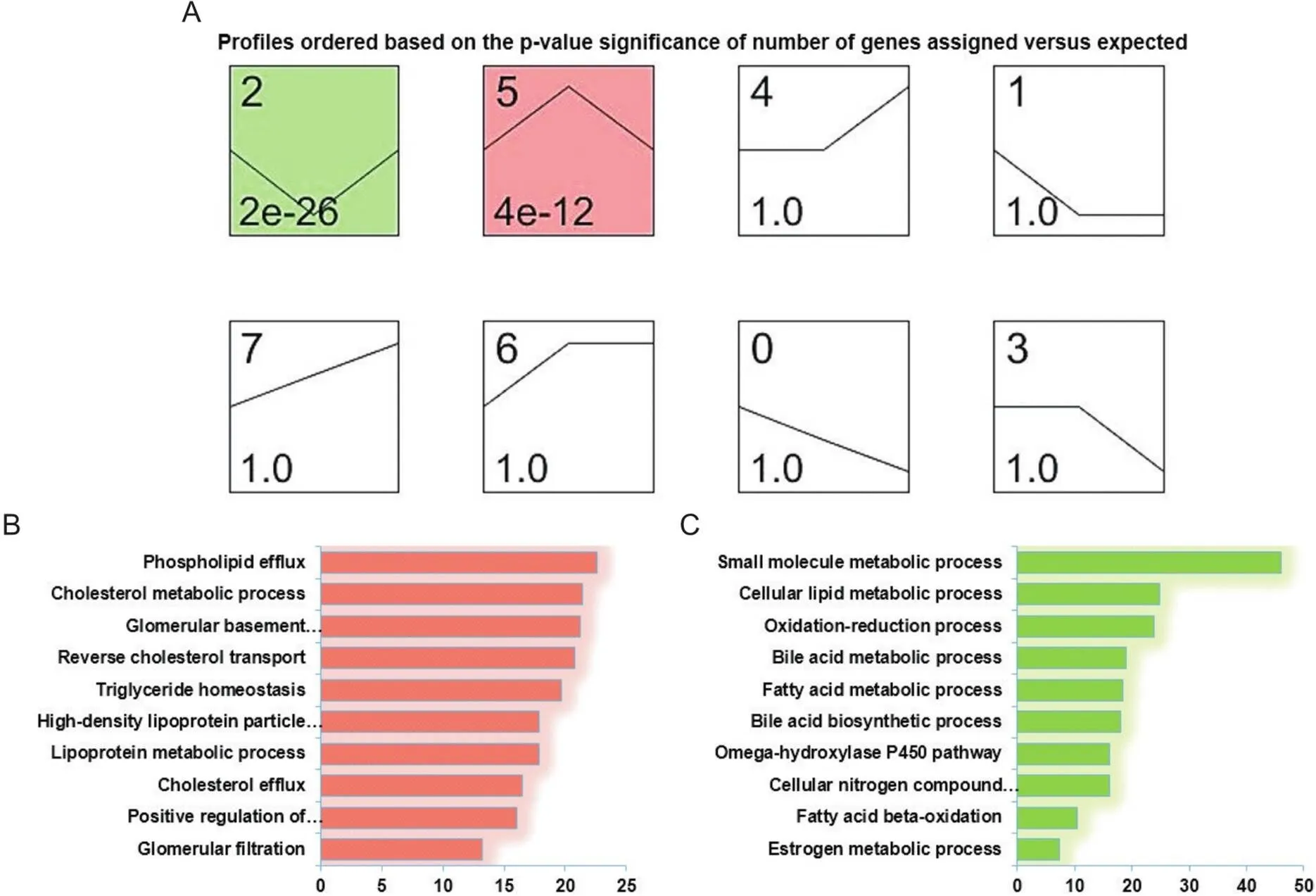
Figure 4. Gene expression tendencies and gene ontology (GO) analysis. A: series-cluster analysis for gene expression profiles of DEGs; B: enriched GO terms in Profile 5; C: enriched GO terms in Profile 2.
