Preparation of ammonium nitrate-based solid composite propellants supplemented with polyurethane/nitrocellulose blends binder and their thermal decomposition behavior
2022-11-28SriTouidjineMouliKrimBoulkdidDjllTrcheSmirBelkhiriAderrhmneMezrouMohmedIslmAlegAffBelkeiche
Sri Touidjine ,Mouli Krim Boulkdid ,*,Djll Trche ,Smir Belkhiri ,Aderrhmne Mezrou ,Mohmed Islm Aleg ,Aff Belkeiche
a Energetic Propulsion Laboratory,Teaching and Research Unit of Energetic Processes,Ecole Militaire Polytechnique,BP 17,Bordj-El-Bahri,16046,Algiers,Algeria
b Energetic Materials Laboratory,Teaching and Research Unit of Energetic Processes,Ecole Militaire Polytechnique,BP 17,Bordj-El-Bahri,16046,Algiers,Algeria
Keywords:Composite propellants Polyurethane Nitrocellulose Ammonium nitrate Decomposition kinetics Thermal decomposition
ABSTRACT To improve the performance of solid composite propellants(SCPs)supplemented with ammonium nitrate(AN)as an oxidizer,the incorporation of energetic ingredients such as explosives,energetic binders or catalysts is a common effective approach.For this purpose,polyurethane(PU),a typical inert binder,was mixed with nitrocellulose(NC)as an energetic polymer.Numerous composite solid propellant compositions based on AN and NC-modified polyurethane binder with different NC ratios were prepared.The prepared formulations were characterized using Fourier transform infrared spectroscopy(FTIR),RAMAN spectroscopy,X-ray diffraction(XRD),electron densimetry,thermogravimetric(TG)analysis,and differential scanning calorimetry(DSC).A kinetic study was then performed using the iterative Kissinger-Akahira-Sunose(It-KAS),Flynn-Wall-Ozawa(It-FWO),and non-linear Vyazovkin integral with compensation effect(VYA/CE)methods.The theoretical performances,such as theoretical specific impulse,adiabatic flame temperature,and ideal exhaust gaseous species,were also determined using the NASA Lewis Code,Chemical Equilibrium with Application(CEA).Spectroscopic examinations revealed the existence of NC and full polymerization of PU in the prepared propellants.According to density tests,the density of the propellant increases as the nitrocellulose component increases.According to the thermal analysis and kinetics study,the increase in NC content catalyzed the thermal decomposition of the AN-based composite solid propellants.Based on the theoretical study,increasing the amount of NC in the propellant increased the specific impulse and,as a result,the overall performance.
1.Introduction
Nowadays,the development of environmentally friendly solid propellants has become a need that has generated immense interest in ammonium nitrate(AN)-based propellants[1,2].A solid composite propellant is composed of a fuel and an oxidizer bound together by a matrix of a synthetic or plastic binder.This category of propellants is considerably employed to produce propulsive energy for diverse applications in rockets,satellites and cruise missiles.
Ammonium nitrate(AN)has several uses in the field of energetic materials.Indeed,it is the main component of several explosives,including Ammonals,Ammonites,Dynamites and ANFO[3].It also plays the role of oxidizer in solid composite propellants(SCPs)[4-12].AN has different advantages over other oxidizers,such as,availability,low cost,low hazard,strong gas production,eco-friendly combustion products,and comparatively low sensitivity.Nevertheless,AN possesses several disadvantages as an oxidizer like room temperature crystallographic phase transition,hygroscopicity,and low burning rate.
Several investigations have been accomplished to ameliorate the burning properties of AN-based propellants,including the utilization of catalysts[13-15],the introduction of metals[16-18],and the utilization of energetic binders[19].The use of an energetic binder is an efficient method with respect to the enhancement of the performance of AN-based propellants.Nonetheless,the synthesis procedures of energetic binders are complex and expensive,and consequently,it is complicated to produce these binders industrially.Thus,the development and the assessment of new systems is crucial.
Polyurethane(PU)elastomers are largely used in the field of high-energy composites such as SCPs and high-energy polymerbonded explosives(PBXs)due to their distinguished characteristics[20-23].Conventional PU binders are mostly non-energetic materials,and consequently reduce the energy performance significantly.Nitrocellulose(NC)is an energetic polymer widely used as an ingredient in propellants,explosives,fireworks,and gas generators[24,25],which may be introduced in PU-based compositions to overcome their performance drawback.Therefore,it could be an efficient binder to improve the burning properties of composite propellants.Additionally,during these last two decades,different research teams[26-31]mixed the polyurethane with NC in order to improve the mechanical and energetic properties.Subsequently,it is predicted that a PU/NC blend could be a promising binder component for increasing the performance of AN-based composite propellants.To the best of our knowledge,the preparation,characterization and thermal decomposition kinetics investigation of AN-based SCPs have never been accomplished.
Therefore,in the present work,we prepared and studied a series of AN-based SCPs supplemented with PU/NC.The physicochemical and thermal properties of the obtained SCPs were investigated.Additionally,based on NASA Lewis Code,Chemical Equilibrium with Application(CEA)[32,33],comparative examination of the theoretical specific impulse,of the adiabatic flame temperature,as well as of the ideal exhaust gaseous species has been done.
2.Experimental and methods
2.1.Preparation of samples
This part of the experimental work consists in developing different formulations of composite solid propellant with a total mass of 7.5 g,containing ammonium nitrate,aluminum(Al)and PU,as well as PU mixed with nitrocellulose.
The size distributions of the AN and Al powders used in the SCPs formulations were determined by the CILAS 1190 laser particle size analyzer.The average diameters of AN and Al are,respectively,equal to 160 and 28 μm.
