Recent advances in the synthesis and energetic properties of potassium-based potential green primary explosives
2022-11-28QmrunNisTriqSirMnzoorMherunNisTriqWenLiCoJinGuoZhng
Qmr-un-Nis Triq ,Sir Mnzoor ,Mher-un-Nis Triq ,Wen-Li Co ,Jin-Guo Zhng ,*
a State Key Laboratory of Explosion Science and Technology,Beijing Institute of Technology,Beijing,100081,China
b Department of Chemistry,University of Okara,Okara,56300,Pakistan
Keywords:Green primary explosives Heterocyclic compounds Synthesis Energetic properties
ABSTRACT Primary explosives are utilized as a reliable initiator for secondary explosives in an extensive range of military and civilian operations.Heavy-metal-based primary explosives are moderate performing,more sensitive,and environmentally hazardous,posing a direct and indirect threat to health and safety.Therefore,heavy-metal-based primaries have been replaced by environment-friendly metal-based primary explosives,such as potassium complexes.This review presents not only a summary of the current progress of new-generation potassium-based primary explosives and their methods of preparation,energetic properties,and applications,but also a further comparison with traditional primary explosives.In addition,this work discusses the necessity of heavy metal-free primary explosives and the major challenges faced in replacing traditional primary explosives.
1.Introduction
In the past few decades,researchers have paid great attention to the production of energetic materials,such as propellants,pyrotechnics,and explosives,due to their applications in civilian and military areas[1].Primary explosives(primaries)are defined as energetic materials that possess remarkably high initiation sensitivities to different stimuli,such as impact,friction,electrostatic discharge,heat,and shock[2].For initiations of devices in industries,primary explosives are extensively used.Primary explosives are very sensitive and reach detonation rather quickly via encountering any of these initiating events[3].The initiation of primary explosives,usually in the form of shockwaves or heat energy,produce a large amount of energy used to initiate the less sensitive energetic materials,including pyrotechnics,propellants,and secondary explosives[4].