接下来,我们对Profile 2和Profile 5进行了GO富集分析。结果显示,Profile 5所包含基因主要和磷脂输出[载脂蛋白C3(apolipoprotein C3, APOC3)和载脂蛋白A5(apolipoprotein A5, APOA5)等]、胆固醇代谢[ATP结合盒转运体G1(ATP binding cassette transporter G1, ABCG1)和载脂蛋白E(apolipoprotein E, APOE)等]、甘油三酯平衡[SREBF1、甘油磷酸肌醇锚定高密度脂蛋白结合蛋白(glycosylphosphatidylinositol anchored high density lipoprotein binding protein, GPIHBP1)和MLXIPL等]、肾小球基底膜发育[IV型胶原A3(collagen type IV alpha 3, COL4A3)和IV型胶原A4(collagen type IV alpha 4, COL4A4)等]和肾小球过滤[硫酸酯酶2(sulfatase 2, SULF2)和免疫球蛋白J(immunoglobulin J, IgJ)等]等生物学过程有关;而Profile 2所包含基因则主要与小分子代谢[细胞色素P450家族成员2C49(cytochrome P450 2C9, CYP2C49)、长寿保证同源物4(longevity assurance homologue 4, LASS4)和烟酰胺单核苷酸腺苷转移酶1(nicotinamide mononucleotide adenylyl transferase 