人参皂苷Rg1通过TLR4/MyD88/NF-κB p65通路调控小鼠急性肾损伤诱导的急性肝损伤的机制研究*
2023-03-10迟晓晨曹瀛心包翠芬李婷钰阎丽菁
迟晓晨, 曹瀛心, 包翠芬, 李婷钰, 阎丽菁
人参皂苷Rg1通过TLR4/MyD88/NF-κB p65通路调控小鼠急性肾损伤诱导的急性肝损伤的机制研究*
迟晓晨1, 曹瀛心1, 包翠芬2△, 李婷钰1, 阎丽菁2△
(1锦州医科大学组织胚胎学教研室,辽宁 锦州 121001;2锦州医科大学基础医学实验教学中心,辽宁 锦州 121001)
探讨人参皂苷Rg1(GRg1)对小鼠急性肾损伤所诱导的急性肝损伤的保护作用及其调控机制。昆明小鼠随机分为假手术(sham)组、模型(model)组、GRg1组和necrostatin-1 (Nec-1)组,每组10只。制备急性肾损伤模型,24 h后收集血液。采用生化试剂盒检测小鼠血清肌酐(SCr)、血尿素氮(BUN)、天冬氨酸转氨酶(AST)、谷氨酸转氨酶(ALT)、丙二醛(MDA)和超氧化物歧化酶(SOD)水平。采用ELISA法检测炎症因子白细胞介素1β(IL-1β)、IL-6、IL-8和肿瘤坏死因子α(TNF-α)的表达。HE染色观察组织病理学改变,采用免疫组织化学和Western blot法检测TLR4、MyD88和NF-κB p65蛋白的表达水平。与假手术组比较,model组小鼠出现明显的肝细胞坏死、肝肾功能减退,血清中SCr、BUN、AST和ALT均显著升高(<0.01),MDA含量显著上升,SOD活性显著降低(<0.01),且血清中炎症因子IL-1β、IL-6、IL-8和TNFα含量显著升高(<0.01),TLR4、MyD88和NF-κB p65蛋白表达显著增高(<0.01);与model组相比,GRg1和Nec-1组处理后小鼠肝细胞坏死减轻,肝肾功能显著改善(<0.01),血清中SCr、BUN、AST和ALT水平显著降低(<0.01),MDA含量显著降低,SOD活性显著增高(<0.01),血清中炎症因子IL-1β、IL-6、IL-8和TNF-α含量显著降低(<0.01),TLR4、MyD88和NF-κB p65蛋白表达显著降低(<0.01);GRg1组和Nec-1组小鼠上述指标比较差异无统计学意义。GRg1可以改善小鼠急性肾损伤所致急性肝损伤的肝肾功能,其机制可能与抑制TLR4/MyD88/NF-κB p65信号通路有关。
人参皂苷Rg1;急性肾损伤;急性肝损伤;TLR4/MyD88/NF-κB p65信号通路
急性肾损伤(acute kidney injury, AKI)多见于原位移植、急性缺血性创伤等疾病,尤其是在肾移植、肾切除等低灌注类大手术时,发生率极高[1]。AKI发生后,机体出现毒素积累、酸碱平衡失调、电解质失调、氧化应激等一系列炎症级联反应,引起其它脏器受累,如急性肝损伤(acute hepatic damage, AHD)[2]。上述因素可导致肝脏代谢负担加重,促进肝脏细胞变性、坏死,炎症渗出物增多,引发AHD。
炎症反应是维持机体稳态的重要环节。Toll样受体(Toll-like receptor, TLR)是机体固有免疫系统重要模式识别受体,可以检测并激活炎症信号通路,TLR4可以通过多种通路发起促炎作用,包括核苷酸结合寡聚化结构域样受体蛋白3(nucleotide-binding oligomerization domain-like receptor protein 3, NLRP3)、髓样分化因子88(myeloid differentiation factor 88, MyD88)等[3-5],AKI发生后,肾组织中TLR4表达明显增高,促发炎症反应,从而引发一系列的病理改变。
据目前研究,为了缓解AKI,提升机体机能,连续肾脏替代治疗法能够有效的改善肾脏受损程度,但由于治疗程序繁琐也会对其他器官造成副作用,有实验研究采用中、西药改善AKI[6-7],但对于同时能够缓解远端器官损伤的药物研究相对较少,人参皂苷Rg1(ginsenoside Rg1, GRg1)是传统中草药人参的主要活性成分,具有抗凋亡、改善认知功能和保护神经系统功能等多种药理作用[8-9]。有研究显示,GRg1可以通过TLR4/NF-κB/NLRP3通路抑制心肌细胞凋亡和炎症反应[10],但在肝损伤治疗的机制有待进一步研究,本项工作拟通过制备动物模型,并使用GRg1进行干预治疗,以探讨GRg1对小鼠AKI所诱导的AHD的保护作用及其可能的调控机制。
材料和方法
1 动物
SPF级昆明小鼠,雄性5~6周,28~32 g,40只,购自锦州医科大学实验动物中心,生产许可证编号为SCXY(辽)2019-0003。本实验中使用的所有方案均得到了实验动物伦理委员会的审核批准(No. 2018011001)。
2 主要试剂
GRg1(吉林大学有机化学教研室);程序性坏死抑制剂necrostatin-1 (Nec-1)购自Sigma;血肌酐(serum creatinine, SCr)、血尿素氮(blood urea nitrogen, BUN)、谷草转氨酶(aspartate aminotransferase, AST)、谷丙转氨酶(alanine aminotransferase, ALT)、超氧化物歧化酶(superoxide dismutase, SOD)和丙二醛(malondialdehyde, MDA)检测试剂盒(南京建成生物工程研究所);白细胞介素1β(interleukin-1β, IL-1β)、IL-6、IL-8和肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)酶联免疫吸附检测试剂盒(上海酶联生物科技有限公司);抗TLR4和NF-κB p65抗体[艾博抗(上海)贸易有限公司];MyD88抗体(武汉爱博泰克生物科技有限公司);HRP标记的Ⅱ抗IgG(北京中杉金桥生物技术有限公司);BCA蛋白浓度测定试剂盒和电化学发光ECL显影液(上海碧云天生物技术有限公司)。