A crucial feature of primary explosives is their rapid deflagration-to detonation transition(DDT),and therefore,they are useful as initiators[5].The term high"initiating efficiency"refers to compounds that can detonate the large mass of secondary explosives.Only detonation tests(Fig.1)can determine this property;compounds with a higher degree of sensitivity are more likely to undergo rapid DDT[6].Primaries exhibit lower detonation heat,detonation pressure,and velocities as compared to secondary explosives.Primary explosives are less potent than secondary explosives,yet they still require extra care while being handled[7].This summary covers the transition from heavy-metal-based(mercury,lead,silver,and copper)primary explosives to potassiumbased primary explosives.Moreover,the new developed potassium-based potential green primary explosives are compared with the existing traditional primary explosives and the results show that these newly developed explosives can be implemented in real applications.
1.1.Heavy-metal-based well-known primary explosives
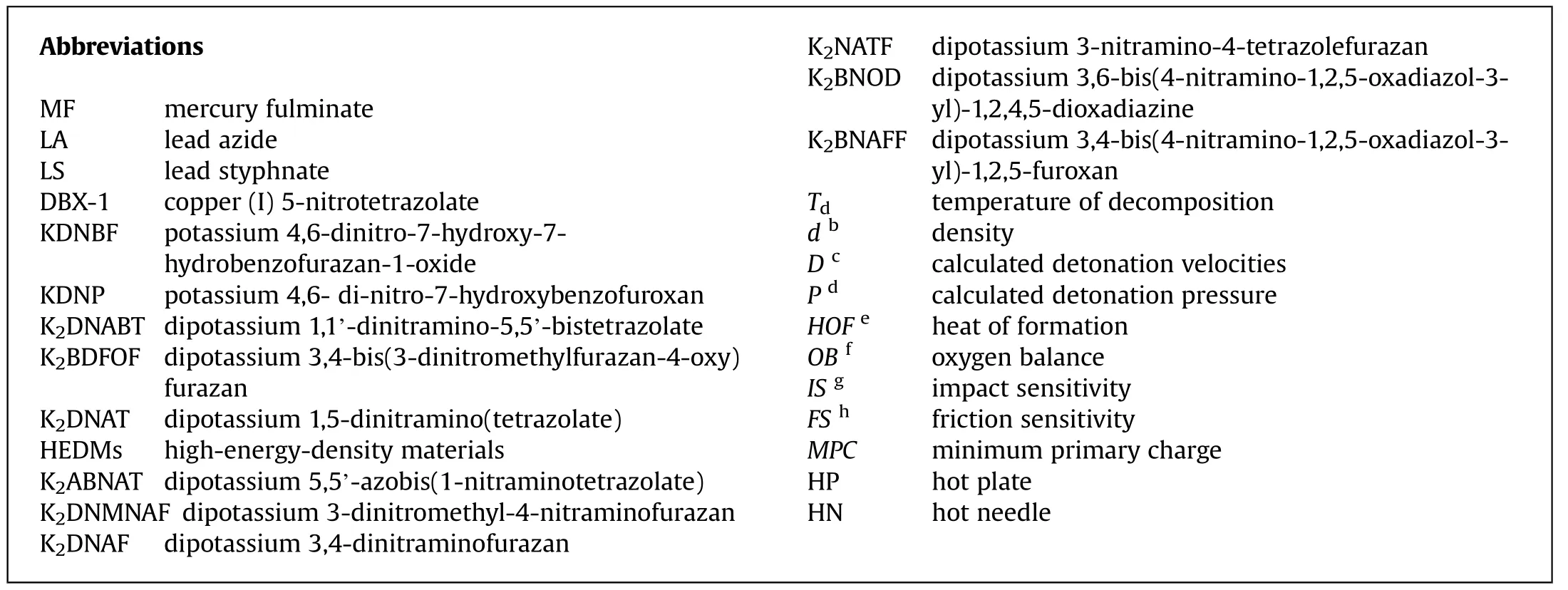
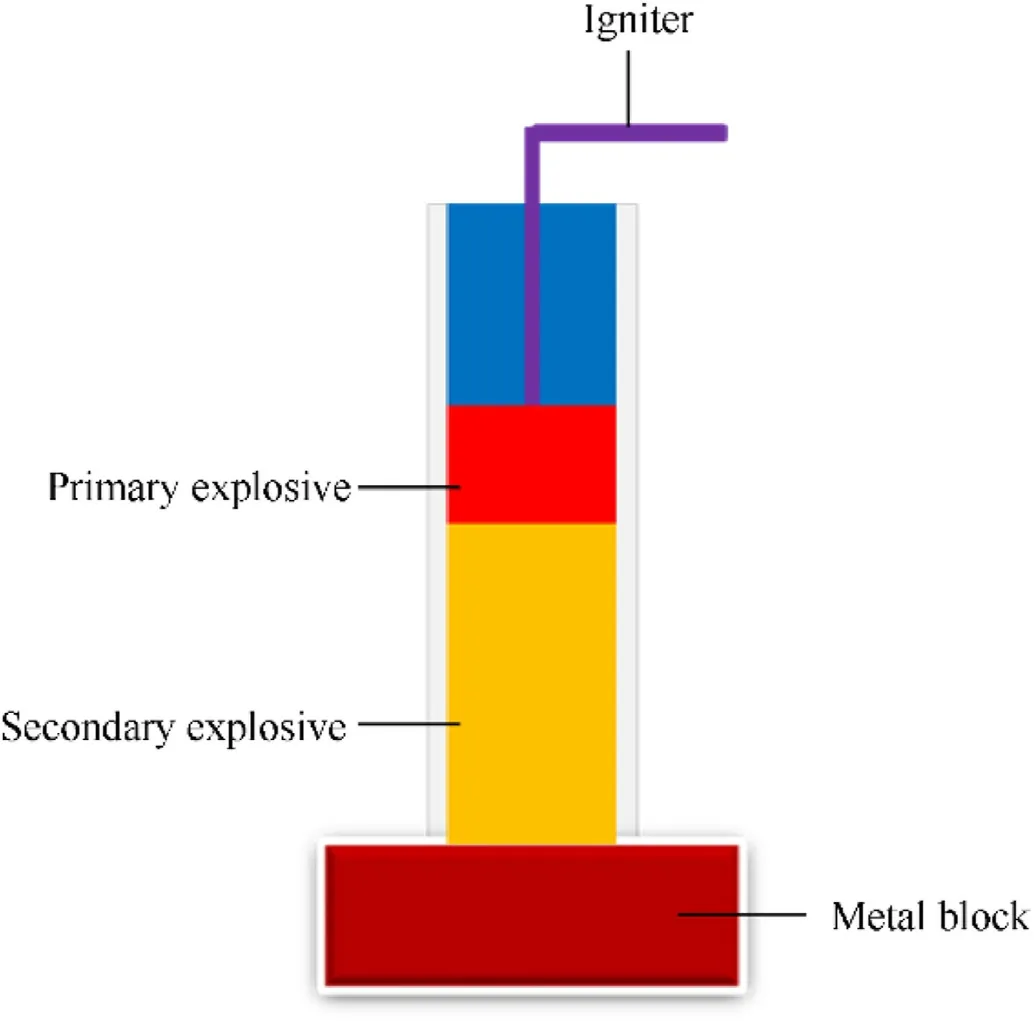
Fig.1.Schematic representation of the detonation test setup.
Initially,primary explosives were composed of high-density material with moderate explosive properties and high sensitivities[8].In the 19th century,mercury fulminate(MF)1 was extensively used as a primary explosive.The preparation of MF(from elemental mercury,ethanol,and nitric acid)is quite easy,but the high cost and toxic nature of mercury are major drawbacks[9].Mercury and its compounds are extremely harmful to biological systems,particularly warm-blooded animals.Mercury cations can easily form complexes with a variety of proteins.As a result,activities of many enzymes are inhibited,and several important metabolic processes are disrupted[10].Mercury compounds have also shown toxic effects on the kidney,nervous system,eye-sight,and vibration of the extremities.Furthermore,MF is highly sensitive and unable to perform under high-pressure loadings[11].Afterward,MF was replaced with well-known primary explosives such as lead azide(LA)2,which is easy to handle.While dextrincoated modified LA is still being used as a primary explosive in blasting caps and detonators[12].
