1-磷酸鞘氨醇通过提高心脏微血管密度缓解压力负荷诱导的小鼠心力衰竭*
2023-03-10杨星陈铿铨汪璐芸蒋建刚
杨星, 陈铿铨, 汪璐芸, 蒋建刚
·论著·
1-磷酸鞘氨醇通过提高心脏微血管密度缓解压力负荷诱导的小鼠心力衰竭*
杨星, 陈铿铨, 汪璐芸, 蒋建刚△
(华中科技大学同济医学院附属同济医院心血管内科,心血管病遗传与分子机制湖北省重点实验室,湖北 武汉 430030)
探究1-磷酸鞘氨醇(sphingosine-1-phosphate, S1P)通过调节心脏微血管密度对压力负荷诱导的小鼠心力衰竭的作用及相关机制。将8周龄的雄性C57BL/6小鼠随机分为4组:假手术(sham)组、sham+2-乙酰基-5-四羟基丁基咪唑(2-acetyl-5-tetrahydroxybutyl imidazole, THI; S1P裂解酶抑制剂)组、主动脉弓缩窄术(transverse aortic constriction, TAC)组和TAC+THI组。TAC手术后1周给予THI灌胃处理,实验终点检测各组小鼠血浆和心脏匀浆组织中S1P水平;心脏超声和Millar导管检测心功能;HE染色检测各组小鼠心脏肥大程度,Masson染色观察各组小鼠心脏间质和管周纤维化程度,CD31和麦胚凝集素免疫荧光染色观察各组小鼠心肌细胞横截面积和心脏微血管密度;RT-qPCR检测心房钠尿肽(atrial natriuretic peptide, ANP)、脑钠肽(brain natriuretic peptide, BNP)、I型胶原蛋白(collagen type I)、III型胶原蛋白(collagen type III)、CD31、血管性血友病因子(von Willebrand factor, vWF)和血管生成素1(angiopoietin 1, Ang1)的mRNA表达水平;Western blot检测各组小鼠心脏组织中血管内皮生长因子(vascular endothelial growth factor, VEGF)、VEGF受体(VEGF receptor, VEGFR)、磷酸化VEGFR、蛋白激酶B(protein kinase B, PKB/Akt)和磷酸化Akt的蛋白水平。TAC小鼠体内S1P水平明显降低,给予THI可显著升高小鼠血浆和心脏匀浆组织中S1P水平。S1P能显著改善心衰小鼠心功能,减轻心肌肥大和纤维化表型,抑制ANP、BNP、collagen type I和collagen type III的表达,上调血管内皮标志物CD31、Ang1和vWF的表达,激活VEGF-VEGFR-Akt信号通路,促进心脏微血管生成。S1P通过VEGF-VEGFR-Akt信号通路提高心衰小鼠心脏微血管密度,从而缓解压力负荷诱导的心力衰竭。
1-磷酸鞘氨醇;心力衰竭;心脏肥大;心脏微血管密度
心力衰竭是各种心血管疾病的终末期表现,由于其发病率、住院率以及死亡率居高不下,给全世界的临床和公共卫生系统带来了严重的经济负担[1],现有的治疗方法和疗效有限,新的治疗方案亟待开发。随着社会人口的老龄化,高血压、心脏瓣膜退行性变等压力超负荷是引起心肌肥大、心力衰竭的主要原因之一[2]。病理性心肌肥大的程度与心脏微血管密度密切相关[3]。在压力负荷增加的早期,主要表现为向心性心肌肥大,心脏微血管生成正常或增加,维持着肥大心肌的营养和氧耗,射血分数保留;随着压力负荷时间延长,心肌细胞凋亡、坏死,室壁厚度逐渐变薄,心脏微血管密度下降,进一步导致心肌组织持续缺氧,心肌细胞坏死、心脏纤维化、室壁僵硬、心功能下降,最终进展为射血分数下降的心力衰竭[3-5]。Sano等[6]在慢性压力负荷诱导的心力衰竭小鼠模型中发现,心力衰竭小鼠心脏中p53蛋白累积,并通过减少缺氧诱导因子1α表达抑制心脏微血管生成,因此加剧心衰进展。心脏微血管生成由多种血管生成因子调节,如血管内皮生长因子家族(vascular endothelial growth factors, VEGFs)、血管生成素1/2(angiopoietin 1/2, Ang1/2)[7]、成纤维细胞生长因子家族(fibroblast growth factors, FGFs)[8]、血小板源性生长因子家族(platelet-derived factors, PDGFs)[9]等,其中VEGFs在心脏微血管生成过程中起着主要作用[10]。上述研究表明,促进心脏微血管生成有望延缓甚至逆转慢性心力衰竭的进展[11]。
1-磷酸鞘氨醇(sphingosine-1-phosphate, S1P)是细胞膜鞘磷脂在代谢过程中产生的一种具有多种生物学活性的小分子磷脂类物质,可在胞外刺激作用下,由S1P激酶1/2(S1P kinase 1/2, SPHK1/2)催化鞘氨醇(sphingosine)磷酸化生成,主要经血管内皮细胞、红细胞和血小板合成并分泌到胞外,参与调节多种心血管系统疾病[12-14]。S1P裂解酶(S1P lyase, SPL)参与了S1P主要的降解过程,被认为是调控S1P水平的关键酶[15]。SPL在应激情况下激活,Bandhuvula等[16]发现,在缺血性心衰模型中SPL活性上调,不可逆地降解S1P,从而使心脏S1P含量减少并促进细胞凋亡,而2-乙酰基-5-四羟基丁基咪唑(2-acetyl-5-tetrahydroxybutyl imidazole, THI)作为SPL抑制剂,可显著升高小鼠体内S1P水平,明显减轻病理损伤[16-22]。THI治疗还可促进小鼠心脏骤停后的复苏和生存[23]。S1P除了显著的心肌细胞保护作用外,在调控血管生成、维持内皮稳定性及调节血管张力中亦发挥着重要作用[24],并可调节心肌梗死后修复性巨噬细胞释放VEGF,促进心肌梗死后血管生成[25]。
基于以上证据,我们提出升高S1P水平可能通过增加心肌微血管密度缓解压力超负荷诱导的心功能障碍。本研究拟通过构建压力超负荷诱导的心力衰竭模型,给予THI升高小鼠体内S1P水平,探究S1P是否促进心脏微血管形成,进而缓解心力衰竭,并对其机制进行初步探索。
材料与方法
1 实验动物
SPF级8周龄的雄性C57BL/6小鼠购自江苏集萃药康生物科技股份有限公司,饲养于华中科技大学同济医学院实验动物中心。适应性饲养1周后,采用随机数字表法将小鼠分为假手术(sham)组、主动脉弓缩窄术(transverse aortic constriction, TAC)组、sham+THI组和TAC+THI组。小鼠接受TAC和假手术(手术过程同TAC,但不进行主动脉弓缩窄)1周后,通过灌胃的方式给予THI(10 μg,每天2次),对照组给予等体积的药物溶剂(生理盐水),连续给药干预7周。
2 主要试剂
THI购自Cayman Chemical;CD31抗体(1∶150)、蛋白激酶B(protein kinase B, PKB/Akt)抗体(1∶1 000)和磷酸化Akt(phosphorylated Akt, p-Akt)抗体(1∶1 000)均购自Cell Signaling Technology;VEGF抗体(1∶1 000)购自Santa Cruz;VEGF受体(VEGF receptor, VEGFR)抗体和磷酸化VEGFR(phosphorylated VEGFR, p-VEGFR)抗体(1∶1 000)均购自Abclonal;GAPDH抗体(1∶20 000)购自Proteintech;辣根过氧化物酶(horseradish peroxidase, HRP)标记的山羊抗兔IgG和山羊抗小鼠IgG(1∶10 000)均购自Jackson ImmunoResearch;Cy3标记的山羊抗小鼠IgG(1∶100)和4′,6-二脒基-2-苯基吲哚(4′,6-diamidino-2-phenylindole, DAPI)购自Servicebio;麦胚凝集素(wheat germ agglutinin, WGA)染液(1∶500)购自Sigma;RT-qPCR试剂购自南京诺唯赞生物科技有限公司;心房钠尿肽(atrial natriuretic peptide, ANP)、脑钠肽(brain natriuretic peptide, BNP)、I型胶原蛋白(collagen type I)、III型胶原蛋白(collagen type III)、CD31、Ang1、血管性血友病因子(von Willebrand factor, vWF)和GAPDH引物均由武汉奥科生物科技技术有限公司合成,序列见表1。

表1 RT-qPCR引物
ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; Ang1: angiopoietin 1; vWF: von Willebrand factor.