1, NMNAT1)等]、氧化还原反应[脱氢酶/氧化还原酶家族成员7(dehydrogenase/reductase family member 7, DHRS7)、乙醇脱氢酶7(alcohol dehydrogenase 7, ADH7)和细胞色素B5(cytochrome5type B,CYB5B)等]、胆汁酸代谢[法尼酯X受体(farnesoid X receptor, FXR/NR1H4)、固醇携带蛋白2(sterol carrier protein 2, SCP2)和溶质转运27家族成员2(solute carrier family 27 member 2, SLC27A2)等]、脂肪酸氧化[ATP结合盒转运蛋白D3(ATP-binding cassette transporter D3, ABCD3)和长链酰基辅酶A脱氢酶(acyl-CoA dehydrogenase long chain, ACADL)等]和雌激素代谢[17β-雌二醇脱氢酶IV(17β-hydroxysteroid dehydrogenase IV, HSD17B4)和雌激素受体1(estrogen receptor 1, ESR1)等]有关(图4B、表2)。
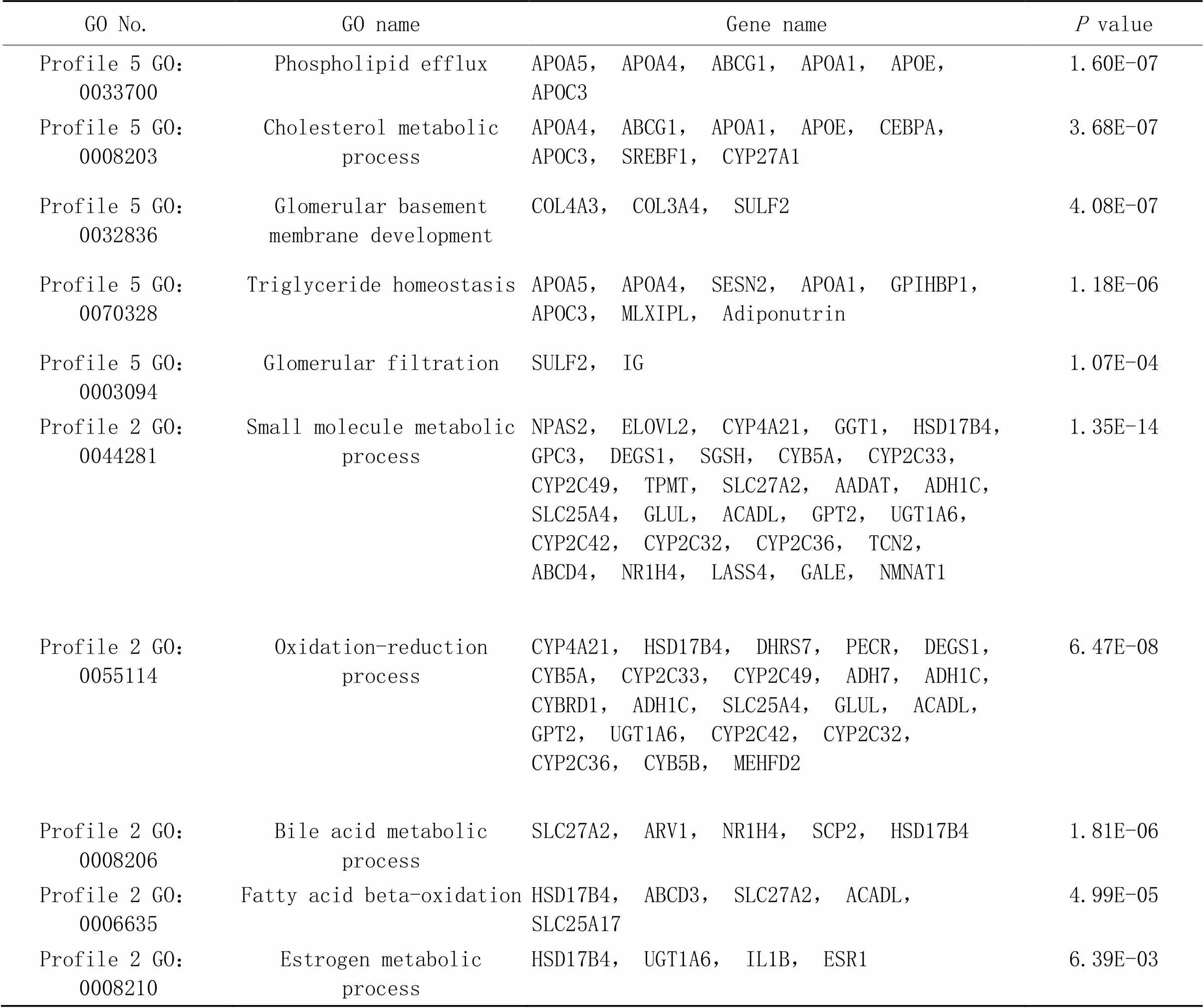
表2 基因术语及相关基因
5 肾脏基因和蛋白表达验证
从前面分析可知,CM小型猪肾脏胆汁酸和雌激素代谢相关基因表达下调,而甘油三酯沉积相关基因表达上调,提示雄激素缺乏诱导肾脏脂质沉积可能和上述途径有关。我们选择上述部分DEGs,包括ESR1、NR1H4、SREBF1、MLXIPL和SCD,采用RT-qPCR和Western blot方法进行了验证。