In 1915,a British patent covered the first practical application of lead styphnate(LS)after several years of LA applications in the field of explosive materials[13].It comes in two types:LS(lead(II)2,4,6-trinitrobenzene-1,3-diolate hydrate)(3)and basic LS(di(lead(II)hydroxide)-2,4,6-trinitrobenzene-1,3-diolate)4(Fig.2).LS is easy to initiate and use in primer applications,but not as effective as LA[14].Basic LS is used to prevent the hydrolytic degradation of LA in detonator blends.The inherent drawbacks of LA include its incompatibility with copper and its alloys(encapsulate the primary explosive formulations),high friction sensitivity,and hydrolytic instability[15].Ingestion or inhalation of lead,a highly toxic heavy metal,can damage several tissues and organs,including bones,heart,kidneys,intestines,renal,and nervous systems[16].Besides health and environmental issues,LA produces highly toxic hydrazoic acid(HN3).At ambient conditions,LA reacts with H2O and CO2to produce HN3and basic lead carbonate.HN3reacts with copper wires and surfaces,thus generating highly sensitive and deadly copper azide(Cu(N3)2).This accidental generation of(Cu(N3)2)is the primary cause of many military accidents[17].
Silver and copper are more environmentally and toxicologically acceptable than lead.Except in large amounts,it is not usually harmful to humans or other vertebrates[18].The synthesis of silver azide(SA)is straightforward and relatively similar to the synthesis of LA.SA has several advantages,including enhanced explosive performance,chemical stability,and better applicability in microscale detonation devices[19].In addition to the high price and light sensitivity,the main disadvantage of SA is the uncontrolled release of silver into the environment,which is toxic to many freshwater aquatic microorganisms and also poses a threat to the environment[20].
Fronabarger et al.[21]investigated copper(I)5-nitrotetrazolate(DBX-1)5 as a potential primary explosive because it has more applications and properties than LA,such as thermal stability,and initiability.In existing detonator applications,DBX-1 is used as a“drop-in”replacement for LS[22].DBX-1 is resistant to oxidation compared to LA,so it can work at relatively high temperatures and humidity.DBX-1 synthesis is quite simple and facile,but its transportation and handling are not easy tasks due to its highly sensitive nature toward impact(IS:0.04 J)and friction(FS:0.1 N)[23].It is necessary to state that copper is also toxic to microorganisms and therefore not yet desirable[24].
1.2.Potassium-based traditional primary explosives
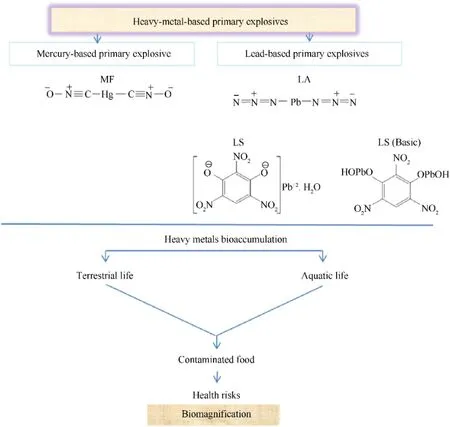
Fig.2.Heavy-metal-based well-known primary explosives and their impact on environment and human life.
In the past, potassium 4,6-dinitro-7-hydroxy-7-hydrobenzofurazan-1-oxide(KDNBF)(6)was enlisted as a heavy metal-free green primary explosive,and it is still known as a commercially available LS alternative used in“green”primary explosives[28].KDNBF is a Meisenheimer adduct between potassium hydroxide and 4,6-dinitrobenzofuroxan;it was synthesized through the reaction of 4,6-dinitrobenzofuroxan with potassium bicarbonate.KDNBF cannot be considered a universal alternative to LS due to its lower thermal stability as compared to lead-based compounds.Small structural modification in KDNBF leads to the synthesis of potassium 4,6-dinitro-7-hydroxybenzofuroxan(KDNP)(7),which varies only in the absence of a hydrogen atom,and KDNP is more stable due to the aromaticity restoration in the benzofuroxan ring(Fig.3).The decomposition temperature of KDNP is 285°C,and its properties are comparable to LS,but the major drawback is its high sensitivity[29].
Up to 2014,several primary explosives were synthesized and reported,heavy metals are being replaced by environment-friendly substituents,but these primaries are still relatively weaker than LA.To fulfill the criterion for green primary explosives,the compound must be free of toxic metals,be safe to synthesize,be easy to handle,be capable of long-term storage,have a stable chemical structure,be insensitive to humidity and light,be thermally stable up to 180°C,have good stability,and have better density with excellent detonation properties[30,31].The latest developments in potassium-based green primary explosives materials since 2014 are covered in this review.A potassium-based primary explosive is a combination of an energetic ligand and environment-friendly potassium metal.Energetic ligands consist of two parts:a backbone,such as aliphatic,cyclic,aromatic,or heterocyclic frameworks,and energetic groups(N2,N3,NF2,N-oxide,NO2,ONO2,NHNO2,C(NO2)2H,C(NO2)3,etc.)that can be covalently bonded to the backbone.In this work,we have selected three reference compounds for comparison purposes(MF,LA,and LS),as shown in Table 1.The review is expected to furnish a summary of the basic methodology for the synthesis of potassium-based green primary explosives.

Table 1Energetic and physicochemical properties of four well-known primary explosives.

Fig.3.Two well-known potassium-based traditional primary explosives.