3 主要方法
3.1TAC小鼠称重,按50 mg/kg浓度腹腔注射戊巴比妥钠诱导麻醉,小鼠取仰卧位固定,用异氟烷维持麻醉。使用静脉留置针外鞘管作为气管插管接呼吸机,控制潮气量2~3 mL,呼吸频率90~110 min-1。剪毛后用碘伏消毒手术区域,以左侧第2肋为中心在小鼠胸前行纵行切口剪开皮肤,钝性分离肌肉组织,断第2肋。使用开胸器撑开纵膈,分离胸腺,暴露主动脉弓。在右颈总动脉分出大约3 mm处,用7-0丝线绕在主动脉弓处,用27号钝性针头放置于丝线和主动脉弓之间垫扎,制造70%左右狭窄。确认结扎后抽出针头,逐层关闭纵膈。待小鼠自主呼吸恢复后,拔除气管插管,放入饲养笼内饲养。假手术组过程同手术组,只是不进行主动脉弓缩窄。
3.2小鼠心脏超声检测1.5%的异氟烷麻醉小鼠后,使用配有30 MHz高频探头的VisualSonic Vevo 770型超声仪获取5个以上连续心动周期M型超声影像,采集影像后使用分析软件进行分析。
3.3小鼠Millar导管检测小鼠腹腔注射戊巴比妥钠(50 mg/kg)诱导麻醉后,用异氟烷维持麻醉,固定于小动物手术台,颈部正中剪一长约2 cm的竖形切口,钝性分离各层组织,游离颈动脉;结扎颈动脉远心端,动脉夹夹闭近心端;在远心端作一斜形向心切口,将前端带有电极的P-V微导管(SPR-838, Millar Instruments)经右颈总动脉插入至主动脉,导管通过压力-容积传导系统(Millar instruments)连接PowerLab/4SP A/D转换器与MS-302多媒体生物信号采集分析系统连接,动态观察压力波形变化,稳定数分钟后记录动脉波形;将导管缓慢向左心室送入,根据监视仪上显示的波形变化,直至出现较好的压力-容积环图形,记录血流动力学指标。
3.4小鼠心脏及血浆中S1P含量检测在实验终点取小鼠血浆和心脏匀浆组织,根据小鼠S1P ELISA检测试剂盒(购自上海江莱生物科技有限公司)技术手册测定各组小鼠体内S1P含量。用缓冲液将S1P抗体稀释至1~10 mg/L,每孔加入100 µL,4 ℃过夜。次日,弃去孔内溶液,并用洗涤缓冲液洗3次,每次5 min。按1∶10的稀释比稀释小鼠血浆和心脏匀浆组织,取50 µL待测样品与标准品加入已包被的反应孔中,37 ℃孵育1 h。洗涤液洗涤3次,每次5 min。于各反应孔中,加入稀释好的酶标二抗50 µL,37 ℃孵育1 h,洗涤液洗涤3次,每次5 min。加入新鲜配置的3,3',5,5'-四甲基联苯胺(3,3',5,5'-tetramethylbenzidine, TMB)底物100 µL,37 ℃避光孵育30 min后,于各反应孔中加入终止液50 µL,15 min之内用酶标仪于450 nm检测样本和标品的吸光度,并根据标品绘制标准曲线,计算出小鼠心脏组织和血浆中S1P含量。
3.5HE染色取小鼠心脏组织,在4% 中性多聚甲醛固定24 h后,经过石蜡包埋,使用石蜡切片机进行切片,厚度约5 µm。心脏组织切片经过二甲苯脱蜡和梯度乙醇复水后,使用HE染色试剂盒进行染色。在中性树胶封片后,在显微镜下对各组小鼠心脏组织切片进行观察并拍照。
3.6Masson染色取小鼠心脏组织,用4%多聚甲醛固定24 h后,脱水、石蜡包埋,使用石蜡切片机将心脏组织切成5 µm左右的切片。心脏组织切片经二甲苯脱蜡和梯度乙醇复水后进行Masson染色。使用中性树胶封片后,在相同参数下,用光学显微镜对各组小鼠心脏组织切片进行观察并拍照。