3 主要仪器设备
PowerPacTMHC电泳仪(Bio-Rad);MK3酶标仪(Thermo Fisher Labsystems);VibraCellVcx105超声粉碎机(Sonics);ST 16R超速离心机(ThermoFisher);AMERSHAM IMAGER600凝胶成像系统(日本通用电气公司)。
4 主要方法
4.1动物分组与处理采用随机数字法将小鼠分为4组,假手术组(sham组)、AKI模型组(model组)、GRg1组和Necrostatin-1抑制剂组(Nec-1组),每组10只。GRg1组术后腹腔注射GRg1(56 mg/kg[11]),Nec-1组注射Nec-1(1.65 mg/kg[12]),sham组和model组分别注射等量生理盐水。
4.2动物模型制备采用20%乌拉坦麻醉小鼠,沿背正中线剪开皮肤,先于脊柱右侧肌肉开一小口,双线结扎法结扎右肾后进行切除,消毒切口并缝合。然后于脊柱左侧肌肉开一小口,探寻左侧肾脏,游离肾蒂,剥离表面被膜,小心分离肾动静脉,使用无损伤动脉夹夹闭肾动脉,计时,观察到肾脏表面颜色逐渐加深,由粉红色变为暗红色,肾脏体积明显变大,45 min后,松开动脉夹[13],观察肾脏血流恢复,颜色逐渐转为粉红色后,将肾脏回纳腹腔,消毒切口并逐层缝合,sham组只游离并剥离肾动静脉,做挑起肾动脉的动作,但不夹闭,术后注意动物保暖,正常喂养,24 h后灌流取材[14]。
4.3标本收集采集各组小鼠心尖血液,离心力1 370 ×,4 ℃离心15 min后,取上清液于-20 ℃冻存;取血后小鼠生理盐水灌流,取部分肝组织放入10%中性缓冲福尔马林溶液中固定,用于制备形态学标本;其余肝组织置于-80 ℃冻存。
4.4血清肝肾功能指标检测按照试剂盒说明书检测各组小鼠血清SCr、BUN、AST和ALT水平。
4.5氧化应激水平检测按照试剂盒说明书检测各组小鼠血清MDA含量和SOD活性。
4.6ELISA法检测炎症因子表达按照试剂盒说明书检测各组小鼠血清中IL-1β、IL-6、IL-8和TNF-α炎症因子表达,测450 nm波长吸光度()值,以标准品浓度为横坐标,吸光度值为纵坐标,制作标准曲线,计算待测样本实际浓度值。
4.7HE染色观察肝脏损伤程度将各组固定在10%中性缓冲福尔马林液的标本常规脱水后制备石蜡标本。切片后进行HE染色,光学显微镜下观察肝脏病理学改变。
4.8免疫组织化学染色观察肝脏病变位置TLR4/MyD88/NF-κB p65蛋白表达将上述制备好的石蜡切片,采用Envision法进行免疫组织化学染色。石蜡切片常规脱蜡水合后,置于pH 6.0的柠檬酸缓冲液中,于高压锅内修复2.5 min。采用3% H2O2处理以灭活内源性过氧化物酶,滴加Ⅰ抗工作液(TLR4 1∶500,MyD88 1:500,NF-κB p65 1∶200)4 ℃孵育过夜,滴加辣根酶标记的Ⅱ抗多聚体37 ℃孵育30 min。每步之间采用磷酸盐缓冲液(phosphate buffer saline,PBS)进行漂洗。DAB显色,苏木精复染,中性树胶封固。采用PBS替代Ⅰ抗作为阴性对照。
4.9Western blot法检测TLR4/MyD88/NF-κB p65蛋白表达小鼠灌流后,将取下的肝脏组织剪碎,放入电动匀浆器中匀浆,加入组织裂解液和最终浓度为1mmol/L的丝氨酸蛋白酶抑制剂苯甲基磺酰氟(phenylmethanesulfonyl fluoride,PMSF),在冰上裂解30 min后,离心力16 099 ×,4 ℃离心30 min,取上清液置于新的Ep管中,-20 ℃冻存。取少量上清液,以BCA试剂盒进行蛋白定量检测,以最低浓度为标准制定样本。SDS-PAGE后转至聚偏二氟乙烯(polyvinylidene fluoride,PVDF)膜上,1%山羊血清封闭1 h,三乙醇胺缓冲盐水溶液(tris buffered saline and tween20,TBST)清洗后加入Ⅰ抗(TLR4 1∶2 000,MyD88 1∶2 000,NF-κB p65 1∶2 000)4 ℃摇床过夜,TBST洗膜,加入HRP标记的Ⅱ抗IgG,室温摇床孵育1 h,TBST洗膜,ECL发光显色液显影,以β-actin为内参照,采用ImageJ图像处理分析计算各指标灰度值。
5 统计学处理
采用SPSS 25.0软件对各组数据进行分析,计数资料以均数±标准差(mean±SD)形式表示,多组之间数据比较采用单因素方差分析,以<0.05为差异有统计学意义。
结果
1 GRg1对小鼠肾功能、肝功能和氧化应激的影响
与sham组相比,model组小鼠BUN和SCr水平显著升高(<0.01);与model组相比,GRg1组和Nec-1组BUN和SCr水平则显著降低(<0.01),见图1A、B。与sham组相比,model组小鼠血清AST和ALT水平显著升高(<0.01);与model组相比,GRg1组和Nec-1组小鼠血清AST和ALT水平显著降低(<0.01),见图1C、D。与sham组相比,model组小鼠血清MDA含量显著升高(<0.01),SOD活性显著降低(<0.01);与model组相比,GRg1组和Nec-1组小鼠血清MDA含量显著降低(<0.01),SOD活性显著升高(<0.01),见图1E、F。

Figure 1. The BUN level (A), SCr level (B), serum AST activity (C), serum ALT activity (D), serum MDA content (E) and serum SOD activity (F) of mice in each group. Mean±SD. n=3 in A, B, D and F; n=4 in C and E. **P<0.01 vs sham group; ##P<0.01 vs model group; △P<0.05 vs GRg1 group.