2.Heavy-metal-free modern primary explosives
For the development of next-generation green primaries,environmental safety is a key factor.Environment-friendly primary explosives minimize environmental pollution via detonation process as they release green detonation products.For designing primary explosives,sensitivity to detonation and stability are the key factors[32].Furthermore,green primary explosives show many good features over traditional primary explosives such as:(i)high decomposition temperature,(ii)insensitivity to humidity and light,(iii)being free from heavy toxic metals such as mercury,lead,cadmium,antimony,etc.,(iv)can be stored for a long time due to stable structure,(v)they are easy and safe to synthesize[33].As a satisfactory precursor for the development of the next-generation high-performance primary explosives,heterocyclic compounds containing environmental-friendly metal ions have been recommended.Nitrogen-rich,heterocyclic energetic ligands play a key role in improving thermal stability,detonation heat,and density.Additionally,they have nitrogen gas as the main detonation product.
3.Potassium-based modern primary explosives
As a substitute for lead-based primary explosives,energetic salts are being prepared by combining heterocyclic-based nitrogen-rich energetic ligands with environmentally friendly potassium metal.Potassium coordinates well with energetic ligands[34].Production of modern higher-performance primaries,oxygen and nitrogenrich compounds,and their potassium-based energetic salts is gaining wide acceptance because of their higher heat of formation and eco-friendly nature[35].In recent years,many potassiumbased energetic materials have been synthesized.The necessary energetic and physicochemical properties of a compound can be obtained by combining an energetic ligand with environmentally friendly potassium metal.A variety of potassium-based primary explosives have been synthesized by using ligand synthesis to develop the potassium salt.
3.1.Pyrazole
In the area of energetic materials,azole-based compounds,especially pyrazole,are regarded as promising building blocks.Selecting pyrazole rings as a fundamental component of energetic compounds leads to high density(1.1 g/cm3),good nitrogen content(41.1%),and large enthalpy of formation(177.4 kJ/mol)[36].Further functionalization can be introduced at the three catenated carbon atoms in the pyrazole ring.For ideal energetic materials,polynitro groups containing compounds are one of the attractive family,and dinitromethyl functionalized compounds are superior energetic compounds with excellent energetic and physicochemical properties,such as high energy,positiveOB,and high density[37,38].The nitro derivatives of pyrazole have huge real-world applications in the areas of modern dyes,agrochemicals,pharmaceuticals,condensation monomers,non-linear optics,and energetic materials.The starting material 3-nitro-4-cyanopyrazol was synthesized by a reported method,and the cyano group 3-nitro-4-cyanopyrazole was subjected to reaction with an equimolar ratio of aqueous hydroxylamine to synthesize pyrazole-3-nitro-4-hydroximoylamine.4-chloroximenitropyrazole was synthesized by chlorinating hydrochloric acid and sodium nitrite,and its nitrated product was obtained by using 100%HNO3and TFAA.The nitrated product was further treated by KI in methanol to produce potassium 3-nitro-4-dinitromethyl-2H-pyrazole(8)as a yellow solid with a 31.7% yield(Fig.4)[39].Compound(8)consists of 4-nitropyrazole as the backbone and dinitromethyl as an explosophoric group.The two structures are joined to achieve a balance between sensitivity and explosive properties.Compound(8)possesses better detonation properties(Dc:8132 m/s;Pd:29.5 GPa),high density 2.04 g/cm3,and a reasonable sensitivity to impact(4 J)and friction(36 N),but it decomposes at 171°C(Table 2).The initiating efficiency,specifically the principal parameter,determines the ability of a primary explosive to initiate secondary explosives.The detonation test was carried out by using 60 mg of primary explosive(LA)to detonate the 500 mg of the secondary explosives(RDX)with nonel and the same experimental conditions were used for compound(8)as well.According to the data,the diameter of the lead indentations of(8)and LA was 15 mm and 21 mm,respectively.So,(8)is an interesting potential green primary explosive and it can detonate RDX easily[39].
Coupled N-heterocyclic moieties and bicyclic compounds are regarded as promising building blocks for enhancing physicochemical and explosive properties[36].Novel bicyclic potentially energetic material based on 4,4′,5,5′-tetranitro-2H,2′H-3,3′-bipyrazole(TNBP)is considered to be a perfect precursor,and it was prepared in the manner reported in the literature[40].To synthesize the N-nitramino-functionalized compound,an effort was made to nitrate the aminated compound(4,4′,5,5′-tetranitro-2H,2′H-[3,3′-bipyrazole]-2,2′-diamine)by using NO2BF4in the presence of CH3CO2K.However,because of the weaker N-NH2bonds in the aminated compound,and even though nitronium tetrafluoroborate is a mild nitrating reagent,only the potassium 4,4′,5,5′-tetranitro-2H-3,3′-bipyrazolate monohydrate(9)was obtained,with a 70%yield.Dipotassium 4,4′,5,5′-tetranitro-3,3′-bipyrazolate(10)was prepared by reacting TNBP with KHCO3,and air-stable salt was readily isolated with a good yield(77%)(Fig.5)[41].Both these monopotassium salt(9)and dipotassium salt(10)may observe their applications in the area of green primaries,particularly for(10)due to its excellent thermal stability(323°C),high density(2.03 g/cm3)and good detonation performance(Table 2).However,no detonation/initiation test was performed in the corresponding article[41].