In order to develop the reference composite propellant(SCPREF)based on the PU binder,several steps were followed in a careful manner,namely,the preparation of the binder,preparation of the solid filler,mixing of the ingredients and the molding followed by the curing process.The PU binder is composed of several ingredients,the resin,the crosslinking agent,the plasticizer,the surfactant and the chain extender.The resin used in this study is a hydroxyl terminated polyester prepolymer(HTPS),the crosslinking agent is isophorone diisocyanate(IPDI),the plasticizer is desavin,the surfactant is silicon oil and the chain extender is glycerin.An amount of the plasticizer was added to the HTPS resin to reduce its viscosity,followed by the addition of the surfactant(silicon oil),the chain extender(glycerin)and the isophorone diisocyanate(IPDI).These components are mixed at a temperature of(60°C-80°C)until a homogeneous mixture is obtained.The mixing operation is an essential step in the manufacture of composite propellants.It must be long enough to create a homogeneous paste ready for pouring.This operation was carried out at a temperature of(60°C-80°C)by mixing the prepared binder with the solid filler,which is added in small quantities in a Teflon beaker until a homogeneous mixture is obtained(a duration of 40 min is necessary).The propellant pastes were then casted under vacuum in a Crawford mold.Subsequently,the mold was left at room temperature for 18 h and then placed in an oven at 60°C for 2 days.The purpose of this step is to accelerate the reaction of the crosslinking of the binder.The cooling of the propellant was carried out gradually to avoid thermal shock as shown in Fig.S1(Supporting Information).Table 1 summarizes the mass fractions of the components used for the preparation of SCP-REF.
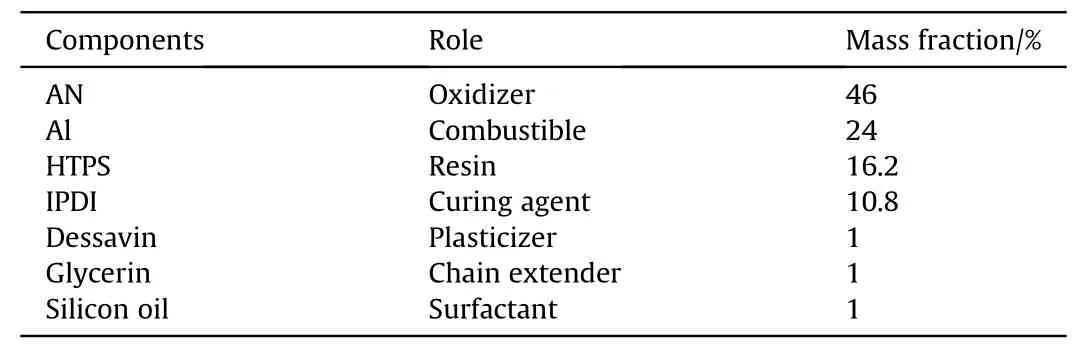
Table 1The mass fractions of the components used for the preparation of SCP-REF.
To obtain the solid composite propellant formulations based on the polyurethane mixed with the NC(SCP-NCxx,where the symbol“xx”corresponds to the percentage of NC in the formulations),we followed several steps,namely,the preparation of the modified binder with the NC,preparation of the solid filler,mixing of the ingredients and the molding followed by the curing.It is worthy to note that the only difference with the preparation method of SCPREF is the step of preparing the binder,this is why in the following we will only detail this step.For the preparation of PU-NC mixtures,NC fibers without stabilizers(having a nitrogen content equal to 13.0%)were solubilized in acetone with stirring at a speed of 750 rpm in a Teflon beaker.Then the PU binder ingredients were added in the following order(prepolymer,plasticizer,chain extender,surfactant,diisocyanate)to the solubilized NC.The mixture was subsequently stirred(750 rpm)for 10 min to ensure homogenization as shown in Fig.S2(Supporting Information).The mass fractions of the NC as well as those of the ingredients of the PU binder are given in Table 2.It is noted that for the chain extender,surfactant and plasticizer,we used a mass fraction of 1% for each component.
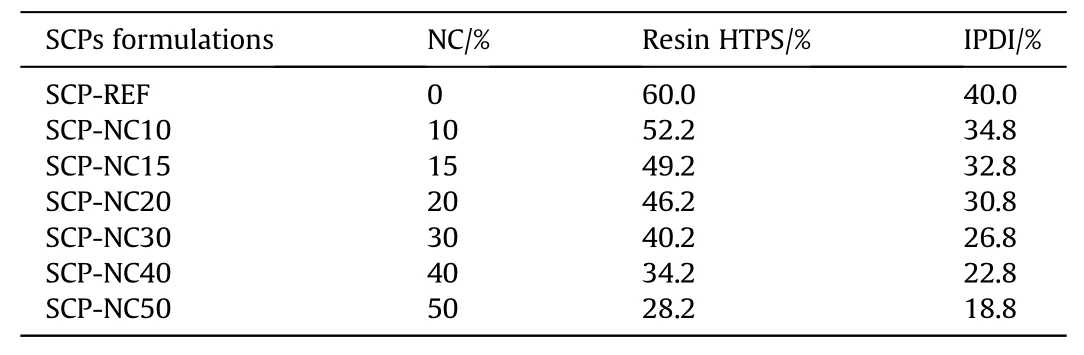
Table 2The mass fractions of the NC as well as those of the ingredients of the PU binder for different prepared SCPs formulations.
2.2.Characterization of the samples
The prepared formulations were characterized through,Fourier transform infrared(FTIR)spectroscopy,X-ray diffraction(XRD),density measurements and differential scanning calorimetry(DSC).
The FTIR spectra were obtained using a Fourier transform spectrometer of the“PerkinElmer”type Spectrum One model.The spectra were obtained by preparing pellets containing a mixture of powders of dried potassium bromide and 1% w/w of the studiedsystems.Noting that to obtain the samples in powder form,they were ground.The spectra were collected in the transmission mode with a resolution of 4 cm-1,in the range of 4000-600 cm-1,where the main characteristic peaks of polyurethane and nitrocellulose are found.An accumulation of 32 scans,to achieve an acceptable signal-to-noise ratio,was taken within this interval.
The RAMAN measurements were carried out using a RAMAN spectrometer(Thermo Fisher Scientific)equipped with a laser source with an excitation wavelength of 532 nm.The samples were analyzed in the frequency range of 500-3500 cm-1.
The XRD diffractograms were acquired employing a PANalyticalX"pert PRO MultiPurpose diffractometer with Cu Kα radiation.An X"celerator detector was exploited to obtain data over an angular range of 5-80°/2θ with a step size of 0.017°/2θ and a count time of 50.1650 s at each step.The voltage was selected to be 45 kV with a current of 40 mA.The specimens were charged on aluminium sample containers by employing a side loading tool.
The densities of all samples were also determined employing an electronic densimeter.The densimeter apparatus comprises a computer command unit,a bottle of helium gas and a densimeter type gas pycnometer Accupyc 1340 II.Three measurements were performed for each sample.