2 GRg1对小鼠血清炎症因子水平的影响
与sham组相比,model组小鼠血清IL-1β、IL-6、IL-8和TNF-α水平显著升高(<0.01);与model组相比,GRg1组和Nec-1组小鼠血清IL-1β、IL-6、IL-8和TNF-α水平显著降低(<0.01),见图2。
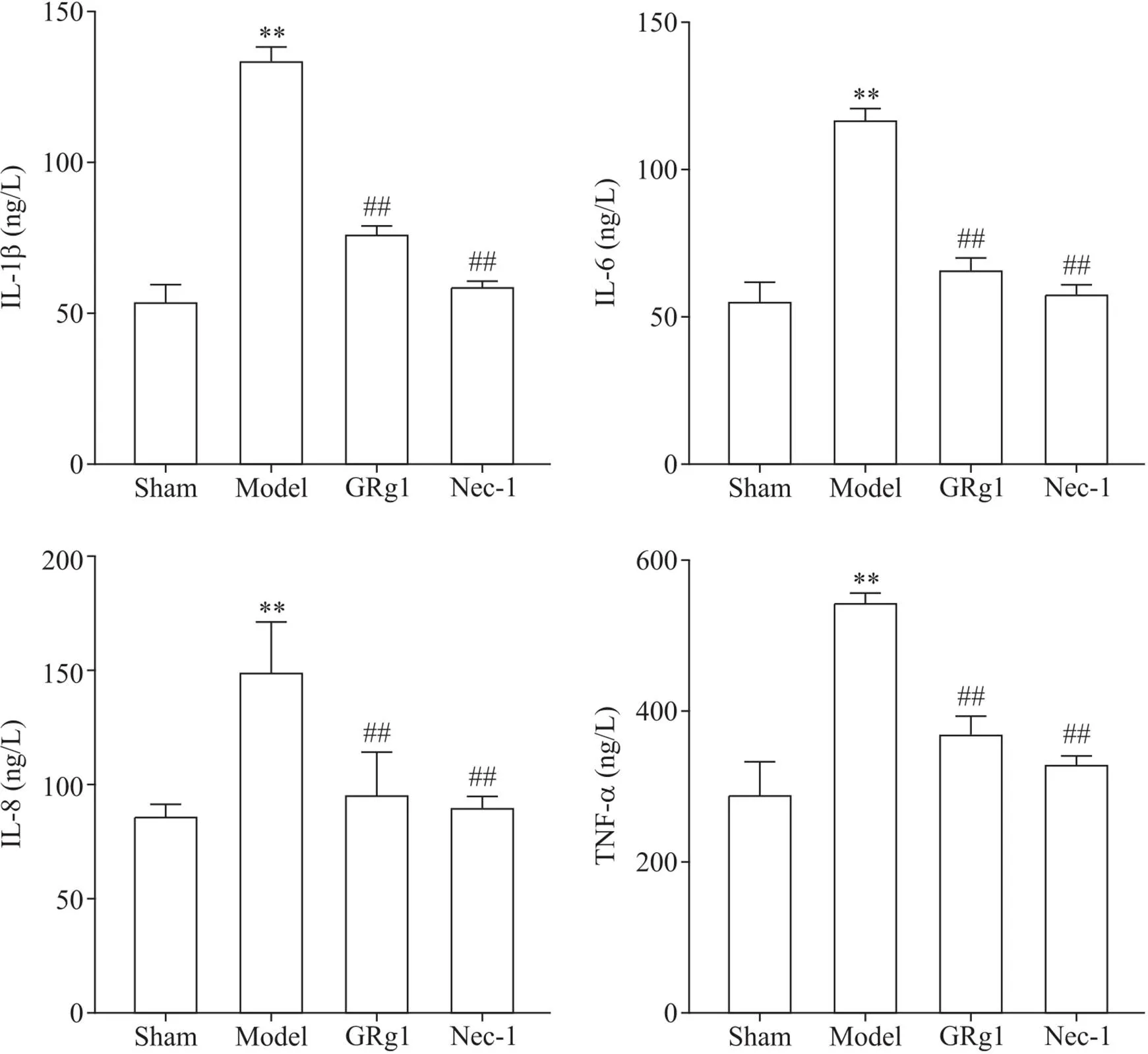
Figure 2. IL-1β, IL-6, IL-8, and TNF-α contents of mice in each group. Mean±SD. n=4. **P<0.01 vs control group; ##P<0.01 vs model group.