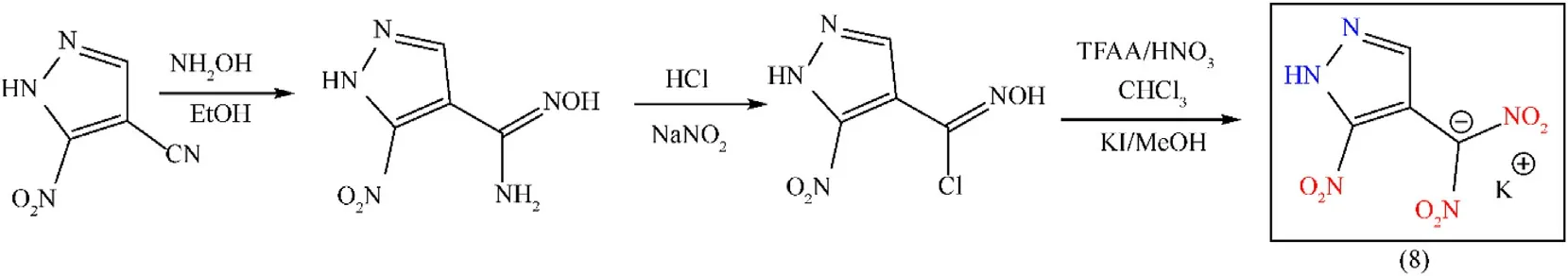
Fig.4.Synthesis scheme of compound(8).

Table 2Energetic and physicochemical properties of compounds(8)to(13).
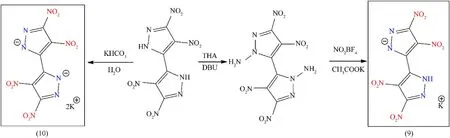
Fig.5.Synthesis scheme of compound(9)and(10).
Tetrazole is a common building block for improving the performance of energetic materials[42].3,4-Dinitro-1H-pyrazol-5-amine(5-ADP)was combined with tetrazole to obtain a novel coupled bicyclic compound that has a low environmental impact while producing a desirable detonation.Combining the highly energetic nitrogen-rich ligand with a non-toxic metal produces sensitive and greener metallic salts that can replace LA.The potassium salt of 3,4-dinitro-1-(1H-tetrazol-5-yl)-1H-pyrazol-5-amine(KANTP)(11)was prepared by C-N coupling of pyrazole-tetrazole bicyclic moiety 3,4-dinitro-1-(1H-tetrazol-5-yl)-1H-pyrazol-5-amine(HANTP).HANTP was obtained by regioselective nucleophilic reaction of 5-ADP at the N1position with cyanogen azide.Treating HANTP with AgNO3led to production of its silver salt,and this was further treated with KCl to obtain KANTP with a 76%yield(Fig.6)[43].Compound(11)has a higher nitrogen content,no toxic metal,a higher decomposition temperature of 253°C,a high positive heat of formation of 630 kJ/mol,good detonation performance(Dc:8380 m/s,Pd:31.4 GPa),and better sensitivities(IS:4 J,FS:144 N)than lead-based primary explosives(Table 2).The comprehensive consideration of physicochemical properties,salt(11)is predicted to be a green primary explosive.While related to initiation ability,no data is found in the relevant article[43].
Fused heterocyclic compounds are a unique class of π-conjugated systems consisting of two or more rings that share two atoms and the bond between the rings[44].To develop a novel class of energetic materials,combining the explosophoric nitro group with the fused-heterocycle ring is of particular interest[45].Forming energetic salts through the NH-acidic group is a uniquely promising technique for modifying the structure of fused heterocyclic compounds[46].Fused heterocyclic-based energetic salts exhibit enhanced friction and impact stability,and their detonation properties and density are not competitive with a parent molecule.Moreover,the oxygen balance and nitrogen content of the resulting energetic salts are firmly restricted by the parental fused molecule[47].Many multi-function energetic compounds can be prepared by structurally modifying 3,6-dinitropyrazolo-[4,3-c]-pyrazole.DipotassiumN,N"-(3,6-dinitropyrazolo[4,3-c]pyrazole-1,4-diyl)dinitramidate(12)was synthesized with a high yield(93%)by using a versatile N-functionalized strategy and an acid-base reaction(Fig.7).The N-nitramino and N-nitro moieties enhance theOBand density greatly(db:2.11 g/cm3;OB:16.2%).Potassium salt(12)featured good thermal stability(208°C),better density,and a highly sensitive nature towards friction and impact stimuli(IS:2 J,FS:20 N)(Table 2),entitled it a competitive candidate as a green primary explosive.The article does not contain any data relevant to the initiation test[48].