The thermogravimetric(TG)analysis was carried out by thermogravimetric analyzer model Q500.Each sample mass(about 5 mg)was heated under nitrogen atmosphere from 50 to 350°C at 10°C/min.
The differential scanning calorimeter measurements were accomplished by employing a DSC 8000(PerkinElmer),within a temperature domain of 50-300°C,at distinct heating rates(5,10,15,and 20°C/min)under nitrogen atmosphere(20 cm3/min).Small mass of samples,around 1.5 mg with less than 10% variation between sample masses,was charged in closed aluminium pans and employed for each analysis.The calibration of the DSC has been made following the procedure described in the technical guide provided by the manufacturer.We followed the different calibration phases,namely the calibration of the baseline,the temperature and the enthalpy of reaction.To evaluate the precision and accuracy of the instrument,analysis with high purity indium and zinc were performed.The uncertainty of the measured temperatures were less than 0.2°C.Moreover,data acquisition and processing were completed by Pyris software version 10.1.
2.3.Theoretical performance analyses
To evaluate the performance of the prepared composite solid propellants,the NASA-CEA chemical equilibrium software was employed,fixing the chamber pressure value at 7 MPa,the ideal expansion to 0.1 MPa,and the supersonic section ratioAe/Atat 10,whereas the initial temperature is 298 K.The main assumption to calculate the theoretical performances are detailed elsewhere[34].The analyzed parameters are as follows:the specific impulse(Isp),the adiabatic flame temperature(Tf),as well as the decomposition product mole fractions.The physical and thermodynamic data of distinct components employed are showed in Table 3.
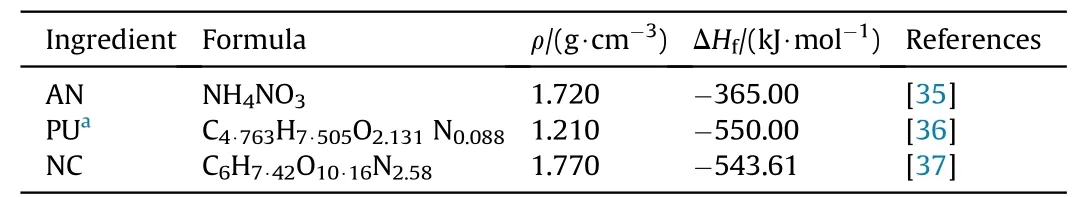
Table 3Physical and thermodynamic data of propellant components utilized for thermochemical evaluation.
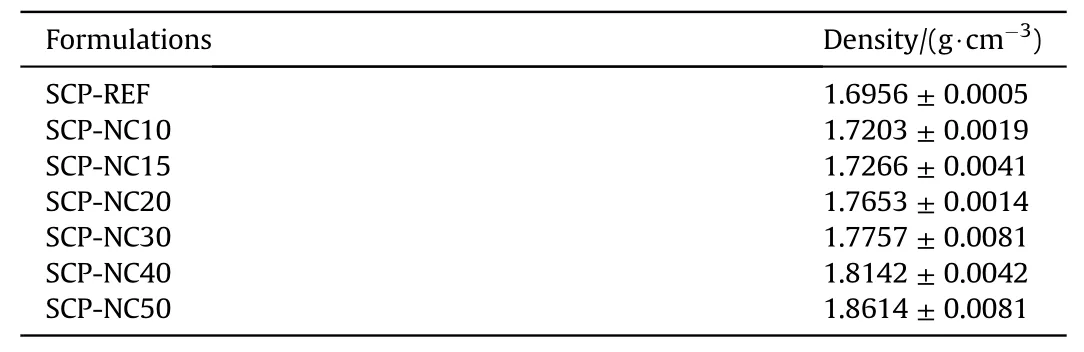
Table 4The densities of the composite propellants produced with the pure polyurethane binder and those with mixtures of PU-NC binders.
3.Results and discussion
3.1.Characterization
3.1.1.FTIR analysis
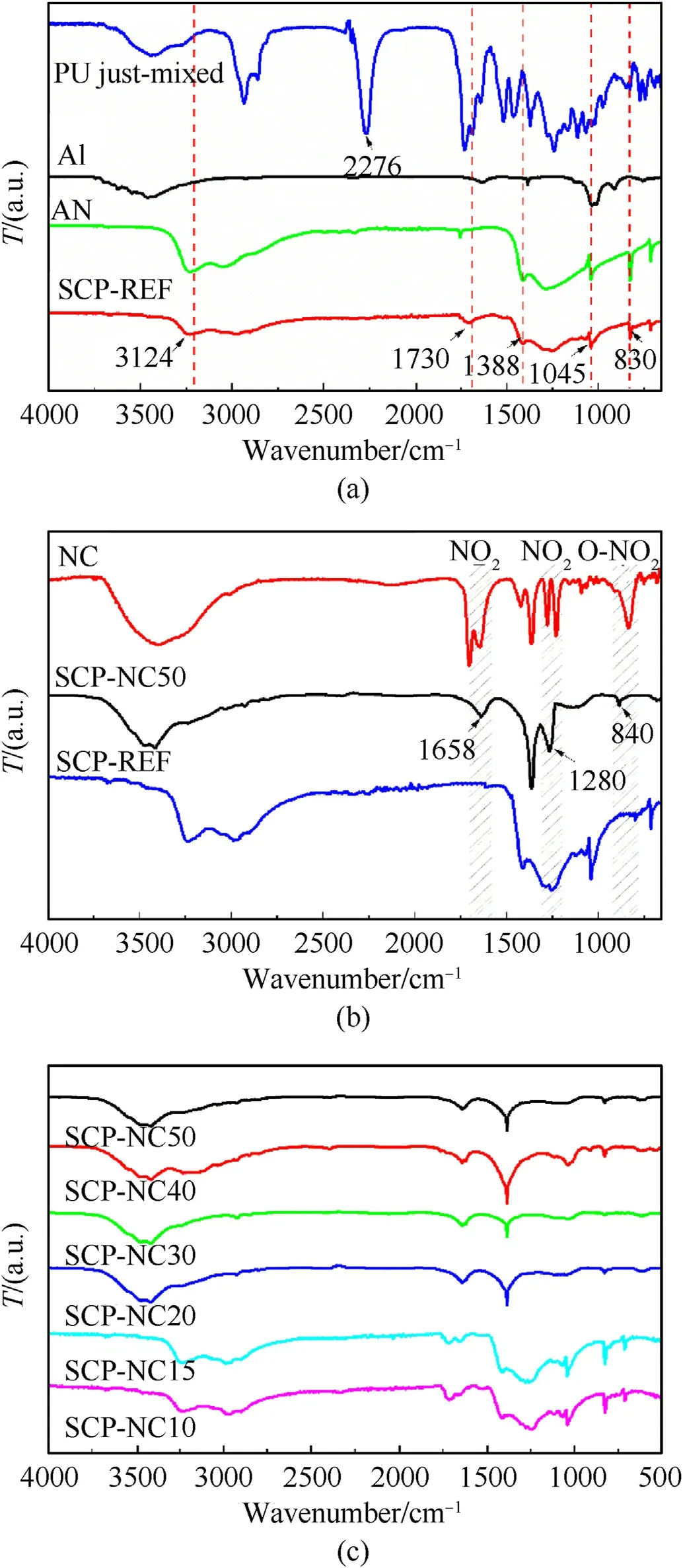
Fig.1.FTIR spectra of nitrocellulose,polyurethane,aluminum,pure ammonium nitrate and composite propellants.