3 GRg1对小鼠肝脏组织病理学改变的影响
HE染色结果显示,sham组小鼠肝脏呈现正常组织表现;model组肝脏于被膜下及肝小叶均可见明显的局灶状坏死,有的肝细胞的细胞质淡染、空泡状为脂肪样变性(黑色箭头),有的肝细胞的细胞质呈明显的嗜酸样变,与周围细胞着色明显不同,细胞可见核固缩(红色箭头)。近血管处有的可见明显的炎症细胞浸润(*);GRg1和Nec-1组小鼠可见少量肝细胞的细胞质呈嗜酸样变,肝损伤细胞数量明显减少,未见脂肪样变性及明显的炎症细胞浸润,见图3。
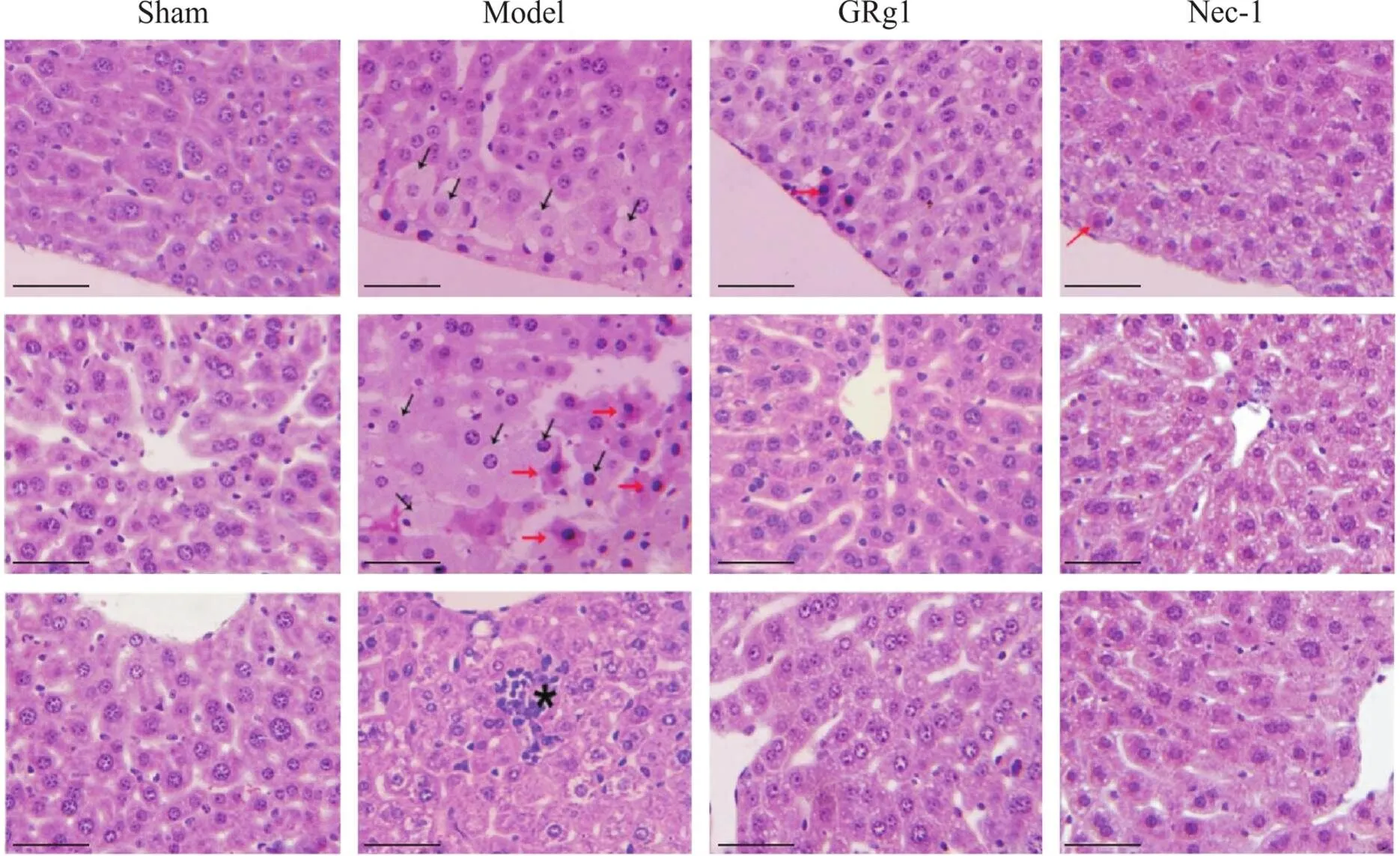
Figure 3. Pathological changes in the liver of mice in each group (HE staining, scale bar=50 μm). The black arrow shows hepatocyte steatosis. The red arrow shows the eosin ophilic change. * shows inflammatory infiltration.
4 GRg1对小鼠肝脏组织TLR4/MyD88/NF-κB p65通路蛋白表达的影响
4.1TLR4表达情况免疫组化染色显示,sham组仅见少量表达微弱的阳性细胞;model组可见较多的肝细胞的细胞质和肝血窦内呈明显的黄色或棕黄色阳性表达;GRg1组和Nec-1组的阳性表达细胞数量较少,肝细胞的细胞质着色呈淡黄色或黄色,见图4。Western blot结果显示,与sham组相比较,model组呈现明显TLR4表达条带,显著高于sham组;与model组比较,GRg1组和Nec-1组的表达条带明显减弱;而GRg1组和Nec-1组之间则无显著差异,见图5。

Figure 4. The expression of TLR4 protein in the liver of mice in each group observed by immunohistochemical staining. Positive cells are indicated by arrows.