3.7免疫荧光染色心脏组织切片经过脱蜡和梯度乙醇复水后,用柠檬酸缓冲液进行抗原修复,再用0.3%的Triton X-100通透15 min,磷酸盐缓冲液洗3次,每次5 min。用5%牛血清白蛋白在室温下封闭1~2 h后,向切片中加入抗CD31抗体,放入湿盒中4 ℃孵育过夜。次日取出切片,并于室温复温30 min,TBST洗3次,每次5 min。随后加入Cy3标记的山羊抗小鼠荧光Ⅱ抗和WGA染液,室温孵育1~2 h,TBST洗3次,每次5 min。最后DAPI复染核后,用抗荧光淬灭封片剂封片,荧光显微镜下观察并拍照。
3.8RT-qPCR用Trizol法提取各组样本心肌组织的RNA,逆转录成cDNA,按照说明书进行RT-qPCR,检测ANP、BNP、collagen type I、collagen type III、CD31、vWF和GAPDH的mRNA表达水平。RT-qPCR扩增程序:95 ℃ 30 s;95 ℃ 5 s,60 ℃ 30 s,循环40次。以GAPDH为内参照,使用2-ΔΔCt方法计算mRNA的相对表达量。
3.9Western blot预先将组织研磨仪和离心机调至4 ℃预冷,按1∶100比例于蛋白裂解液中加入100×蛋白酶抑制剂和100×磷酸酶抑制剂,混匀后置于冰上。切取10 mg心脏组织于研磨管中,加入适量裂解液和研磨珠,研磨3 min;4 ℃、12 000 r/min离心15 min,取上清并用BCA法测定蛋白浓度。加入相应体积的loading buffer后,于100 ℃水浴加热变性5 min。经10% SDS-PAGE分离蛋白后转膜,5%脱脂牛奶封闭2 h,加入相应Ⅰ抗,4 ℃摇床孵育过夜。次日取出,TBST洗膜3次,每次5 min。加入相应Ⅱ抗,室温摇床孵育1 h,TBST洗膜3次,每次5 min。ECL试剂盒显影蛋白条带,ImageJ图像分析软件测定目的蛋白和内参照蛋白的灰度值,计算两者之间的比值,并进行统计分析。
4 统计学分析
实验过程中的所有实验数据均以均数±标准差(mean±SD)的形式表示,使用GraphPad Prism 8软件进行数据分析。组间均数比较采用单因素方差分析(one-way ANOVA),进一步两两比较采用LSD-检验。以<0.05为差异有统计学意义。
结果
1 THI显著升高TAC小鼠体内S1P水平
TAC术后8周,与sham组相比,TAC组小鼠血浆和心脏中S1P水平明显下降(<0.01),给予THI干预可明显升高基线水平和TAC术后小鼠心脏和血浆中S1P的含量(<0.01),见图1。
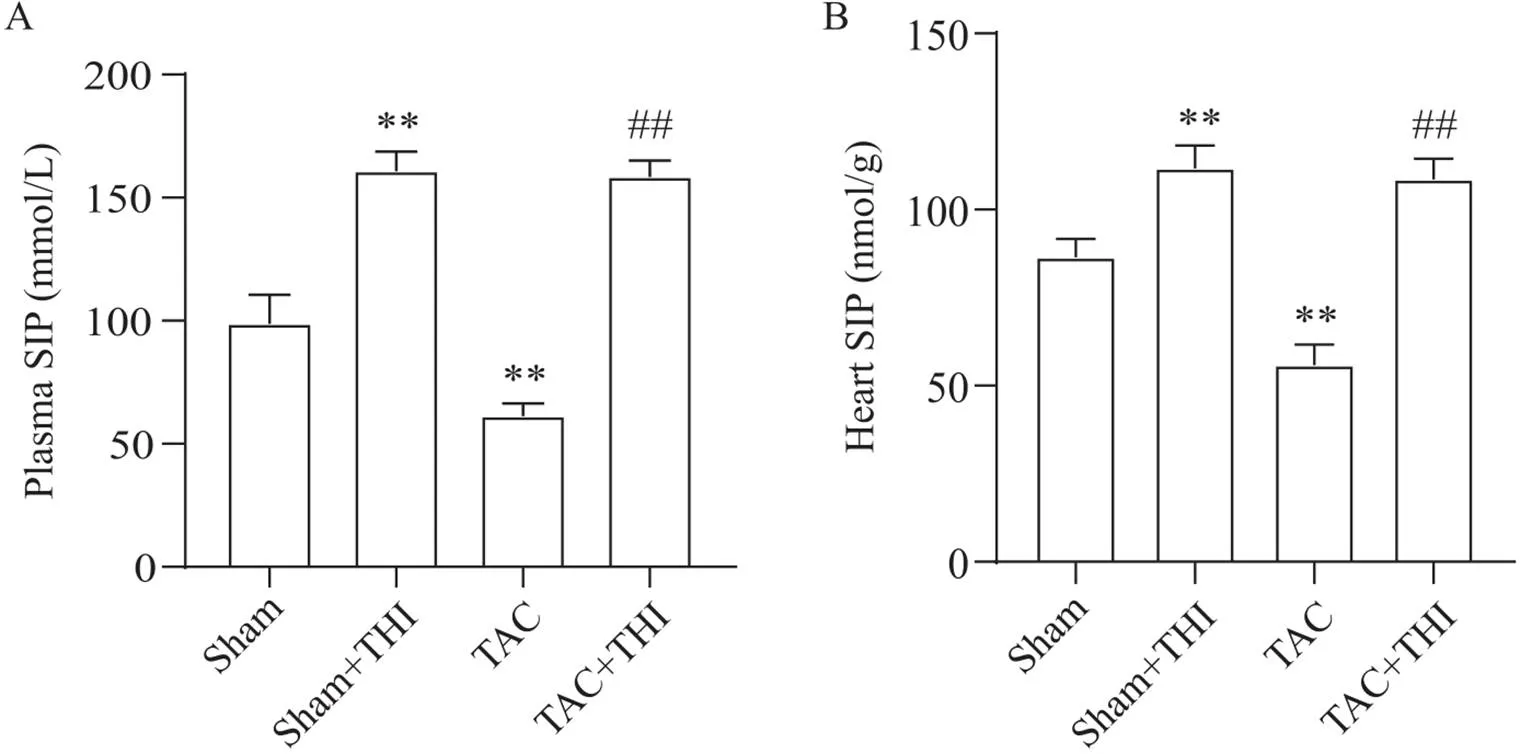
Figure 1. Effects of transverse aortic constriction (TAC) and 2-acetyl-5-tetrahydroxybutyl imidazole (THI) on sphingosine-1-phosphate (S1P) levels in mouse plasma and heart. A: eight weeks after TAC, the concentration of S1P in plasma of mice in each group was detected by ELISA; B: eight weeks after TAC, the content of S1P in the heart of mice in each group was detected by ELISA. Mean±SD. n=6. **P<0.01 vs sham group; ##P<0.01 vs TAC group.