RT-qPCR验证结果表明,相比IM组和CMT组小型猪,CM小型猪肾脏、和基因表达显著上调,而和基因表达显著降低。我们选择了3个表达差异未达到显著的基因进行RT-qPCR验证,结果证实,微小异源二聚体(small heterodimer partner, SHP/NR0B2)、FASN和ACC在组间表达无显著差异(图5A、B),这与测序结果基本一致。Western blot检测发现,CM小型猪肾脏SREBF1和SCD蛋白表达明显高于IM和CMT小型猪(图5C~E),这从蛋白水平上证实了测序结果一致性。
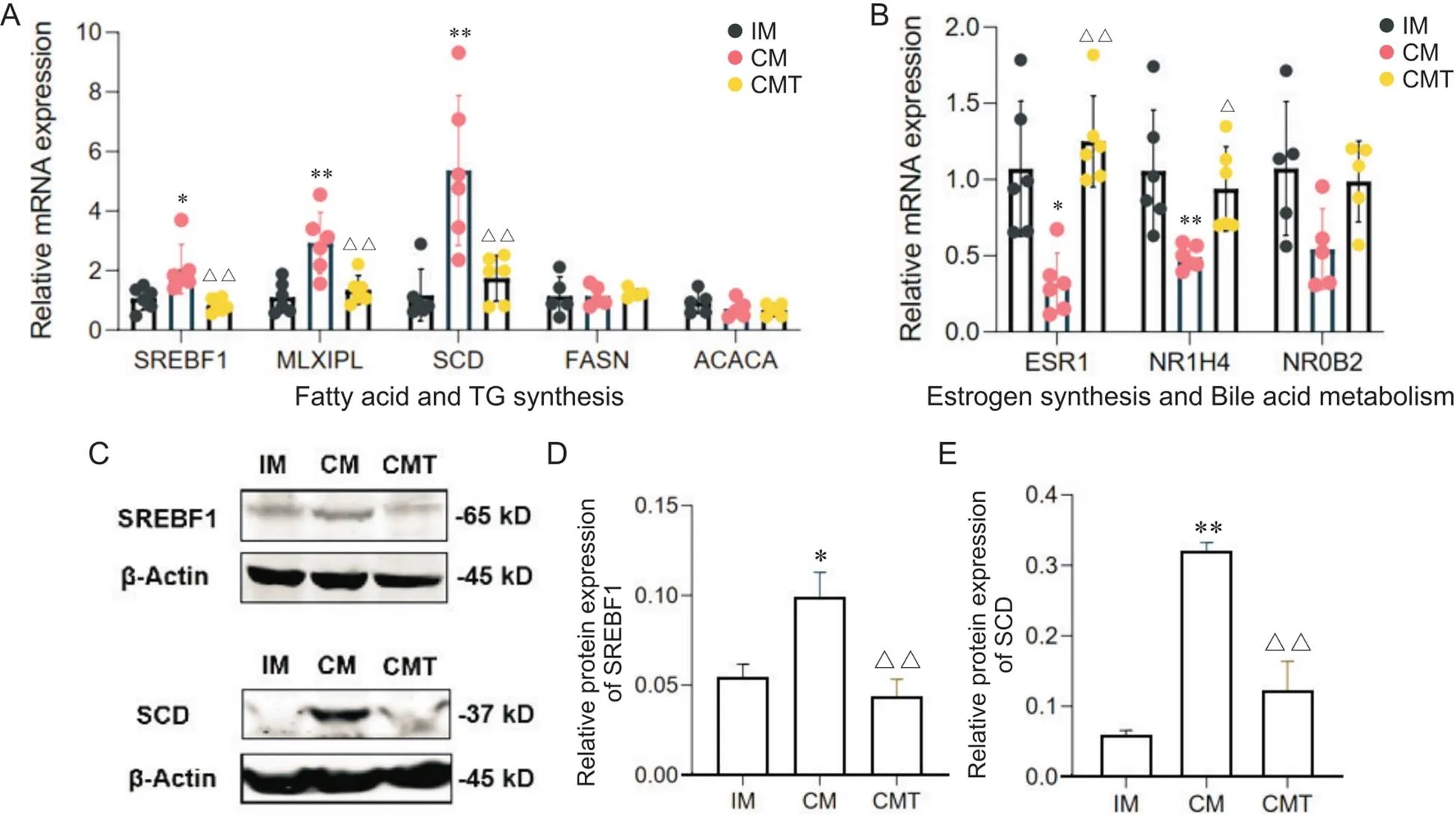
Figure 5. Expression of DEGs involved in fatty acid and TG synthesis, estrogen synthesis and bile acid metabolism. A: expression of fatty acid and TG synthesis-related genes detected by RT-qPCR; B: expression of estrogen synthesis and bile acid metabolism-related genes detected by RT-qPCR; C~E: Western blot analysis of SREBF1 and SCD. Mean±SD. n=3. *P<0.05, **P<0.01 vs IM group;△P<0.05, △△P<0.01 vs CM group.