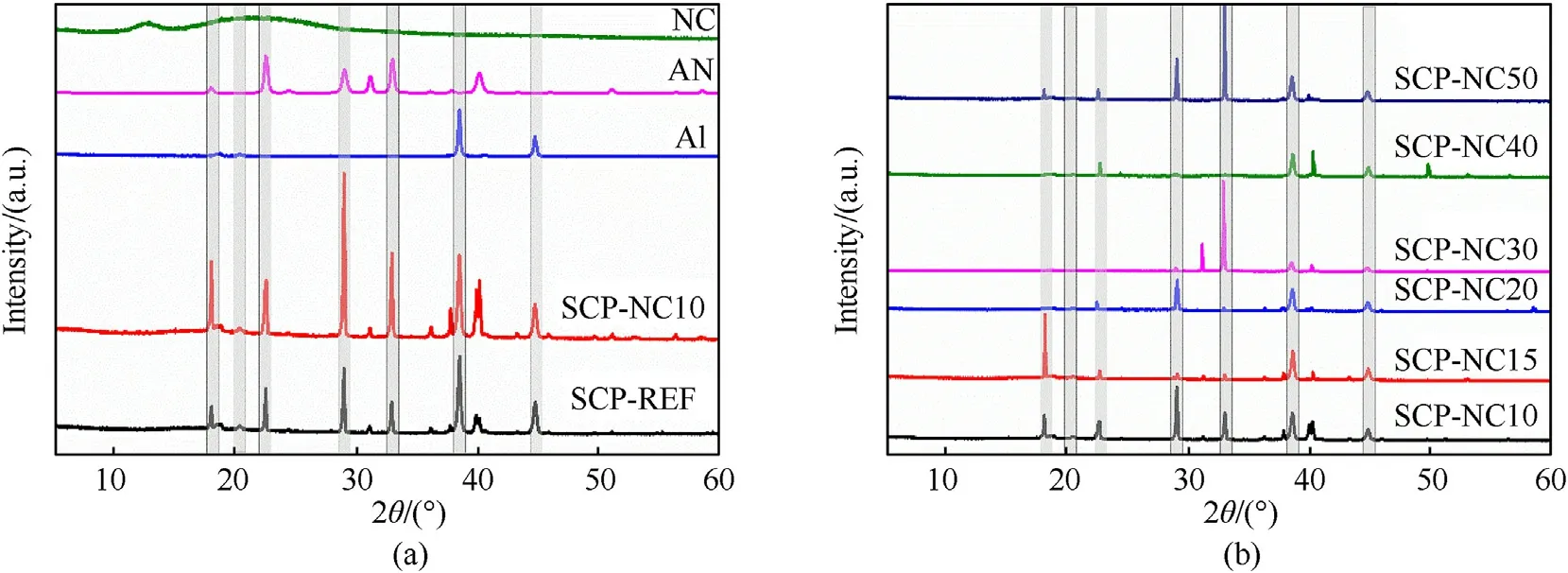
Fig.2.X-ray diffractograms of(a)nitrocellulose,aluminum,pure ammonium nitrate and(b)composite propellants.
To identify the chemical groups of the different constituents of the propellants produced,we performed FTIR analysis for the different samples.Figs.1-3 show,respectively,the results obtained by FTIR of nitrocellulose,polyurethane,aluminum and pure ammonium nitrate as well as those of composite propellants(SPCREF,SPC-NC10,SPC-NC15,SPC-NC20,SPC-NC30,SPC-NC40 and SPC-NC50).All the characteristic bands are found and correspond to what available in the literature[35-38].The spectra of the elaborate composite propellants show the appearance of the characteristic urethane peak at 1730 cm-1and the absence of the isocyanate peak at about 2276 cm-1,which confirms the complete crosslinking of the binder,generated by the polymerization reaction between the IPDI and the HTPS prepolymer.From the superposition of the spectra,we can clearly verify that the characteristic bands of AN,pure Al are all found in the spectra of the produced propellants.Obviously,the characteristic peak at 1388 cm-1corresponding to the asymmetric elongation vibration of NH4+and NO3-,the peak at 830 cm-1of the out-of-plane deformation of NO3and the peak at 3124 cm-1are assigned to the symmetrical elongation of NH4+and the vibration of Al a 1045 cm-1.Moreover,from these results,we note that the characteristic bands of the characteristic groups of the NC such as the asymmetric and symmetrical elongation vibrations of NO2,which appeared,respectively,at 1280 cm-1and 1680 cm-1,and the vibration corresponding to the flexion of the O-NO2group at 824 cm-1,can be easily seen in the SPC-NCxx,without appearing in the PPC-REFs.These results confirm the presence of NC in the propellants produced with a mixture of PU/NC binders.