3.2.Triazole
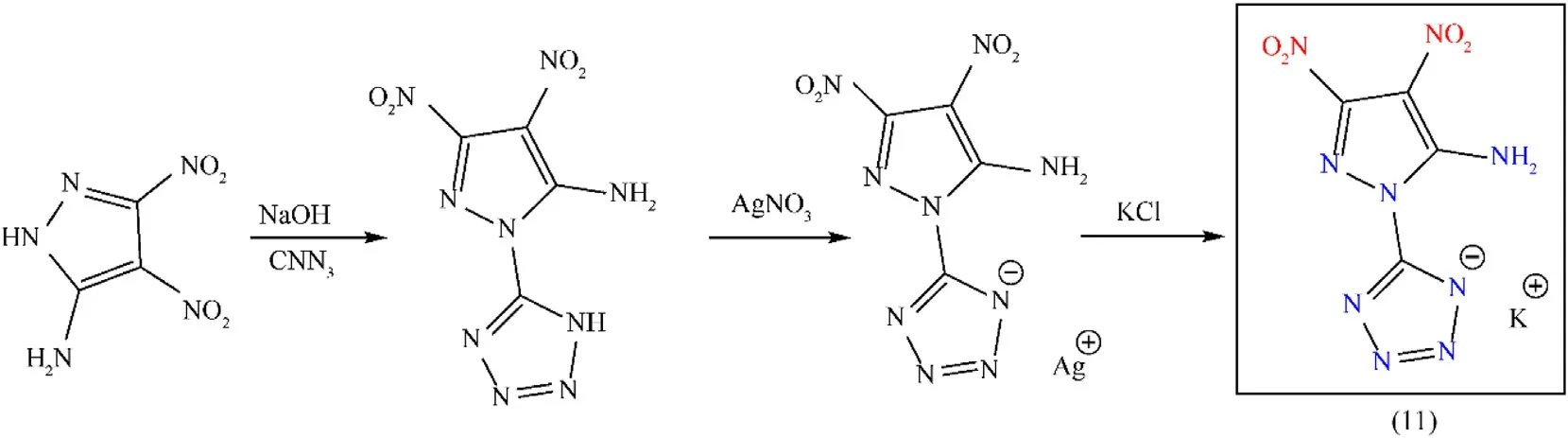
Fig.6.Synthesis scheme of KANTP(11).
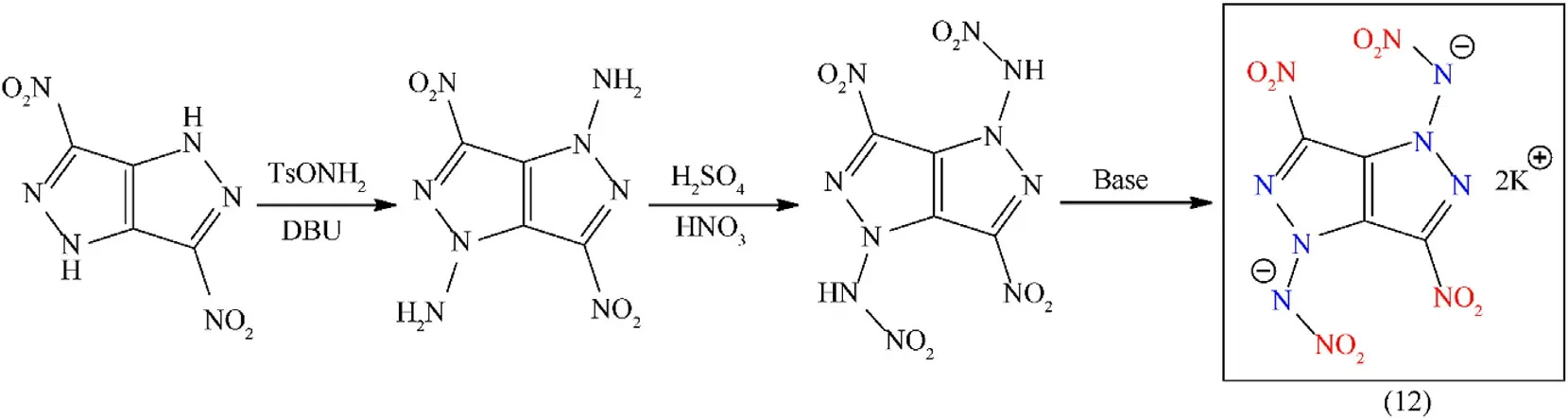
Fig.7.Synthesis scheme of compound(12).
As compared to pyrazole,1,2,4-triazole has a higher density(1.15 g/cm3),higher nitrogen contents(60.8%),and larger enthalpy of formation(192.7 kJ/mol)than pyrazole[36].Oxygen and nitrogen-rich substituted triazole and their potassium-based salts have achieved significant attention because of their environmentally friendly nature and higher heat of formation[49].To produce novel HEDMs,the well-known compound 3-amino-5-nitro-1,2,4-triazole(ANTA)was structurally modified.By introducing various explosophoric groups with a 1,2,4-triazole framework,the primary explosive was obtained,with various improved properties such as better oxygen balance,density,and detonation properties[50].Potassium N-(3-nitro-1-(trinitromethyl)-1H-1,2,4-triazol-5-yl)nitramidate hydrate(13)was prepared with a high yield(97%)through three steps:(i)preparing N-alkyl-functionalized 3-amino-5-nitro-1,2,4-triazole by a reaction of bromoacetone with ANTA under alkaline conditions,(ii)nitrating the N-alkyl-functionalized moiety with mixed acid(conc.H2SO4/fuming HNO3),(iii)forming energetic salt by treating the nitrated product(13a)with a base(Fig.8)[51].Compound(13)is a novel two-dimensional(2D)energetic MOF that was synthesized by combining an oxygen-rich energetic ligand with an environmentally friendly potassium ion.Compound(13),which possesses superior detonation performance(Dc:8739 m/s;Pd:30.90 GPa),higher oxygen contents(46.5%),goodOB(14.8%),and high density(2.00 g/cm3),equivalent to CL-20,and satisfactory sensitivity towards to impact(2 J)and friction(32 N)(Table 2).The higher density is due to two factors:1)strong coordination interactions between ligands and potassium ions,and 2)the“zigzag”stacking of the trinitro-methyl group,which maximizes the use of void space.Its high sensitivity is explained by the extensive O…O closed-shell interactions of the nitramine-and trinitromethyl-based energetic materials.In addition,according to the theoretical evaluation,the detonation products of this nitrogenand oxygen-rich energetic material do not contain heavy-metal residue and also show less amount of toxic gases(NO+CO)[25].The compound(13)is a competitive candidate for green primary energetic materials because of its good detonation performance,high sensitivity,and green detonation products.However,still,this article lacks an initiation test of primary explosives[51].