讨论
本研究分析了去势和睾酮处理对高脂诱导下小型猪肾脏脂质沉积和功能指标的影响,结果表明,CM小型猪血清BUN含量和肾脏TG沉积显著高于其他两组小型猪,但血清CREA、TP以及肾脏TC含量却没有显著差异。目前有关雄激素水平影响肾脏脂质沉积和功能的报道较少,不过曾有研究发现,高脂诱导导致大鼠肾脏损伤存在性别差异[13]。还有研究报道,卵巢摘除引起的雌激素缺乏可以促进高胆固醇血症小鼠肾脏脂质沉积和肾功能紊乱[14]。可见,性激素对高脂饮食或高胆固醇血症对肾脏脂质沉积和功能具有重要调控作用。Liu等[15]采用高脂饮食饲喂5个月能够诱导巴马小型猪肾脏脂质沉积,但对血和尿肌酐水平并无显著影响。Li等[16]采用高脂饮食饲喂长达23个月来诱导巴马小型猪代谢综合症模型,发现模型组小型猪不仅肾脏出现脂质沉积,血清肌酐和尿素氮也显著升高。由此可见,雄激素缺乏促进短期高脂诱导的小型猪肾脏脂质沉积,但肾功能出现严重损伤可能需更长时间饮食诱导。
转录组测序分析表明,CM小型猪肾脏中许多与甘油三酯平衡、胆固醇代谢以及脂蛋白代谢有关的基因表达上调,例如、、、patatin样磷脂酶域蛋白3(patatin-like phospholipase domain-containing 3,)、、和脂滴包被蛋白4(perilipin 4,)等。SREBF1作为脂质代谢调节关键核转录因子,近年来被发现在许多因素引起的肾脏脂质沉积有重要调节作用。值得一提的是,Jiang等[6]研究曾发现,衰老诱导的小鼠肾脏脂质沉积增加过程中伴随着基因表达升高,这和我们的研究结果相似。该研究还发现,衰老小鼠肾脏胆固醇合成途径限速酶羟甲基戊二酰辅酶A还原酶(3-hydroxy-3-methylglutaryl coenzyme A reductase,)基因表达升高,这和我们的结果却不太一致,原因可能在于CM小型猪肾脏胆固醇沉积较IM组差异不明显。Braun等[17]分析了与Jiang等[5]研究相近年龄的同品系小鼠肾脏却发现,衰老小鼠肾脏基因表达并未上调反而下调,可见衰老导致的肾脏脂质(特别是胆固醇)沉积相关研究仍未定论。
本研究还发现,CM小型猪肾脏中胆汁酸代谢和雌激素合成等相关基因表达下调,包括、、和等。FXR属于代谢性核受体超家族成员,它激活后能够诱导SHP的表达进而抑制SREBP-1c及下游脂质合成基因的表达来减少脂质沉积[18]。有研究证实,采用FXR激动剂处理/小鼠能够诱导其肾脏的表达,并抑制及其下游等基因的表达,从而减少肾脏脂质沉积[19];反之将敲除,糖尿病小鼠肾脏及其靶基因(、和等)表达会显著上调,并且肾脏脂质沉积增加[20]。这些研究结果和本研究发现的CM小型猪肾脏和表达下调,而和表达上调的结果部分相符,提示FXR介导的SREBP-1c脂质合成途径可能参与雄激素缺乏诱导的高脂饮食小型猪肾脏脂质沉积过程。另有研究发现,雌激素能通过其受体ESR1激活肝细胞SHP的表达并抑制SREBP-1c及其靶基因,进而减少肝脏脂质沉积[21]。本研究发现,CM小型猪肾脏基因表达显著下调,表明睾酮可能通过芳香化为雌激素参与调节肾脏脂质沉积过程。雌激素还通过ESR1抑制肝脏和基因表达[22-23]。因此,有关性激素调控肾脏脂质沉积的作用可能涉及FXR以及雌激素受体依赖和非依赖作用,今后需要在细胞水平上进一步开展深入研究。
综上所述,雄激素缺乏能够促进短期高脂饮食诱导的小型猪肾脏脂质沉积,并且在雄激素缺乏小型猪肾脏组织中,甘油三酯合成和脂蛋白代谢相关基因表达上调,而胆汁酸代谢、脂肪酸氧化和雌激素合成相关基因表达下调。ESR1和NR1H4可能通过影响SREBF1脂质合成途径参与高脂诱导的雄激素缺乏小型猪肾脏脂质沉积过程,这为老年男性慢性肾脏疾病防治提供了实验依据。
[1] Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the role of fetuin-A, adiponectin, and AMPK[J]. J Am Soc Nephrol, 2010, 21(3):406-412.