3.1.2.XRD analysis
XRD analysis is used to determine the crystallographic structure of samples of nitrocellulose,ammonium nitrate,aluminum,the reference propellant formulation(SCP-REF)and other formulations of propellant based on the binders composed of the mixture of PU/NC.Thus,an identification of the effect of the interaction during mixing by superimposing the diffractograms is carried out.Fig.2 represents the diffractograms obtained by the XRD analysis for the different samples:SCP-REF,SCP-NC10,SCP-NC15,SCP-NC20,SCP-NC30,SCP-NC40 and SCP-NC50,as well as that of pure NC,AN and Al.The characteristic peaks of AN as oxidizer at around of 53.3°,24.9°,23.8°and 19.5°are all preserved in the diffractograms of the produced composite propellant formulations.The obtained results show that the SCP-NCxx formulations display a characteristic peak at 19.0°,which is attributed to the amorphous region of cellulosic chain[39],indicating the presence of nitrocellulose in the propellants supplemented with a mixture of PU/NC binders.Furthermore,all the characteristic peaks of aluminum at 38.4°and 44.8°are found in the XRD patterns of composite propellants.
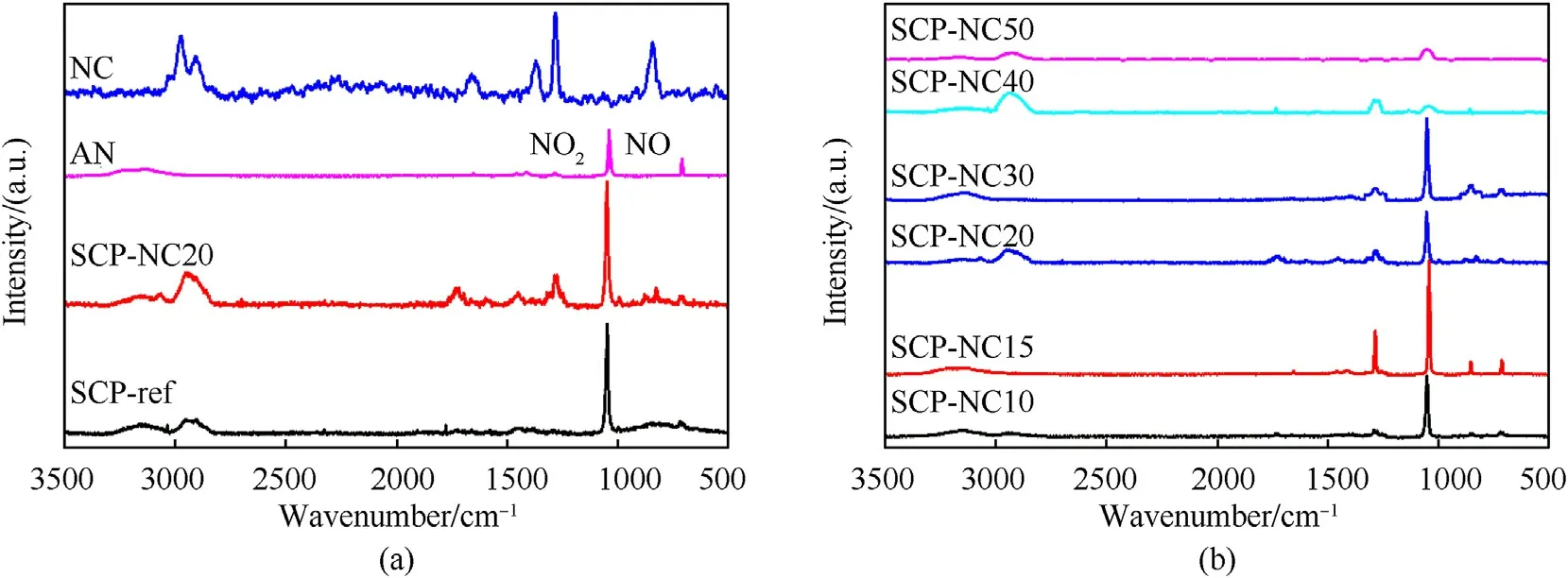
Fig.3.RAMAN spectra of(a)nitrocellulose,pure ammonium nitrate and(b)composite propellants.

Fig.4.TG/DTG curves of the produced composite propellants:(a)SCP-REF;(b)SCPNC30 and(c)SCP-NC50 at fixed heating rate(β=10 °C/min).
3.1.3.Raman analysis

Fig.5.DSC thermograms of the produced composite propellants:(a)SCP-REF;(b)SCPNC30 and(c)SCP-NC50 at different heating rates.
Raman spectroscopy,which is a complementary technique to infrared spectroscopy,also measures vibrational energy levels.The characterizations by RAMAN spectroscopy of the reference formulation(SCP-REF)as well as the other developed formulations(SCP-NC10,SCP-NC15,SCP-NC20 and SCP-NC30)were carried out in order to highlight the different links that exist and to confirm the structure of the chemical mixture obtained with nitrocellulose and ammonium nitrate.The results obtained from this analysis for the various composite propellants produced,nitrocellulose and ammonium nitrate are shown in Fig.3.The absorption bands indicative of the allocation of the functional groups of the NC,the AN and the composite propellants produced,are defined from previous studies[38,40-43].From the results obtained by RAMAN,we notice that the characteristic bands of polyurethane,AN are all found.As regards the formulations of propellants based on binders prepared on the basis of admixture PU/NC(SCP-NC10,SCP-NC15,SCP-NC20 and SCP-NC30),the presence of peaks characteristic of NC is noted.In addition,the 3100 cm-1absorption band is due to the elongation vibrations of the NH group.The band around 2933 cm-1is attributed to the CH group(Vibration of elongation of the C-H group).The absorption band at 1732 cm-1is due to a stretching vibration of the carbonyl(amide band I)of the urethane bonds.The symmetrical elongation vibrations of the NO2group are attributed to the 1285 cm-1band.The absorption band at 853 cm-1is assigned to the symmetrical elongation vibrations of the C-O-C ester group.The peaks at 1043 cm-1and 714 cm-1are,respectively,related respectively to the elongation of the N-O group and to the strain vibration of the N-H group,which are present in the AN.The results obtained by RAMAN are in agreement with those derived from the FTIR.Indeed,the presence of urethane functions confirms the complete polymerization of PU in the prepared propellant formulations.In addition,they contain nitro groups due to nitrocellulose and AN.