3.3.Tetrazole
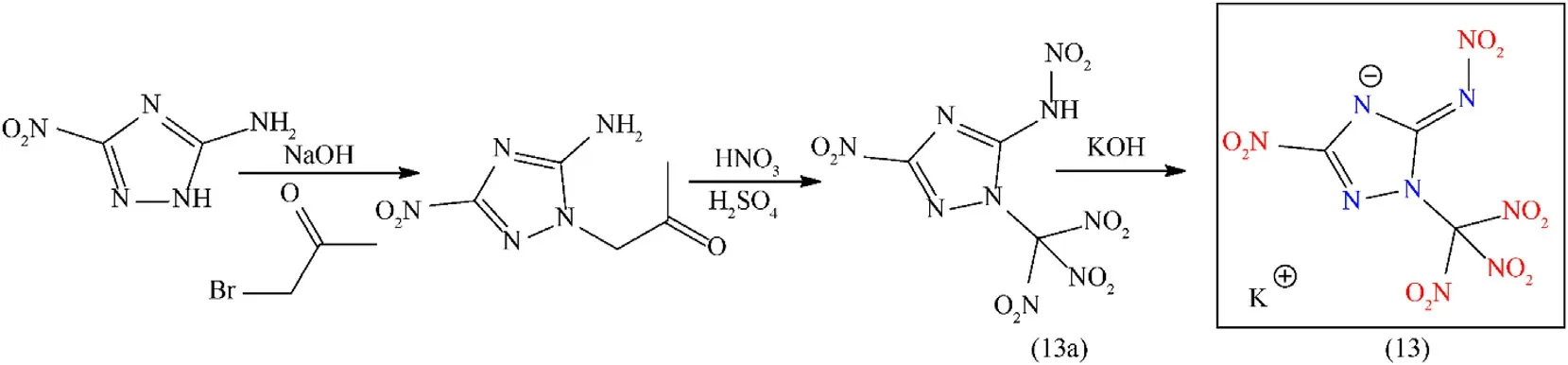
Fig.8.Synthesis scheme of compound(13).
The tetrazole ring exhibits higher nitrogen contents(79.8%),high positive heat of formation(ΔHf)(320 kJ/mol),and greener detonation products such as molecular nitrogen[36].To the production of high-energy-density materials(HEDMs),the backbone of 1-nitraminotetrazoles is considered for particular interest because they possess good oxygen balance and large positive heat of formation[52,53].C-nitraminotetrazoles are typically prepared through acid-based nitrating reagents(100% HNO3)and N-nitramino tetrazoles(usually produced by nitrating the corresponding N-aminotetrazoles with slightly strong nitrating agents i.e.,N2O5and NO2BF4).N-methoxycarbonyl is considered a novel indicatory compound of the following class and the development of the following compounds has been a desirable target in the field of HEDMs due to its exceptional energetic and physicochemical properties.Several attempts at direct nitration have been unsuccessful yet[54].It was synthesized from commercially available reagents dimethylcarbonate and hydrazine monohydrate reacted to give methyl carbazate.Cyanogen azide was treated with methyl carbazate to produce 1,5-diaminotetrazole and further nitrated with N2O5in the presence of acetonitrile.1,5-Diaminotetrazole is considered one of the effective synthetic explosives,but the use is limited due to its high sensitivity and low decomposition temperature.To overcome this drawback,its potassium salt was prepared by decomposition of nitramide in the presence of aqueous KOH to give dipotassium 1,5-dinitramino(tetrazolate)(K2DNAT)(14)as a white solid in high yield 98%(Fig.9)[55].Compound(14)featured high thermal stability(240°C),promising performance values(Dc:1011 m/s;Pd:52.2 GPa),and exhibits ideal behavior for use in primary compositions(replacement of tetrazene).In a detonation test,50 mg of(14)was initiated using a standard pyrotechnical igniter.The shockwave formed by(14)was stronger enough to detonate 500 mg of hexogen(RDX).The test was repeated 50 mg(14)without RDX,only a very little dent in the copper plate was detected[55].