Figure 5. The expression level of TLR4 in the liver of mice in each group. Mean±SD. n=4. **P<0.01 vs sham group; ##P<0.01 vs model group.
4.2MyD88表达情况免疫组化染色显示,sham组可见肝血窦内有少量呈淡黄色或黄色的阳性细胞;model组可见部分肝血窦以及部分肝细胞的细胞质内有黄色、棕黄色或褐色颗粒,为阳性表达细胞;GRg1组和Nec-1组的阳性细胞数量较少,且表达细胞呈色为淡黄色或黄色,见图6。Western blot结果显示,model组呈现明显MyD88表达条带,显著高于sham组;与model组比较,GRg1组和Nec-1组的表达条带明显减弱;而GRg1组和Nec-1组之间则无显著差异,见图7。
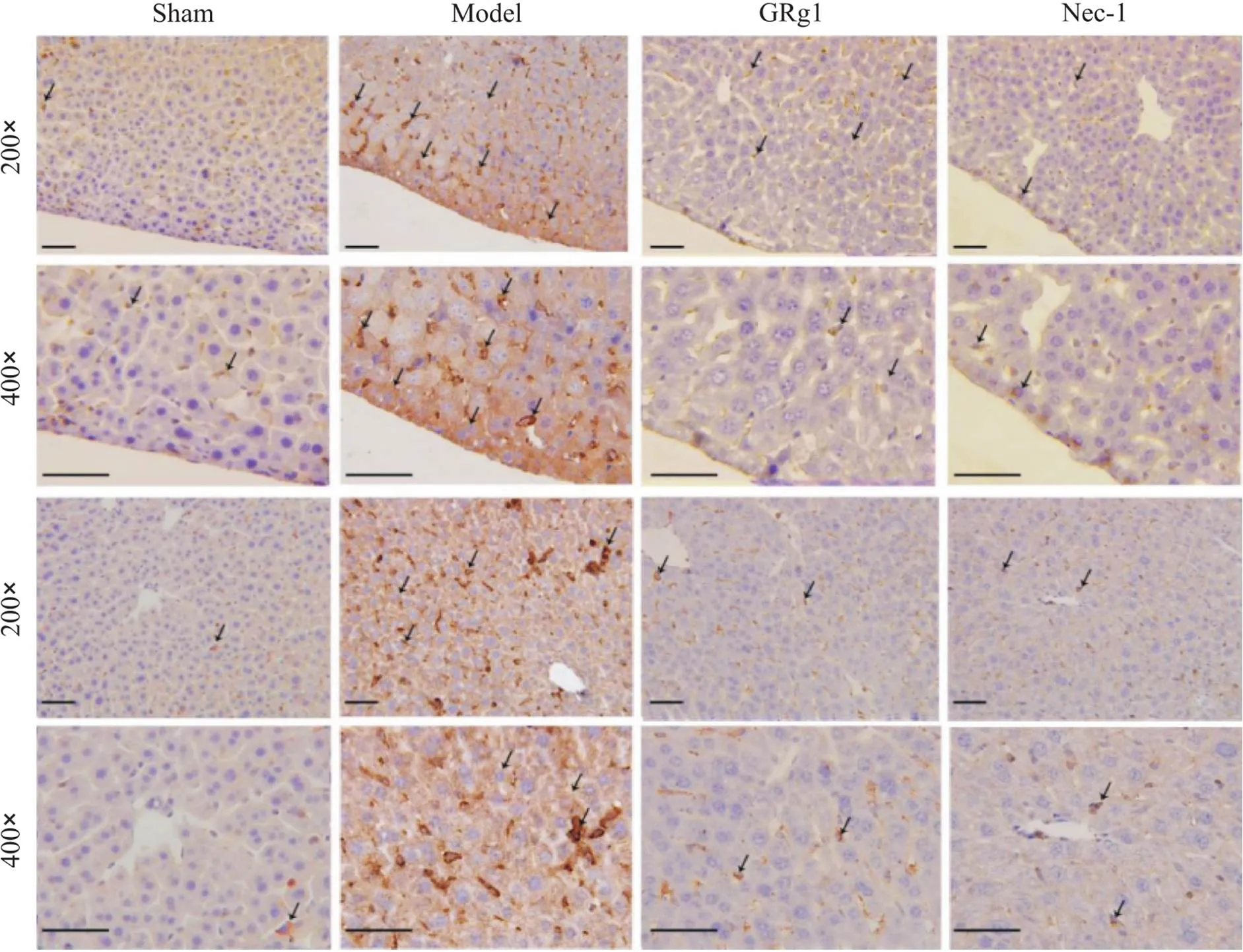
Figure 6. The expression of MyD88 protein in the liver of mice in each group observed by immunohistochemical staining. Positive cells are indicated by arrows.

Figure 7. The expression level of MyD88 in the liver of mice in each group. Mean±SD. n=4. **P<0.01 vs sham group; ##P<0.01 vs model group.
4.3NF-κB p65(phospho S536)表达情况p-NF-κB p65(Ser536)为NF-κB p65的活化形式。NF-κB p65被激活后从细胞质移入到细胞核内发挥其转录作用,因此主要定位于细胞核。免疫组化染色观察sham组可见少量肝细胞的细胞核呈淡黄色或黄色,肝血窦的窦腔内也有少量阳性表达细胞;model组可见较多肝细胞的细胞核呈黄色或棕黄色,肝血窦窦腔内的阳性表达细胞也较多;而GRg1组和Nec-1组NF-κB p65活化细胞数量较少,见图8。Western blot结果显示,与sham组比较,model组呈现明显p-NF-κB p65表达条带,显著高于Sham组;与model组比较,GRg1组和Nec-1组的表达条带明显减弱;而GRg1组和Nec-1组之间则无显著差异,见图9。

Figure 8. The expression of NF-κB p65 protein in the liver of mice in each group was observed by immunohistochemical staining. Positive cells are indicated by arrows.