3.1.4.Density analysis
The densities of the composite propellants produced with the pure polyurethane binder as well as those with mixtures of PU-NC binders were measured.The values obtained are presented in Table 4.The results showed that as the content of the NC increases,the density of the propellant increases;this trend can be explained by the higher density of nitrocellulose compared to the substituted polyurethane,revealing the positive effect of NC on the overall density of propellant since it,closely affect the find performance.
3.1.5.TG analysis
Fig.4 shows TG/DTG curves of SCP-REF,SCP-NC30 and SCP-NC50 samples at heating rate of 10°C/min.It can be observed that the introduction of NC in the formulation catalyze the thermal decomposition of the solid composite propellant.In fact,according to DTG curves,it is obvious that the decomposition of SCP-NCxx took place at a lower temperature than that of SCP-REF.This may be due to the effect of the exothermic decomposition of the nitrocellulose,which could promote the decomposition of the propellant.
3.1.6.DSC analysis
In order to study the thermal properties of the produced propellants,we used the DSC analysis.The obtained results after performing the tests at different heating rates(β=5,10,15 and 20°C/min)for the several formulations which are SCP-REF,SCP-NC30 and SCP-NC50,are illustrated in Fig.5.According to the obtained thermograms,for SCP-REF,we note the appearance of a single exothermic peak around 255°C,which corresponds to the decomposition of the propellant.For the SCP-NC30 and SCP-NC50 formulations,the appearance of two overlapping exothermic peaks is observed;the first peak corresponds to the decomposition of the NC,while the second peak is related to the main decomposition of the propellant.The superposition of the thermograms allows to notice a shift towards low temperatures with the increase in the percentage of the NC in the propellant binder.Therefore,the higher amount of NC promotes the decomposition of the propellant.The early decomposition of SCP-NC30 and SCP-NC50 samples may be due to the effect of exothermic decomposition of nitrocellulose,which could promote the decomposition process of the propellant.It is also important to mention that some endothermic peaks appeared as well.They,correspond to the solid-solid transitions of AN as well as its melting.
Table S1(Supporting Information)presents a comparative survey of the onset and peak decomposition temperatures.Compared to the reference propellant formulation(SCP-REF),it can be seen that the prepared PU/NC binders promoted effectively the thermal decomposition of composite solid propellant with lower decomposition temperature.Additionally,the energetic performance of the SCPs have been enhanced.Indeed,Fig.S3(Supporting Information),which exhibits the heat release at different heating rates,revealed that as the content of the NC increases the energy release increases.
3.2.Kinetic parameters
Different models of thermal kinetic analysis are available to assess the kinetic triplet namely,activation energy,pre-exponential factor and reaction model.In the framework of this study,we applied the iterative methods of Kissinger-Akahira-Sunose(It-KAS),Flynn-Wall-Ozawa(It-FWO)and the nonlinear Vyazovkin integral with compensation effect(VYA/CE),using the results provided by DSC to investigate the decomposition kinetics of the prepared composite propellants based on PU and the mixture of PU-NC binders.More details regarding the kinetic approaches employed can be found in a previous study[39,45].In addition,all the calculations,which will be mentioned hereafter,were carried out using a kinetic calculation interface compiled under the MATLAB programming software.It is worthy to mention that to well understand the effect of the variation of the NC content in the binder employed for the different propellant formulations,we have selected three formulations that contain a higher and medium content,in addition to the benchmark based propellant.

Fig.6.Kinetic parameters and their errors bars as function of conversion rate(α)for the SCP-REF sample determined by It-FWO and It-KAS methods.
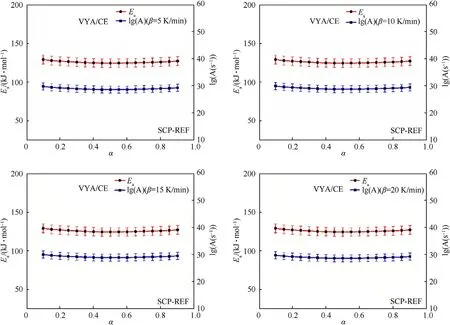
Fig.7.Kinetic parameters and their errors bars as function of conversion rate(α)for the SCP-REF sample determined by Vyazovkin"s method.
3.2.1.SCP-REF
Figs.6 and 7 give the variation of the kinetic parameters as a function of the conversion rate(α)for the SCP-REF.
3.2.2.SCP-NCxx
In the case of SCPs prepared on the basis of PU/NC mixtures,two thermal decomposition peaks were observed(Fig.5).Consequently,the determination of the kinetic parameters was carried out separately for each peak.For this purpose,Fig.8 shows an example of the deconvolution of the decomposition peaks of the SCP-NC30 by the Gaussian method,taking the correlation coefficientR2>0.98 for all the peaks.The same method was followed for the sample SCP-NC50.
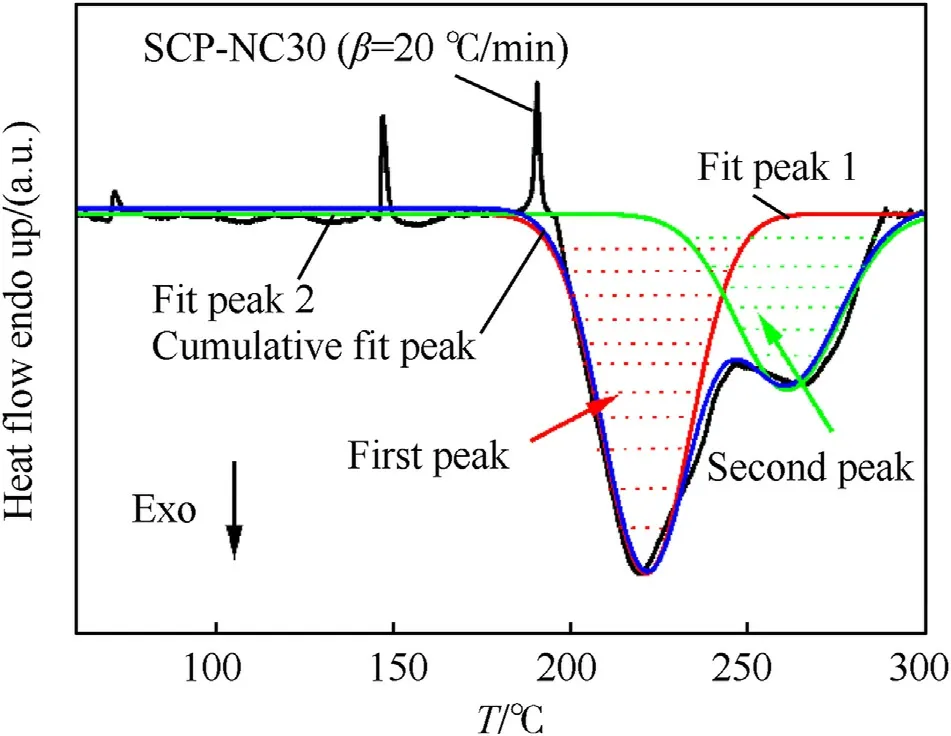
Fig.8.Deconvolution of the decomposition peak of the SCP-NC30 at heating rate(β=20 °C/min).