2 S1P可改善TAC小鼠的心功能
心脏超声结果显示,TAC术后8周,小鼠心室肥大明显,舒张末期左室内径(left ventricular internal dimension at end-diastole, LVIDd)、收缩末期左室内径(left ventricular internal dimension at end-systole, LVIDs)、舒张期左室前壁厚度(left ventricular anterior wall thickness during diastole, LVAWd)和收缩期左室前壁厚度(left ventricular anterior wall thickness during systole, LVAWs)均显著增大,射血分数(ejection fraction, EF)和缩短分数(fractional shortening, FS)较sham组明显下降;给予THI能显著减轻TAC小鼠心肌肥大和心脏收缩功能障碍,见图2A。同样的,小鼠心脏Millar导管结果显示,与sham组相比,TAC组小鼠d/dmax和d/dmin显著下降;升高S1P水平后,小鼠心脏收缩功能明显改善,见图2B。
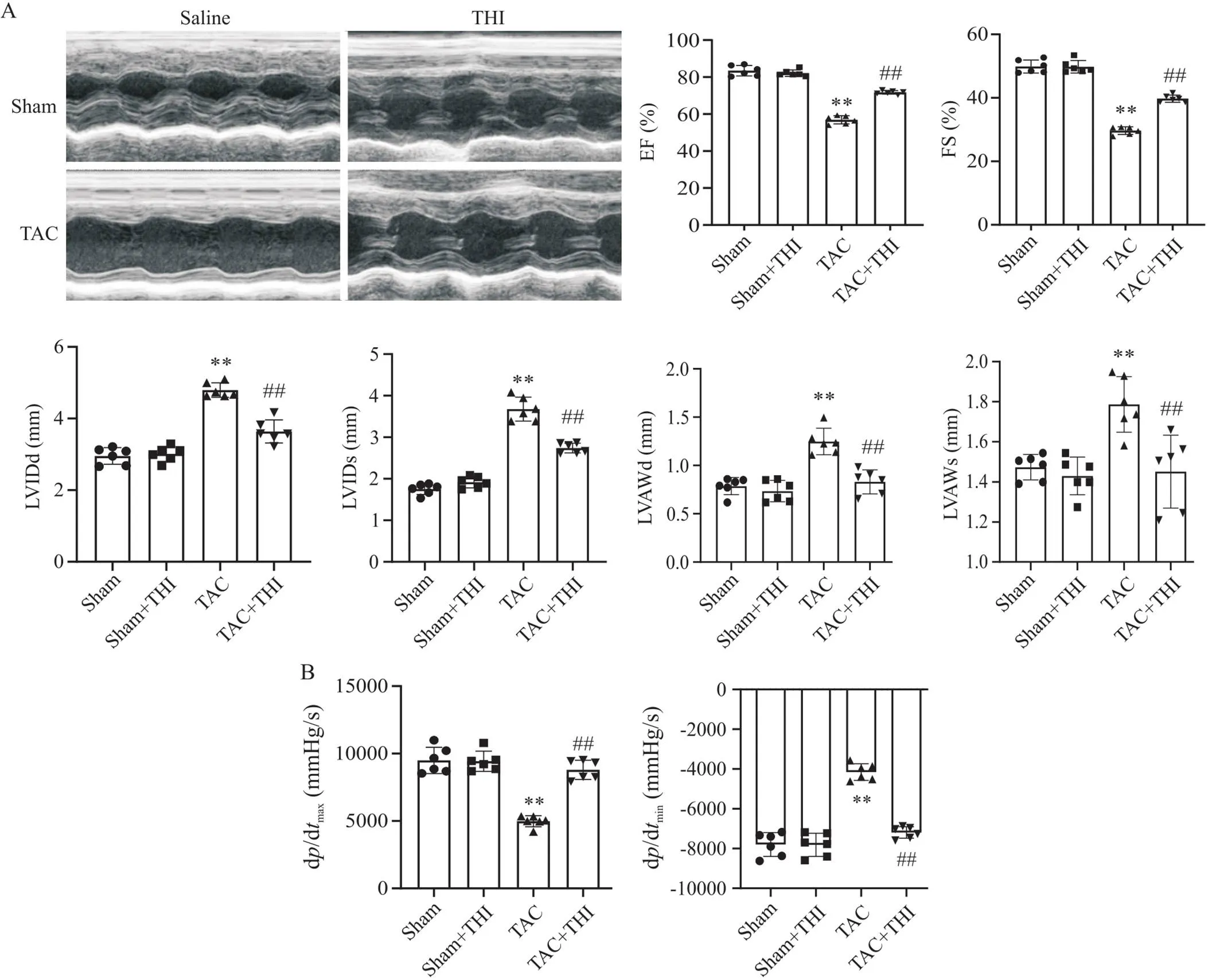
Figure 2. Sphingosine-1-phosphate (S1P) improved cardiac function of transverse aortic constriction (TAC) mice. A: representative images of echocardiography of mice in each group, and statistical charts of ejection fraction (EF), fractional shortening (FS), left ventricular internal dimension at end-diastole (LVIDd), left ventricular internal dimension at end-systole (LVIDs), left ventricular anterior wall thickness during diastole(LVAWd) and left ventricular anterior wall thickness during systole (LVAWs) of mice in each group; B: Millar catheter was used to detect dp/dtmax and dp/dtmin of mice in each group. Mean±SD. n=6. **P<0.01 vs sham group; ##P<0.01 vs TAC group.
3 S1P减轻TAC诱导的心肌肥大
在TAC术后8周,心脏大体图和HE染色显示,TAC组小鼠心脏肥大明显,心肌细胞横截面积显著增大;升高小鼠体内S1P水平可明显减轻TAC小鼠心肌肥大,见图3A。RT-qPCR检测各组小鼠ANP和BNP的mRNA表达量,结果显示,TAC组小鼠心脏ANP和BNP表达上调,而S1P可抑制TAC诱导的心肌肥大指标的表达,见图3B。

Figure 3. Sphingosine-1-phosphate (S1P) attenuated transverse aortic constriction (TAC)-induced hypertrophy. A: representative images of gross mouse heart specimens and HE staining (scale bar=50 µm), and statistical chart of the cross-sectional area of cardiomyocytes in each group (n=3); B: relative mRNA levels of cardiac hypertrophic markers, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), in mouse heart tissues of each group (n=4). Mean±SD. **P<0.01 vs sham group; #P<0.05, ##P<0.01 vs TAC group.