Figure 9. The expression level of p-NF-κB p65 in the liver of mice in each group. Mean±SD. n=4. **P<0.01 vs sham group; ##P<0.01 vs model group.
讨论
1 GRg1对AKI诱导的AHD的保护作用
AKI是由各种原因导致的肾功能快速下降,伴随一系列连贯的细胞事件发生,包括活性氧释放、凋亡、坏死、炎症细胞的浸润和活性介质的释放,导致肾组织或其它组织损伤[15- 16]。本实验通过阻断肾动脉血流45 min后复灌24 h的方法制备AKI模型,结果显示,经过缺血再灌注处理的小鼠血清肌酐和尿素氮的含量显著升高,肾脏形态学病理检测可见部分肾小管上皮细胞变性、坏死,肾小管管型形成,有的可见脱落细胞,提示小鼠出现AKI。在此基础上,检测小鼠肝脏功能变化,结果显示血清谷草转氨酶和谷丙转氨酶含量显著增高。肝脏病理切片观察显示,model组肝脏可见明显的局灶状坏死,部分肝细胞胞质淡染甚至空泡状。有的肝细胞胞质呈明显的嗜酸样变,与周围细胞明显不同。细胞核有的固缩,近血管处有的可见明显的炎性细胞浸润。上述结果均提出小鼠出现了AHD。而经过GRg1处理后,小鼠肝组织的坏死情况明显改善,提示GRg1对小鼠肝损伤具有保护作用。
2 GRg1通过抑制炎症反应发挥对AHD的保护作用
细胞坏死时由于细胞裂解释放出内含物,经常引发炎症反应。炎症因子作为炎症微环境的关键组成部分,在AHD过程中也发挥着关键作用。包括IL-1β、IL-6、IL-8和TNF-α等[17]。IL-1β和TNF-α是重要的炎症因子,参与炎症反应、细胞凋亡、氧化应激等,具有强烈的促炎活性[15, 18-19]。在TNF-α的刺激下白细胞不断加剧炎症反应[20],吞噬细胞和间充质细胞产生IL-8,诱导趋化、胞吐和氧爆发。加剧促炎因子表达[21]。AKI发生后,单核细胞和巨噬细胞快速产生IL-6,并介导远端器官效应[22]。IL-6能够清除感染因子和修复受损组织,对肝细胞稳态至关重要[23]。机体受到损伤后,处于应激状态,由于交感神经兴奋会产生大量的氧自由基,体内氧化与抗氧化平衡被打破,中性粒细胞发生炎症浸润,蛋白酶分泌增加,氧化中间产物大量释放,如白三烯、血栓素A2等,都是促炎介质,加剧炎症反应,恶性循环。
本研究结果显示,AKI诱发肝损伤后,血清中的炎症及坏死相关因子IL-1β、IL-6、IL-8和TNF-α的表达水平显著上调,切片观察结果显示在部分肝细胞呈现变性或坏死状态,近肝血管附近有炎症细胞浸润现象,提示坏死的同时伴随着炎症反应。同时,检测到model组SOD的活性显著降低,MDA的含量显著升高。SOD为自由基清除剂,当体内SOD含量降低,机体清除自由基能力下降,自由基含量升高,其细胞毒性致使脂质过氧化,MDA含量升高,损伤细胞膜,从而加剧炎症反应。而经Nec-1处理后,组织病理改变明显减轻,上述炎症相关因子的表达降低,提示AKI所致肝损伤症状可以被Nec-1抑制。经GRg1处理后,肝脏受损的程度与model组相比明显减轻,肝功能相对恢复,IL-1β、IL-6、IL-8和TNF-α的表达水平显著降低,SOD水平升高,MDA含量减少,表明GRg1处理对AKI所诱导的肝损伤具有保护作用。
3 GRg1通过TLR4/MyD88/NF-κB p65通路调控AKI诱导的AHD的机制研究
TLR4/MyD88/NF-κB p65通路是参与机体固有免疫的重要通路。TLR4通过招募MyD88发挥其功能[24],MyD88是含有TLRs结构域的接头蛋白,是TLR受体家族下游信号通路的典型适配器。MyD88的死亡结构域与IL-1R相关激酶(IL-1R-related kinase, IRAK)的死亡结构域相结合,引起后者磷酸化,进一步诱导IκB的活化,从而导致NF-κB的激活和转位[25],诱导TNF-α表达,TNF-α增高的同时还会促进NF-κB p65活化,两者互相作用,加重损伤,促进多种炎症因子释放,启动程序性坏死,加剧炎症反应进而导致一系列损伤[26-27],有研究表明Nec-1可以降低TLR4的表达,抑制NF-κB p65磷酸化,降低缺血再灌注所致细胞内ROS产生[28-29]。
本实验结果显示,model组小鼠肝组织内有数量较多的TLR4、MyD88和p-NF-κB p65阳性细胞表达,主要表达于实质肝细胞及肝血窦。其中TLR4和MyD88主要定位于细胞质,而p-NF-κB p65为活化的NF-κB p65,主要定位于细胞核。此结果提示由AKI所诱导的AHD过程中启动了TLR4/MyD88/NF-κB p65通路,由于TLR4的表达增高,进一步与MyD88结合后,诱导NF-κB p65磷酸化,从而使其定位改变,从细胞质迅速移入细胞核,启动细胞核转录,进而促进炎症因子IL-1β、IL-6、IL-8和TNF-α等的释放,促发炎症反应,进一步导致细胞坏死等肝损伤。进一步采用GRg1处理后,TLR4、MyD88和p-NF-κB p65蛋白的表达水平明显降低,提示GRg1抑制了TLR4功能,从而减少了TLR4与MyD88的结合,进一步抑制NF-κB p65的磷酸化水平,减少促炎相关因子IL-1β、IL-6、IL-8和TNF-α等的释放,减轻炎症细胞浸润,从而使肝细胞坏死情况明显改善。且应用GRg1处理的疗效与阳性对照药Nec-1的处理效果并无明显差异。上述结果提示,GRg1可能通过抑制TLR4/MyD88通路的激活,干预NF-κB p65磷酸化水平,抑制AHD炎症反应发展、减轻坏死、改善肝功能,从而发挥保护肝功能作用。
[1] Siew ED, Parr SK, Wild MG, et al. Kidney disease awareness and knowledge among survivors ofacute kidney injury[J]. Am J Nephrol, 2019, 49(6):449-459.