3.2.2.1.First peak of decomposition.Figs.9 and 10 show the variation of kinetic parameters as a function of conversion rate α for samples SCP-NC30 and SCP-NC50.
3.2.2.2.Second peak of decomposition.Figs.11 and 12 exhibit the change of the kinetic properties as a function of the conversion rate α corresponding to the second decomposition peak of the propellants SCP-NC30 and SCP-NC50.
3.2.3.Discussions
Tables S2,S3,S4,S5,S6 and S7(Supporting Information)summarize all the results obtained for the propellants SCP-REF,SCPNC30 and SCP-NC50,calculated by the three isoconversional methods,namely,It-KAS,It-FWO and Vyazovkin/CE.

Table 5CEA calculated values of Isp,Tf and Mm of the composite propellants produced with the pure polyurethane binder and those with mixtures of PU-NC binders.
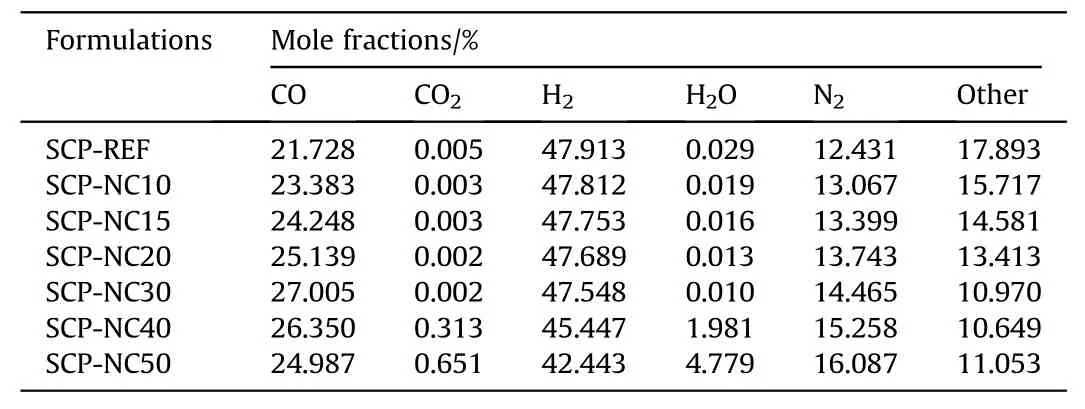
Table 6The calculated mole fractions of combustion products for propellants produced with the pure polyurethane binder and those with mixtures of PU-NC binders.
The comparison between the reference propellant and the propellants based on PU/NC binders,reveals that the mean Ea of the SCP-REF,which is equals to 125 kJ/mol,is lower than that of SCPNCxx.Indeed,the activation energy decreases to 55 kJ/mol for SCP-NC30 and to 41 kJ/mol for SCP-NC50.It can be therefore concluded that the addition of NC has a catalytic effect on the decomposition of the propellant,caused mainly by the earliest decomposition of NC.
The evaluation of the g(α)decomposition model showed that the formulations SCP-REF,SCP-NC30 and SCP-NC50 decompose according to similar mechanisms for the three calculation methods.
The values of Ea and Log(A)vary in the same way.In addition,the results of the activation energies are almost similar regardless of the employed model,which shows that the adopted approach is correct,with a slight difference due to the nature of the used approximations.

Fig.9.Kinetic parameters and their errors bars as function of conversion rate(α)for samples SCP-NC30 and SCP-NC50 determined by It-FWO and It-KAS methods(First peak).
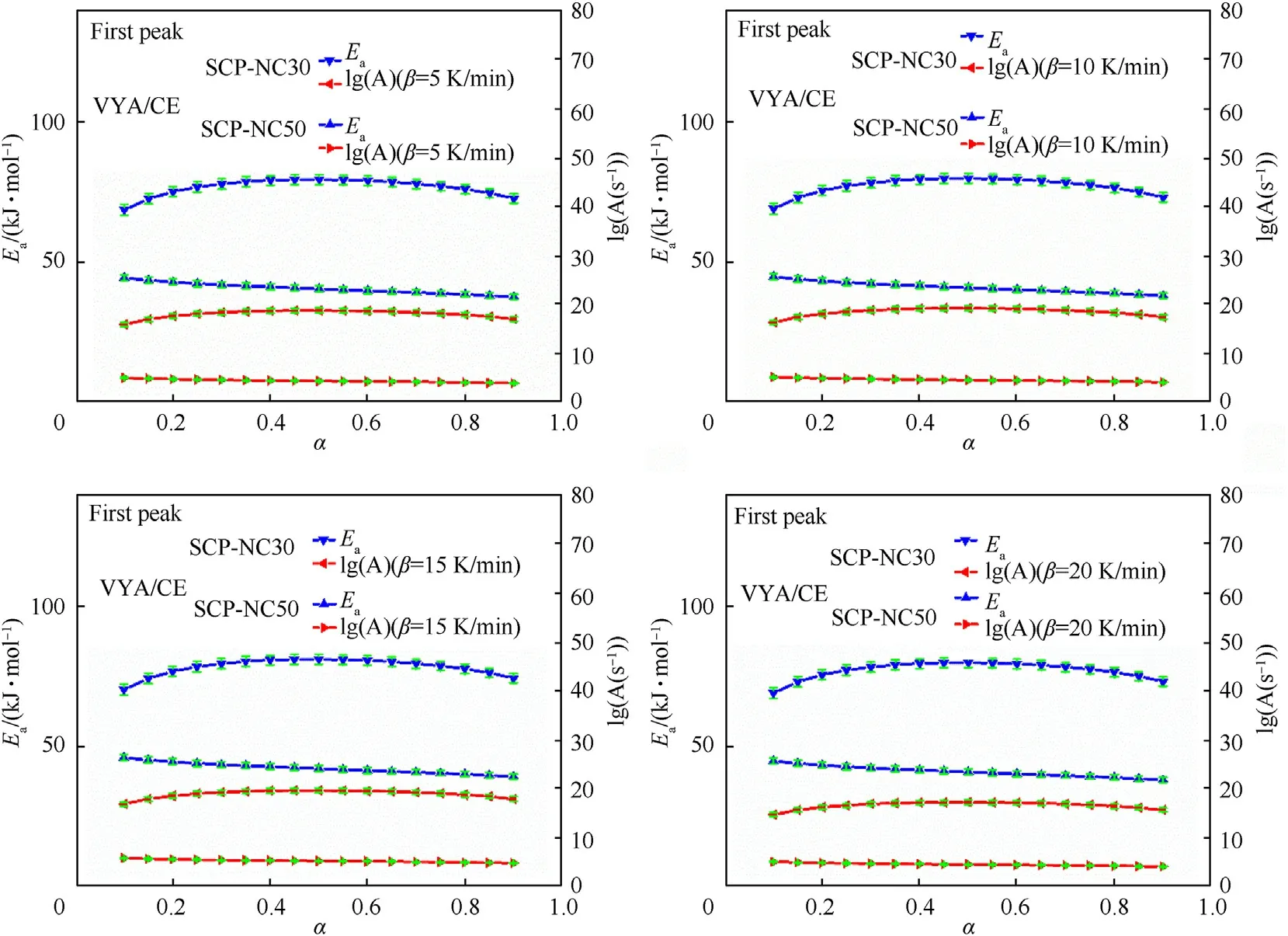
Fig.10.Kinetic parameters and their errors bars as function of conversion rate(α)samples SCP-NC30 and SCP-NC50 determined by Vyazovkin"s method(First peak).

Fig.11.Kinetic parameters and their errors bars as function of conversion rate(α)for samples SCP-NC30 and SCP-NC50 determined by It-FWO and It-KAS methods(Second peak).
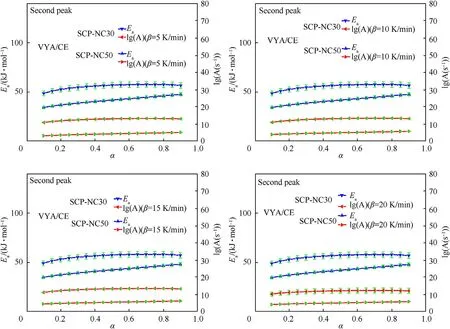
Fig.12.Kinetic parameters and their errors bars as function of conversion rate(α)samples SCP-NC30 and SCP-NC50 determined by Vyazovkin"s method(Second peak).
3.3.Theoretical performance analyses
The results of calculation by the code CEA-NASA of the specific impulse(Isp),adiabatic temperature of the flame(Tf)and the average molar mass(Mm)as well as the mole fractions of the decomposition products of SCPs based on the PU binder modified with the NC,are grouped together in Tables 5 and 6.