[2] Romejko K, Rymarz A, Sadownik H, et al. Testosterone deficiency as one of the major endocrine disorders in chronic kidney disease[J]. Nutrients, 2022, 14(16):3438.
[3] Zhao JV, Schooling CM. The role of testosterone in chronic kidney disease and kidney function in men and women: a bi-directional Mendelian randomization study in the UK Biobank[J]. BMC Med, 2020, 18(1):122.
[4] Noels H, Lehrke M, Vanholder R, et al. Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations[J]. Nat Rev Nephrol, 2021, 17(8):528-542.
[5] Jiang T, Wang Z, Proctor G, et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via sterol regulatory element-binding protein-1c-dependent pathway[J]. J Biol Chem, 2005, 280(37):32317-32325.
[6] Jiang T, Liebman SE, Lucia MS, et al. Role of altered renal fatty lipid metabolism, and the sterol regulatory element-binding proteins in the pathogenesis of age-related renal disease[J]. Kidney Int, 2005, 68(6):2608-2620.
[7] Wang H, Zhu L, Hao J, et al. Co-regulation of SREBP-1 and mTOR ameliorates lipid accumulation in kidney of diabetic mice[J]. Exp Cell Res, 2015, 336(1):76-84.
[8]郝军, 曹延萍, 朱琳, 等. 罗格列酮对高脂喂养大鼠肾小管上皮细胞SREBP-1、TGF-β表达和细胞外基质沉积的影响[J]. 中国病理生理杂志, 2009, 25(12):2430-2435.
Hao J, Cao YP, Zhu L, et al. Effect of rosiglitazone on SREBP-1 and TGF-β expression and accumulation of ECM in renal tubular cells of Wistar rats treated with high fat diet[J]. Chin J Pathophysiol, 2009, 25(12):2430-2435.
[9]郝军, 王晨, 吴海江, 等. SREBP-1和SREBP-2在Ⅰ型糖尿病大鼠肾脏中的表达[J]. 中国病理生理杂志, 2009, 25(3):566-571.
Hao J, Wang C, Wu HJ, et al. Expression of SREBP-1 and SREBP-2 in kidney of type 1 diabetic rats[J]. Chin J Pathophysiol, 2009, 25(3):566-571.
[10] Chung KW, Lee EK, Lee MK, et al. Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging[J]. J Am Soc Nephrol, 2018, 29(4):1223-1237.
[11] Cai Z, Jiang X, Pan Y, et al. Transcriptomic analysis of hepatic responses to testosterone deficiency in miniature pigs fed a high-cholesterol diet[J]. BMC genomics, 2015, 16(1):59.
[12] 蔡兆伟, 凌云, 蔡月琴, 等. 高脂环境下雄激素缺乏对小型猪内脏脂肪蓄积和炎症基因表达的影响[J]. 中国实验动物学报, 2017, 25(1):74-78.
Cai ZW, Ling Y, Cai YQ, et al. Effect of androgen deficiency on visceral fat accumulation and inflammatory gene expression in miniature pigs fed a high-fat diet[J]. Acta Lab Anim Sci Sin, 2017, 25(1):74-78.
[13] Al-Rejaie SS, Abuohashish HM, Alkhamees OA, et al. Gender difference following high cholesterol diet induced renal injury and the protective role of rutin and ascorbic acid combination in Wistar albino rats[J]. Lipids Health Dis, 2012, 11:41.
[14] Carneiro SS, Carminati RZ, Freitas FP, et al. Endogenous female sex hormones delay the development of renal dysfunction in apolipoprotein E-deficient mice[J]. Lipids Health Dis, 2014, 13:176
[15] Liu Y, Wang ZB, Yin WD, et al. Preventive effect of Ibrolipim on suppressing lipid accumulation and increasing lipoprotein lipase in the kidneys of diet-induced diabetic minipigs[J]. Lipids Health Dis, 2011, 10:117.