[2] Choi YJ, Zhou D, Barbosa ACS, et al. Activation of constitutive androstane receptor ameliorates renal ischemia-reperfusion-induced kidney and liver injury[J]. Mol Pharmacol, 2018, 93(3):239-250.
[3] Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling[J]. Cell Mol Life Sci, 2021, 78(4):1233-1261.
[4]许武军, 谢娟娟, 陈仙. H2S通过抑制TLR4/MyD88/PI3K信号通路减轻尿源性脓毒血症诱导的急性肾损伤[J]. 中国病理生理杂志, 2019, 35(2):243-247.
Xu WJ, Xie JJ, Chen X. Hydrogen sulfide attenuates urosepsis-induced acute kidney injury by blocking TLR4/MyD88/PI3K signaling pathway[J]. Chin J Pathophysiol, 2019, 35(2):243-247.
[5] Chen Y, Jin S, Teng X, et al. Hydrogen sulfide attenuates LPS-induced acute kidney injury by inhibiting inflammation and oxidative stress[J]. Oxid Med Cell Longev, 2018, 2018:6717212.
[6] Wang Z, Wu J, Hu Z, et al. Dexmedetomidine alleviates lipopolysaccharide-induced acute kidney injury by inhibiting p75NTR-mediated oxidative stress and apoptosis[J]. Oxid Med Cell Longev,2020, 2020(4):5454210.
[7] Wang J, Zhou L, Yin W, et al. Clinical efficacy of Danhong injection in preventing contrast-induced acute kidney injury based on propensity score matching method[J]. Zhong Nan Da Xue Xue Bao Yi Xue Ban, 2020, 45(10):1193-1198.
[8] Li Y, Wang L, Wang P, et al. Ginsenoside Rg1 rescues stress-induced depression-like behaviors via suppression of oxidative stress and neural inflammation in rats[J]. Oxid Med Cell Longev, 2020, 2020:2325391.
[9] Lin J, Huang HF, Yang SK, et al. The effect of Ginsenoside Rg1 in hepatic ischemia reperfusion (I/R) injury ameliorates ischemia-reperfusion-induced liver injury by inhibiting apoptosis[J]. Biomed Pharmacother, 2020, 129:110398.
[10] Luo M, Yan D, Sun Q, et al. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-κB/NLRP3 pathway[J]. J Cell Biochem, 2020, 121(4):2994-3004.
[11] Xiao Q, Zhang S, Ren H, et al. Ginsenoside Rg1 alleviates ANIT-induced intrahepatic cholestasis in rats via activating farnesoid X receptor and regulating transporters and metabolic enzymes[J]. Chem Biol Interact, 2020, 324(9):109062.
[12] Huang Z, Epperly M, Watkins SC, et al. Necrostatin-1 rescues mice from lethal irradiation[J]. Biochim Biophys Acta, 2016, 1862(4):850-856.
[13] Fu Y, Tang C, Cai J, et al. Rodent models of AKI-CKD transition[J]. Am J Physiol Renal Physiol, 2018, 315(4):F1098-F1106.
[14] Hesketh EE, Czopek A, Clay M, et al. Renal ischaemia reperfusion injury: a mouse model of injury and regeneration[J]. J Vis Exp, 2014, 88(6):51816-52824.
[15] Dasdelen D, Solmaz M, Menevse E, et al. Increased apoptosis, tumor necrosis factor-alpha, and DNA damage attenuated by 3',4'-dihydroxyflavonol in rats with brain ischemia-reperfusion[J]. Indian J Pharmacol, 2021, 53(1):39-49.
[16] Zhao S, Chen W, Li W, et al. LncRNA TUG1 attenuates ischaemia-reperfusion-induced apoptosis of renal tubular epithelial cells by sponging miR-144-3p via targeting Nrf2[J]. J Cell Mol Med,2021, 25(20):9767-9783.
[17] Rong Z, Huang Y, Cai H, et al. Gut microbiota disorders promote inflammation and aggravate spinal cord injury through the TLR4/MyD88 signaling pathway[J]. Front Nutr, 2021, 13(8):702659-702673.
[18] Iwawaki T. From property of IL-1beta to imaging of inflammation[J]. Nihon Rinsho Meneki Gakkai Kaishi, 2017, 40(5):329-336.
[19] 付阳, 董一飞. 白细胞介素33通过增强炎症反应加重脂多糖诱导的急性肾损伤[J]. 中国病理生理杂志, 2022, 38(8):1424-1429.
Fu Y, Dong YF. Interleukin-33 aggravates lipopolysaccharide-induced acute kidney injury in mice by enhancing inflammation[J]. Chin J Pathophysiol, 2022, 38(8):1424-1429.