4 S1P可抑制TAC诱导的心肌纤维化
Masson染色显示,与sham组相比,TAC组心肌间质和管周胶原纤维沉积明显;而在给予THI干预后,可观察到TAC+THI组小鼠心肌间质和管周纤维化明显减轻,见图4A。RT-qPCR结果显示,TAC小鼠心脏collagen type I和collagen type III的mRNA表达显著增多,而升高S1P可减少TAC小鼠心脏胶原蛋白的mRNA表达,见图4B。
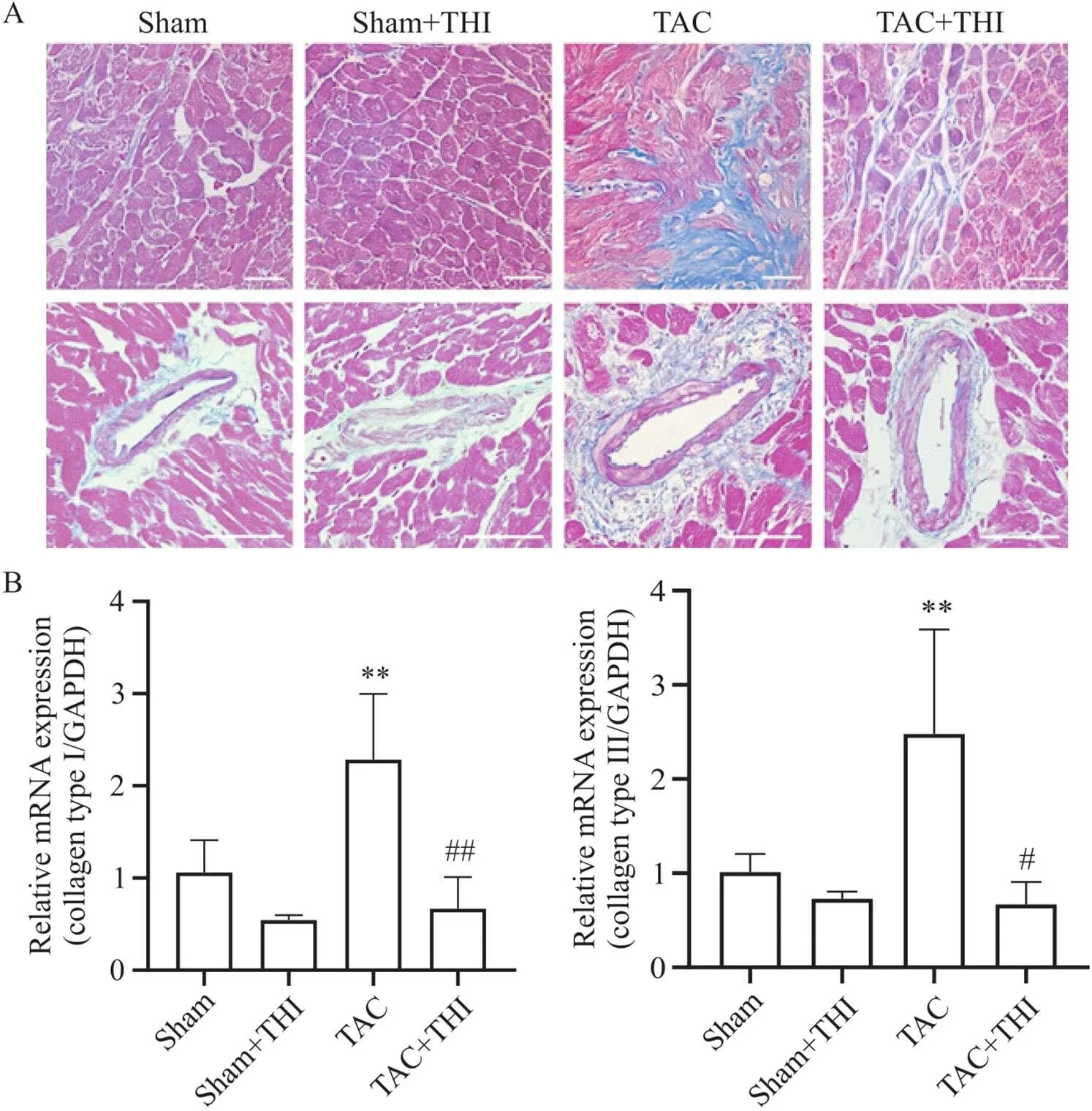
Figure 4. Sphingosine-1-phosphate (S1P) inhibited transverse aortic constriction (TAC)-induced myocardial fibrosis. A: representative Masson staining images of mouse heart tissues in each group (scale bar=50 µm); B: relative mRNA levels of fibrotic markers, collagen type I and collagen type III, in mouse heart tissues of each group. Mean±SD. n=4. **P<0.01 vs sham group; #P<0.05, ##P<0.01 vs TAC group.
5 S1P可促进TAC模型小鼠心脏微血管生成
WGA和CD31免疫荧光染色结果显示,TAC术后,小鼠心脏中微血管密度(microvascular count/area)和微血管/心肌细胞比值(microvascular count/cardiomyocytes)较sham组显著降低;而与TAC组相比,TAC+THI组小鼠心脏组织中微血管密度和微血管/心肌细胞比值显著上调,见图5A。RT-qPCR结果显示,TAC+THI组小鼠心脏血管生成标志物CD31、vWF和Ang1的mRNA表达较TAC组增多,差异有统计学意义(<0.05),见图5B。以上结果表明,升高S1P水平可增加TAC小鼠心脏微血管密度。
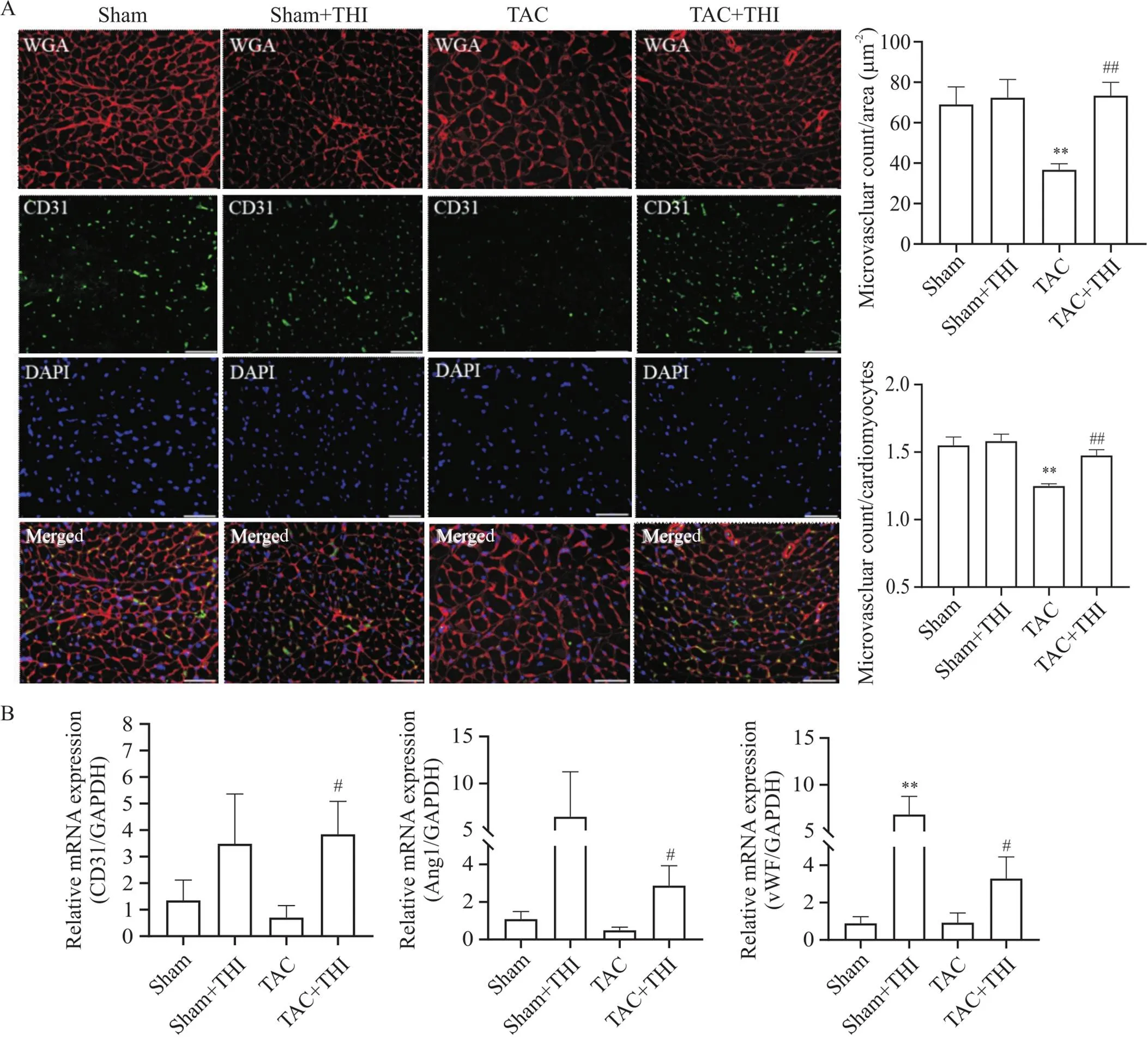
Figure 5. Sphingosine-1-phosphate (S1P) promoted cardiac micro-angiogenesis in transverse aortic constriction (TAC) mice. A: representative images of wheat germ agglutinin (WGA; red) and CD31 (green) immunofluorescence staining of mouse heart tissues (scale bar=50 µm), and statistical charts of cardiac microvascular density (microvascular count/area) and microvascular count/cardiomyocyte ratio of mice in each group; B: relative mRNA levels of vascular endothelial markers, CD31, angiopoietin 1 (Ang1) and von Willebrand factor (vWF), in mouse heart tissues of each group. Mean±SD. n=3. **P<0.01 vs sham group; #P<0.05, ##P<0.01 vs TAC group.