[16] Li L, Zhao Z, Xia J, et al. A long-term high-fat/high-sucrose diet promotes kidney lipid deposition and causes apoptosis and glomerular hypertrophy in Bama minipigs[J]. PLoS One, 2015, 10(11):e0142884
[17] Braun F, Rinschen MM, Bartels V, et al. Altered lipid metabolism in the aging kidney identified by three layered omic analysis[J]. Aging, 2016, 8(3):441-57.
[18] Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nucler sterol-activated receptors LXR and FXR[J]. Nat Rev Mol Cell Biol, 2012, 13(4):213-224.
[19] Jiang T, Wang XX, Scherzer P, et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy[J]. Diabetes, 2007, 56(10):2485-2493.
[20] Wang XX, Jiang T, Shen Y, et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor reactivation in a type 1 diabetes model[J]. Diabetes, 2010, 59(11):2916-2927.
[21] Wang X, Lu Y, Wang E, et al. Hepatic estrogen receptor α improves hepatosteatosis through upregulation of small heterodimer parter[J]. J Heptol, 2015, 63(1):183-190.
[22] Bryzgalova G, Lundholm L, Portwood N, et al. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice[J]. Am J Physiol Endocrinol Metab, 2008, 295(4):E904-E912.
[23] Kim S, Jin Y, Park Y. Estrogen and n-3 polyunsaturated fatty acid supplementation have a synergistic hypotriglyceridemic effect in ovariectomized rats[J]. Genes Nutr, 2015, 10(4):475.
Analysis of key genes regulating renal lipid accumulation in testosterone-deficient miniature pigs fed with high-fat diet
FAN Yingying1, REN Yujie1, LING Yun2, LÜ Dongying2, CAI Zhaowei1,2△
(1,,310053,;2,,,310053,)
To investigate the effect of testosterone deficiency on renal lipid deposition and related gene expression in miniature pigs fed with high-fat diet.Male Wuzhishan miniature pigs were randomly divided into 3 groups: intact male pigs (IM), castrated male pigs (CM) and castrated male pigs with testosterone replacement (CMT). Six pigs in each group were fed with high-fat diet for 12 weeks. Serum renal function parameters and renal triglyceride (TG) and total cholesterol (TC) levels were detected. Oil red O and HE staining was performed to observe lipid deposition and histopathological changes in the kidney. RNA-Seq technology was used to investigate the differential expression of transcriptome in the kidney. RT-qPCR and Western blot were used to verify the differentially expressed genes involved in TG synthesis, bile acid metabolism and estrogen synthesis.The kidney weight was significantly lower in CM group than IM group and CMT group (<0.05). The content of blood urea nitrogen in CM group was increased compared with IM group and CMT group (<0.05). However, serum creatinine and total protein levels showed no significant difference. A large number of lipid droplets and significantly increased TG content in the kidney were observed in CM group compared with IM and CMT groups (<0.05). Compared with IM group and CMT group, the expression of TG synthesis genes including sterol regulatory element binding transcription factor 1 (), carbohydrate-responsive element-binding protein (/) and stearoyl-coenzyme A desaturase () was significantly increased in the kidneys of CM, while the expression of genes related to bile acid metabolism and estrogen synthesis including farnesoid X receptor (/) and estrogen receptor 1 () was significantly decreased. Testosterone replacement reversed the changes of lipid deposition and related gene expression in the kidneys of CM.Theandgenes may be involved in the process of lipid deposition in the kidneys of CM fed with high-fat diet through influencing the SREBF1 lipid synthesis pathway.
kidney; lipid deposition; androgen deficiency; miniature pigs; high-fat diet
R692; R363.2
A
10.3969/j.issn.1000-4718.2023.02.013
1000-4718(2023)02-0305-09
2022-10-11
2022-12-24
[基金项目]浙江省基础公益研究计划项目(No. LGD22C040008);浙江中医药大学自然科学青年探索项目(No. 2021JKZKTS008A)
Tel: 0571-86613782; E-mail: zwcai@zcmu.edu.cn
(责任编辑:宋延君,李淑媛)