[20] Chen T, Zhang X, Zhu G, et al. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-κB and AP-1 signaling pathway[J]. Medicine (Baltimore), 2020, 99(38):e22241.
[21] Skrypnyk NI, Gist KM, Okamura K, et al. IL-6-mediated hepatocyte production is the primary source of plasma and urine neutrophil gelatinase-associated lipocalin during acute kidney injury[J]. Kidney Int, 2020, 97(5):966-979.
[22] Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy[J]. J Hepatol, 2016, 64(6):1403-1415.
[23] Mlachkova A, Popova C, Doseva V. Presence of IL-8 gene polymorphism and IL-8 serum levels in patients with chronic periodontitis-literature review[J]. Folia Med (Plovdiv), 2020, 62(2):253-257.
[24] Zhu X, Liu J, Chen O, et al. Neuroprotective and anti-inflammatory effects of isoliquiritigenin in kainic acid-induced epileptic rats via the TLR4/MYD88 signaling pathway[J]. Inflammopharmacology, 2019, 27(6):1143-1153.
[25] Zhu G, Cheng Z, Huang Y, et al. MyD88 mediates colorectal cancer cell proliferation, migration and invasion via NF-κB/AP1 signaling pathway[J]. Int J Mol Med, 2020, 45(1):131-140.
[26] 陶计委, 南亚强, 周杰, 等. Exendin-4通过抑制Toll样受体4/核因子κB信号通路对帕金森病小鼠保护作用的研究[J]. 中国病理生理杂志, 2022, 38(3):502-508.
Tao JW, Nan YQ, Zhou J, et al. Protective effect of exendin-4 on Parkinson disease mice by inhibiting Toll-like receptor 4/nuclear factor-κB signaling pathway[J]. Chin J Pathophysiol, 2022, 38(3):502-508.
[27] Yan S, Fang C, Cao L, et al. Protective effect of glycyrrhizic acid on cerebral ischemia/reperfusion injury via inhibiting HMGB1-mediated TLR4/NF-κB pathway[J]. Biotechnol Appl Biochem, 2019, 66(6):1024-1030.
[28] Liang S, Lv ZT, Zhang JM, et al. Necrostatin-1 attenuates trauma-induced mouse osteoarthritis and IL-1beta induced apoptosis via HMGB1/TLR4/SDF-1 in primary mouse chondrocytes[J]. Front Pharmacol, 2018, 9(11):1378-1389.
[29] Cao L, Mu W. Necrostatin-1 and necroptosis inhibition: Pathophysiology and therapeutic implications[J]. Pharmacol Res, 2021, 163(1):105297-105313.
Mechanism of ginsenoside Rg1 regulating acute hepatic damage induced by acute kidney injury in mice through TLR4/MyD88/NF-κB p65 signaling pathway
CHI Xiaochen1, CAO Yingxin1, BAO Cuifen2△, LI Tingyu1, YAN Lijing2△
(1,,121001,;2,,121001,)
To investigate the protective effect of ginsenoside Rg1 (GRg1) on acute hepatic damage induced by acute kidney injury in mice and its regulatory mechanism.Kunming mice were randomly divided into sham group, model group, GRg1 group and necrostatin-1 (Nec-1) group, with 10 mice in each group. The acute kidney injury model was prepared, and blood was collected after 24 h. The serum creatinine (SCr), blood urea nitrogen (BUN), aspartate aminotransferase (AST), alanine aminotransferase (ALT), malondialdehyde (MDA), and superoxide dismutase (SOD) levels were measured by biochemical kits. The expression of interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor-α(TNF-α) were measured by ELISA. Histopathological changes were observed by HE staining, the levels of TLR4, MyD88 and NF-κB p65 were detected by immunohistochemistry and Western blot.Compared with sham group, the model group showed significant hepatocyte necrosis and hepatorenal function were significantly decreased. SCr, BUN, AST and ALT were significantly increased (<0.01). The content of MDA was increased and the activity of SOD was decreased dramatically (<0.01), the serum levels of IL-1β, IL-6, IL-8 and TNF-α were remarkably increased (<0.01). The expressions of TLR4, MyD88 and NF-κB p65 were significantly increased (<0.01). Compared with the model group, hepatocyte necrosis was reduced, liver and kidney functions were dramatically improved (<0.01), serum levels of SCr, BUN, AST and ALT were decreased significantly (<0.01). The content of MDA was decreased and the activity of SOD was increased obviously (<0.01). The contents of inflammatory factors IL-1β, IL-6, IL-8, TNF-α were remarkably decreased (<0.01). The expressions of TLR4, MyD88 and NF-κB p65 were significantly decreased (<0.01). There was no significant difference in the above indexes between GRg1 group and Nec-1 group.Ginsenoside Rg1 can improve liver and kidney function in mice with acute hepatic damage induced by acute kidney injury, and the mechanisms may be related to the inhibition of the TLR4/MyD88/NF-κB p65 signaling pathway.
ginsenoside Rg1; acute kidney injury; acute hepatic damage; TLR4/MyD88/NF-κB p65 signaling pathway
R363.2; R692
A
10.3969/j.issn.1000-4718.2023.02.011
1000-4718(2023)02-0287-10
2022-01-24
2022-12-16
[基金项目]国家自然科学基金资助项目(No. 81774116);辽宁省教育厅项目(No. JYTJCZR2020083)
包翠芬 Tel: 13941605408; E-mail: 736466881@qq.com; 阎丽菁 Tel: 18704160516; E-mail: 3212141299@qq.com
(责任编辑:宋延君,李淑媛)