6 S1P调节TAC小鼠心脏中VEGF-VEGFR-Akt信号通路,促进心脏微血管生成
VEGF家族是血管生成过程中最重要的一类细胞因子之一,主要通过和其受体(VEGFR)结合,激活细胞内信号通路,在血管生成中起着主要作用[26]。因此,我们检测各组小鼠心脏组织中VEGF、VEGFR/p-VEGFR的蛋白水平,以及其下游Akt信号通路活化状态。Western blot结果显示,与TAC组相比,THI能显著上调小鼠心脏中VEGF蛋白表达和p-VEGFR水平;与sham组相比,TAC降低了心脏组织中Akt磷酸化水平,而给予THI干预后磷酸化Akt蛋白水平显著升高,见图6。

Figure 6. Sphingosine-1-phosphate (S1P) promoted cardiac micro-angiogenesis through regulating vascular endothelial growth factor (VEGF)-VEGF receptor (VEGFR)-protein kinase B (PKB/Akt) signaling pathway. The protein levels of vascular endothelial growth factor A (VEGFA), VEGFR, phosphorylated VEGFR (p-VEGFR), Akt and phosphorylated Akt (p-Akt) in mouse heart tissues of each group were detected by Western blot. Mean±SD, n=4. *P<0.05, **P<0.01 vs sham group; #P<0.05, ##P<0.01 vs TAC group.
讨论
本实验通过TAC诱导的心力衰竭小鼠模型,证实了S1P通过对心脏微血管密度的调节影响心力衰竭的发展。本研究结果表明,在TAC模型小鼠血浆和心脏组织中S1P的含量都明显下降,而通过给予THI纠正小鼠体内紊乱的S1P水平,可以明显改善TAC小鼠心功能,减轻心肌肥大和心肌纤维化。该作用可能是通过调节VEGF-VEGFR-Akt信号通路介导的心脏微血管生成,增加TAC小鼠心脏微血管密度,从而缓解TAC诱导的心力衰竭。
心脏微血管密度稀疏是心力衰竭发生发展的重要机制,且既往研究表明促进心脏微血管增生可以缓解心力衰竭[27-28]。Flanagan等[29]利用成年羊羔压力超负荷模型中证实,在病理性肥大的心脏中,毛细血管密度在由心肌肥大向心力衰竭转变的过程中降低。这一现象在临床试验中得到了进一步验证,在一项纳入110人的临床研究中,血管内超声结果发现,与无左室肥大的患者相比,伴有左室肥大的高血压患者心脏微血管密度降低,冠状动脉血流储备减少[30]。Wadowski等[4]在临床研究中亦发现,慢性心力衰竭患者心脏组织中微血管密度明显低于健康对照。在移植外周血单个核细胞(peripheral blood mononuclear cells, PB-MNC)治疗下肢缺血的临床试验中,PB-MNC促进缺血区骨骼肌释放大量促血管生成因子,促进新生血管形成,不仅缓解了患者下肢缺血症状[31-32],亦有益于心肌缺血与心功能的改善,这可能得益于大量促血管生成因子对心脏微血管的调控[11]。此外,包括补充诸如VEGF[33-34]、成纤维细胞生长因子5(fibroblast growth factor-5, FGF-5)[35]、基质细胞衍生因子1(stromal cell-derived factor-1, SDF-1)[36]、肝细胞生长因子(hepatocyte growth factor, HGF)[37]等血管生成因子蛋白及基因治疗的试验证实了促血管生成治疗在缓解心力衰竭上的良好应用前景。其中VEGFs由于其强大的促新生血管形成功能,被认为是对血管内皮细胞最有力的刺激物之一[38-39],其通过结合VEGFR(包括VEGFR1/2/3,均属于酪氨酸激酶受体家族),导致受体磷酸化激活,随后激活下游的Akt信号通路,促进内皮细胞的生长、增殖和成熟,最终促使新生血管形成[40-41]。因此,从增加心脏微血管密度为切入点的治疗方案的开发,有望为解决目前临床心力衰竭治疗效果不佳的问题提供新的思路和方法。
S1P是细胞膜鞘磷脂代谢产物,具有重要的心血管保护功能[24, 42]。S1P由鞘氨醇磷酸化产生,主要由SPL分解为磷酸乙醇胺和十六烯醛。由于S1P被SPL不可逆地降解,SPL被认为是调节S1P水平最关键的代谢酶[43]。THI作为SPL的抑制剂,在多项研究中被证明可增加体内外S1P水平,并发挥重要作用[17, 43-44]。Bandhuvula等[16]在缺血再灌注小鼠模型中表明,THI可升高心脏和血浆S1P的水平,并保护小鼠心脏免受缺血再灌注损伤。在我们之前的研究[45]以及本次实验也观察到,TAC术后,小鼠心脏及血浆中S1P水平明显降低,THI纠正了S1P的水平下降,同时缓解了压力负荷模型小鼠心力衰竭,表明S1P具有明显的心脏保护作用。之前的研究显示,S1P是血管功能的重要调节因子,参与了多种病理生理情况下的血管生成及内皮功能调节[46],且在多种疾病研究中被证实参与VEGF信号通路的调控。在风湿性关节炎中,S1P可通过c-Src/FAK信号通路阻断miR-16-5p的合成,增加成骨细胞VEGF的表达与分泌,从而促进内皮祖细胞(endothelial progenitor cells, EPCs)介导的血管生成[47]。在视网膜新生血管性疾病中,S1P-VEGF信号通路亦被证实有重要的应用价值,针对S1P介导的新生血管形成过程被越来越多的基础实验证实具有治疗靶向性[48-49]。但是,S1P是否通过调控心脏微血管密度而缓解心力衰竭尚未见报道。基于以上研究,我们猜想S1P很可能在心力衰竭心脏中微血管生成过程起着重要作用。我们构建了压力负荷诱导的心肌肥大小鼠模型,发现在TAC组小鼠心脏中微血管密度和微血管/心肌细胞比值较sham组明显降低,给予THI升高TAC小鼠体内S1P水平后,小鼠心脏微血管密度增加,且血管生成相关标志物如CD31、Ang1和vWF的mRNA水平升高,并与心功能的改善和心室重构的缓解呈现一致性。进一步的实验证实,S1P可增加TAC小鼠心脏中VEGF和p-VEGFR蛋白水平,并激活其下游的Akt信号通路,发挥促进心脏微血管生成功能,这可能与TAC+THI组小鼠心脏血管生成增加及心力衰竭缓解有关。
综上所述,我们的研究发现S1P可能通过调控VEGF-VEGFR-Akt信号通路,促进心脏微血管的生成,减轻心肌肥大和纤维化,进而缓解压力超负荷所致的心力衰竭。本研究为心力衰竭的治疗提供了新的方向和思路,为S1P及其调控物作为临床上治疗心力衰竭的药物提供了新的理论依据。
[1] Tanai E, Frantz S. Pathophysiology of heart failure[J]. Compr Physiol, 2015, 6(1):187-214.