One can observe that the composite propellants based on PU-NC binder have better performance parameters.In fact,an increase of 14.64 s in the value ofIspis noted following the addition of 50%by mass of NC in the formulation of the binder.Additionally,it can be seen that the fraction of CO has slightly increased with the increase in the percentage of NC introduced into the composition of the propellants for the percentages 10,15,20 and 30%of NC,while for 40 and 50% of NC,the CO fraction decreased,unlike the CO2fractions where a decrease and then a considerable increase is observed in the case of SCP-NC40 and SCP-NC50.Also,the molar fraction of H2O increases unlike that of H2,which decreases with the increase in the mass percentage of NC in the formulations.These trends are explained by a more complete combustion after the addition of the NC,especially for SCP-NC40 and SCP-NC50,which brings an additional quantity of oxygen to the compositions as shown in Fig.13.The fraction of N2increases with the percentage of NC added in the formulation of the propellant.This trend is explained by the additional supply of nitrogen due to the addition of nitrocellulose.
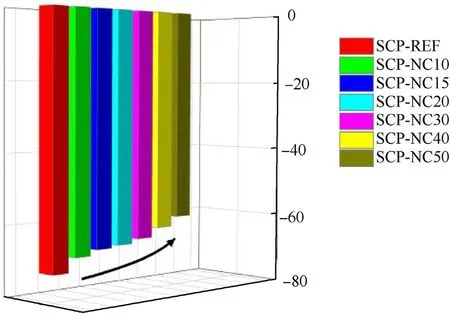
Fig.13.Oxygen balance of solid composite propellant formulations.
4.Conclusions
The synthesis and characterization of ammonium nitrate-based composite solid propellants supplemented with a blend of PU-NC binders were the focus of this research.FTIR,XRD,RAMAN,electronic densimetry,TG,and DSC instruments were utilized to characterize the various samples.A theoretical performance study was also completed using the NASA-CEA algorithm.The spectroscopic results show that the chemical structure of the NC has not changed within the composite propellant through the creation of the polyurethane network.The density of propellants based on PU/NC blends enhances as the NC ratio rises.The results of a study on the non-isothermal degradation and kinetics revealed that the introduction of NC in the formulation of SCPs catalyzed the overall thermal decomposition of the propellants.The theoretical characterization implies that hypothetical AN-based composite solid propellant formulations containing PU/NC blends exhibited better performance.Finally,the current research will undoubtedly pave the way for the development of high-performance solid propellants based on ammonium nitrate as oxidizer.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2021.12.013.
杂志排行
Defence Technology的其它文章
- Modified couple stress and thickness-stretching included formulation of a sandwich micro shell subjected to electro-magnetic load resting on elastic foundation
- Recent advances in the synthesis and energetic properties of potassium-based potential green primary explosives
- Investigation of normal,lateral,and oblique impact of microscale projectiles into unidirectional glass/epoxy composites
- A quasi-isentropic model of a cylinder driven by aluminized explosives based on characteristic line analysis
- Kevlar fabric reinforced polybenzoxazine composites filled with silane treated microcrystalline cellulose in the interlayers:The next generation of multi-layered armor panels
- Experimental study on the cavity evolution and liquid spurt of hydrodynamic ram