[2] Cabassi A, Dancelli S, Pattoneri P, et al. Characterization of myocardial hypertrophy in prehypertensive spontaneously hypertensive rats: interaction between adrenergic and nitrosative pathways[J]. J Hypertens, 2007, 25(8):1719-1730.
[3] Camici PG, Tschöpe C, Di Carli MF, et al. Coronary microvascular dysfunction in hypertrophy and heart failure[J]. Cardiovasc Res, 2020, 116(4):806-816.
[4] Wadowski PP, HüLsmann M, Schörgenhofer C, et al. Sublingual functional capillary rarefaction in chronic heart failure[J]. Eur J Clin Invest, 2018, 48(2):e12869.
[5] Walsh K, Shiojima I. Cardiac growth and angiogenesis coordinated by intertissue interactions[J]. J Clin Invest, 2007, 117(11):3176-3179.
[6] Sano M, Minamino T, Toko H, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload[J]. Nature, 2007, 446(7134):444-448.
[7] Dallabrida SM, Ismail NS, Pravda EA, et al. Integrin binding angiopoietin-1 monomers reduce cardiac hypertrophy[J]. FASEB J, 2008, 22(8):3010-3023.
[8] Kardami E, Detillieux K, Ma X, et al. Fibroblast growth factor-2 and cardioprotection[J]. Heart Fail Rev, 2007, 12(3/4):267-277.
[9] Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine[J]. Genes Dev, 2008, 22(10):1276-1312.
[10] Oka T, Akazawa H, Naito AT, et al. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure[J]. Circ Res, 2014, 114(3):565-571.
[11] Oka T, Morita H, Komuro I. Novel molecular mechanisms and regeneration therapy for heart failure[J]. J Mol Cell Cardiol, 2016, 92:46-51.
[12] Venkataraman K, Lee YM, Michaud J, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate[J]. Circ Res, 2008, 102(6):669-676.
[13] Yatomi Y, Igarashi Y, Yang L, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum[J]. J Biochem, 1997, 121(5):969-973.
[14] Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy[J]. J Clin Invest, 2015, 125(4):1379-1387.
[15] Cartier A, Hla T. Sphingosine 1-phosphate: lipid signaling in pathology and therapy[J]. Science, 2019, 366(6463):eaar5551.
[16] Bandhuvula P, Honbo N, Wang GY, et al. S1P lyase: a novel therapeutic target for ischemia-reperfusion injury of the heart[J]. Am J Physiol Heart Circ Physiol, 2011, 300(5):H1753-H1761.
[17] Klyachkin YM, Nagareddy PR, Ye S, et al. Pharmacological elevation of circulating bioactive phosphosphingolipids enhances myocardial recovery after acute infarction[J]. Stem Cells Transl Med, 2015, 4(11):1333-1343.
[18] Baranowska U, Holownia A, Chabowski A, et al. Pharmacological inhibition of sphingosine-1-phosphate lyase partially reverses spatial memory impairment in streptozotocin-diabetic rats[J]. Mol Cell Neurosci, 2020 107:103526.
[19] Karuppuchamy T, Tyler CJ, Lundborg LR, et al. Sphingosine-1-phosphate lyase inhibition alters the S1P gradient and ameliorates Crohn's-like ileitis by suppressing thymocyte maturation[J]. Inflamm Bowel Dis, 2020, 26(2):216-228.
[20] Nguyen-Tran DH, Hait NC, Sperber H, et al. Molecular mechanism of sphingosine-1-phosphate action in Duchenne muscular dystrophy[J]. Dis Model Mech, 2014, 7(1):41-54.
[21] Bagdanoff JT, Donoviel MS, Nouraldeen A, et al. Inhibition of sphingosine-1-phosphate lyase for the treatment of autoimmune disorders[J]. J Med Chem, 2009, 52(13):3941-3953.
[22] Shirakabe K, Higashiyama M, Furuhashi H, et al. Amelioration of colitis through blocking lymphocytes entry to Peyer's patches by sphingosine-1-phosphate lyase inhibitor[J]. J Gastroenterol Hepatol, 2018, 33(9):1608-1616.
[23] Gorshkova IA, Wang H, Orbelyan GA, et al. Inhibition of sphingosine-1-phosphate lyase rescues sphingosine kinase-1-knockout phenotype following murine cardiac arrest[J]. Life Sci, 2013, 93(9/10/11):359-366.
[24] Jozefczuk E, Guzik TJ, Siedlinski M. Significance of sphingosine-1-phosphate in cardiovascular physiology and pathology[J]. Pharmacol Res, 2020, 156:104793.
[25] Kuang Y, Li X, Liu X, et al. Vascular endothelial S1pr1 ameliorates adverse cardiac remodeling via stimulating reparative macrophage proliferation after myocardial infarction[J]. Cardiovasc Res, 2021, 117(2):585-599.
[26] Giacca M, Zacchigna S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond[J]. Gene Ther, 2012, 19(6):622-629.
[27] Shiojima I, Sato K, Izumiya Y, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure[J]. J Clin Invest, 2005, 115(8):2108-2118.
[28] Mitsos S, Katsanos K, Koletsis E, et al. Therapeutic angiogenesis for myocardial ischemia revisited: basic biological concepts and focus on latest clinical trials[J]. Angiogenesis, 2012, 15(1):1-22.
[29] Flanagan MF, Fujii AM, Colan SD, et al. Myocardial angiogenesis and coronary perfusion in left ventricular pressure-overload hypertrophy in the young lamb. Evidence for inhibition with chronic protamine administration[J]. Circ Res, 1991, 68(5):1458-1470.
[30] Hamasaki S, Al Suwaidi J, Higano ST, et al. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy[J]. J Am Coll Cardiol, 2000, 35(6):1654-1660.
[31] Tateno K, Minamino T, Toko H, et al. Critical roles of muscle-secreted angiogenic factors in therapeutic neovascularization[J]. Circ Res, 2006, 98(9):1194-202.
[32] Moriya J, Minamino T, Tateno K, et al. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia[J]. Circ Cardiovasc Interv, 2009, 2(3):245-254.
[33] Serpi R, Tolonen AM, Huusko J, et al. Vascular endothelial growth factor-B gene transfer prevents angiotensin II-induced diastolic dysfunction via proliferation and capillary dilatation in rats[J]. Cardiovasc Res, 2011, 89(1):204-213.
[34] Huusko J, Lottonen L, Merentie M, et al. AAV9-mediated VEGF-B gene transfer improves systolic function in progressive left ventricular hypertrophy[J]. Mol Ther, 2012, 20(12):2212-2221.
[35] Suzuki G, Lee TC, Fallavollita JA, et al. Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle[J]. Circ Res, 2005, 96(7):767-775.
[36] Sundararaman S, Miller TJ, Pastore JM, et al. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure[J]. Gene Ther, 2011, 18(9):867-873.
[37] Siltanen A, Kitabayashi K, Lakkisto P, et al. hHGF overexpression in myoblast sheets enhances their angiogenic potential in rat chronic heart failure[J]. PLoS One, 2011, 6(4):e19161.
[38] Ylä-Herttuala S. Gene therapy with vascular endothelial growth factors[J]. Biochem Soc Trans, 2009, 37(Pt 6):1198-1200.
[39] 张梦泽, 李国珅, 赵欣童, 等. 血管新生的分子机制与相关疾病[J]. 中国病理生理杂志, 2016, 32(9):1718-1722, 1728.
Zhang MZ, Li GK, Zhao XT, et al. Angiogenesis: molecular mechanism and related diseases[J]. Chin J Pathophysiol, 2016, 32(9):1718-1722, 1728.
[40] Uemura A, Fruttiger M, D'Amore PA, et al. VEGFR1 signaling in retinal angiogenesis and microinflammation[J]. Prog Retin Eye Res, 2021, 84:100954.
[41] 刘继彦, 魏于全. 血管内皮生长因子受体2的结构功能与信号转导[J]. 中国病理生理杂志, 2004, 20(7):192-196.
Liu JY, Wei YQ. Structure, function and signal transduction of vascular endothelial growth factor receptor-2[J]. Chin J Pathophysiol, 2004, 20(7):192-196.
[42] Takuwa Y, Okamoto Y, Yoshioka K, et al. Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system[J]. Biochim Biophys Acta, 2008, 1781(9):483-488.
[43] Schwab SR, Pereira JP, Matloubian M, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients[J]. Science, 2005, 309(5741):1735-1739.
[44] Harris CM, Mittelstadt S, Banfor P, et al. Sphingosine-1-phosphate (S1P) lyase inhibition causes increased cardiac S1P levels and bradycardia in rats[J]. J Pharmacol Exp Ther, 2016, 359(1):151-158.
[45] Chen K, Wang Z, Liu C, et al. Sphingosine-1-phosphate attenuates endoplasmic reticulum stress-induced cardiomyocyte apoptosis through sphingosine-1-phosphate receptor 1[J]. Arch Med Res, 2022, 53(6):562-573.
[46] Mendelson K, Evans T, Hla T. Sphingosine 1-phosphate signalling[J]. Development, 2014, 141(1):5-9.
[47] Huang CC, Tseng TT, Liu SC, et al. S1P increases VEGF production in osteoblasts and facilitates endothelial progenitor cell angiogenesis by inhibiting miR-16-5p expression via the c-Src/FAK signaling pathway in rheumatoid arthritis[J]. Cells, 2021, 10(8):2168.
[48] Alshaikh RA, Ryan KB, Waeber C. Sphingosine 1-phosphate, a potential target in neovascular retinal disease[J]. Br J Ophthalmol, 2022, 106(9):1187-1195.
[49] Eresch J, Stumpf M, Koch A, et al. Sphingosine kinase 2 modulates retinal neovascularization in the mouse model of oxygen-induced retinopathy[J]. Invest Ophthalmol Vis Sci, 2018, 59(2):653-661.
Sphingosine-1-phosphate alleviates pressure overload-induced heart failure via increasing cardiac microvascular density
YANG Xing, CHEN Kengquan, WANG Luyun, JIANG Jiangang△
(,,,,,,430030,)
To investigate the effect of sphingosine-1-phosphate (S1P) on pressure overload-induced heart failure in mice, and to explore its mechanism.Eight-week-old male C57BL/6 mice were randomly divided into 4 groups: sham group, sham+2-acetyl-5-tetrahydroxybutyl imidazole (THI; an S1P lyase inhibitor) group, transverse aortic constriction (TAC) group, and TAC+THI group. The THI was administered by gavage one week after TAC surgery. The S1P level in the plasma and heart were measured by ELISA. Cardiac function of mice was detected by echocardiography and Millar catheter. Cardiac hypertrophy was observed by HE staining. Immunofluorescence staining of CD31 and wheat germ agglutinin was used to indicate the cross-sectional area of cardiomyocytes and cardiac microvascular density. Cardiac fibrosis was evaluated by Masson staining. The mRNA expression levels of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), collagen type I, collagen type III, CD31, angiopoietin 1 (Ang1) and von Willebrand factor (vWF) were detected by RT-qPCR. The protein levels of vascular endothelial growth factor (VEGF), VEGF receptor (VEGFR), phosphorylated VEGFR, protein kinase B (PKB/Akt) and phosphorylated Akt were determined by Western blot.The ELISA results showed that plasma and heart S1P levels were decresed in TAC mice, while THI increased S1P levels in plasma and heart. Treatment with THI improved cardiac function of mice with heart failure. Compared with TAC group, THI increased cardiac microvascular density, while the cross-sectional area of cardiomyocytes was reduced. Masson staining revealed that S1P attenuated myocardial collagen deposition in TAC mice. The RT-qPCR results showed that S1P inhibited the expression of ANP, BNP, collagen type I and collagen type III, but up-regulated the expression of vascular endothelial makers CD31, Ang1 and vWF. Western blot results indicated that S1P activated VEGF-VEGFR-Akt signaling pathway.The S1P increases cardiac microvascular density via activation of VEGF-VEGFR-Akt signaling pathway, thus alleviating pressure overload-induced heart failure in mice.
sphingosine-1-phosphate; heart failure; cardiac hypertrophy; cardiac microvascular density
R541; R363.2
A
10.3969/j.issn.1000-4718.2023.02.001
1000-4718(2023)02-0193-11
2022-07-25
2022-11-02
[基金项目]国家自然科学基金资助项目(No. 81873505);湖北省自然科学基金资助项目(No. 2018CFB552)
Tel: 027-83663607; E-mail: jiangjg618@126.com
(责任编辑:林白霜,罗森)