Till date,a number of valuable potassium containing primary explosives have been synthesized and examined as promising replacements for the ordinarily used lead-based primary explosives[56].Salt formation and detonation properties are significantly improved by the introduction of the nitramino group.There are several steps involved in synthetic procedures may affect the practicability of industrial processes to substitute the commercially available lead-based energetic material[57,58].From the following viewpoints,an easier and shorter synthetic procedure would be preferable.Intellectually separation of symmetrical dipotassium 1,1’-dinitramino-5,5’-bistetrazolate compound into two mononitraminotetrazolate is an easy way to synthesize and exhibits comparable energetic properties to the parental dinitraminotetrazolate molecule.In the past,ammonium and silver salts of 1-(Nnitramino)-5H-tetrazolate and 2-(N-nitramino)-5H-tetrazolate had been synthesized,but these compounds do not get any attention due to limited characterization data,sensitive nature of silver salt,and unacceptable solubility of the ammonium salts[59].Two new isomeric salts potassium 1-(N-nitramino)-5H-tetrazolate(15)and potassium 2-(N-nitramino)-5H-tetrazolate(16)are considered as unique primary explosives and they could be synthesized by simple step through nitrating the 1-amino-5H-tetrazole and 2-amino-5Htetrazole with KOEt(Fig.10)[60].(15)and(16)were obtained in the acceptable yield of 64% and 48% respectively.Both these isomers easily outperform LA in performance parameters such as detonation velocity,the heat of explosion,and gas volume after detonation[4].For determination of the DDT capability,a hot needle(HN)and hot plate(HP)tests were performed.Following the penetration of(15)and(16)with a red-heated needle,a vast detonation occurred,indicating their considerable potential as primary explosives.The HP test is a simple and safe way to see how unconfined samples behave to rapid fast heating.During the HP test,both compounds detonated strongly with a small bending of the plates.By consideration of the HN and HP test,only 5 mg of the(15)or(16)is enough to initiate RDX and PETN reliably[60].Therefore,(15)is considered a lead-free green primary explosive due to its easy synthetic steps(easy availability of starting materials)and better initiation ability(Table 3).
Dipotassium 1,1′-dinitramino-5,5′-bistetrazolate(K2DNABT)(17)was produced through a safe and delicate procedure by using commercially available material(glyoxal and dimethylcarbonate)in 61% yield(Fig.11)[26].Compound(17)undergoes no decomposition or mass loss when it was subjected to 100°C for 48 h.It owns a high density(2.11 g/cm3),high heat of formation(326 kJ/mol),and impressive detonation properties(Dc:8330 m/s,Pd:31.7 GPa);due to such excellent properties it can replace LA from detonators applications.Additionally,it exhibits good initiation power and faster detonation than traditionally lead-based primary explosives while being more environment-friendly.A flame test and a"hot needle test"confirmed quick detonation of K2DNABT in contact with a flame or a hot metal needle,ultralow MPC of 40 mg could easily ignite the 1 g of RDX successfully[26].By considering their good flowability,500 mg of both compounds((17)and LA)was poured into the steel block and ignited unpressed;the indentation of 1.4 mm and 0.2 mm was observed,respectively.The major drawbacks of(17)are its complex and long synthesis route,low yield 61%,and its highly sensitive nature towards impact and friction(1 J and≤1 N,respectively)(Table 3).The challenging field of energetic materials can help to overcome these drawbacks.
Dipotassium 5,5′-azobis(1-nitraminotetrazolate)K2ABNAT(18)consists of potassium metal and two tetrazole rings linked by an azo-bond.Azo-linkage is responsible for the high heat of formation(617 kJ/mol).Compound(18)was prepared by the simple and suitable method based on the three steps by using cyanogen azide and methylcarbazate as starting material to produce N-methoxycarbonyl protected 1,5-diaminotetrazole,which was oxidized in 5,5′-azobis(1-ethoxyformam idotetrazole)by using conc.HCl with KMnO4and its further decomposition leads to the synthesis of K2ABNAT(21.2%yield)(Fig.12)[61].Compound(18)displays good oxygen balance,higher nitrogen contents,good thermal stability(189°C),high density(2.11 g/cm3),higher positive heat of formation and excellent energetic performances(Dc:8367 m/s,Pd:31.5 GPa)(Table 3).So that it can outperform the LA and K2DNABT in all critical detonation parameters such as the temperature of detonation,heat of detonation,detonation velocity,gas volume after detonation,and energy of formation.The compound(18)is a green primary explosive with superior initiation power,but no data is found in this article in favor of initiation efficiency,low yield and lack of initiation test are its major drawbacks[61].
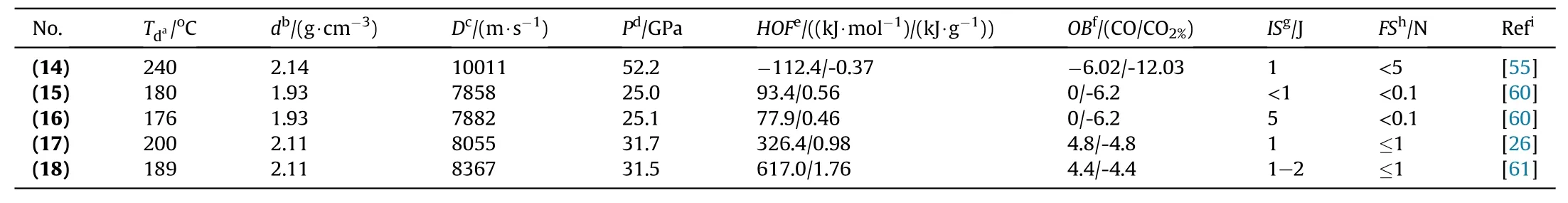
Table 3Energetic and physicochemical properties of compounds(14)to(18).
3.4.Oxadiazole
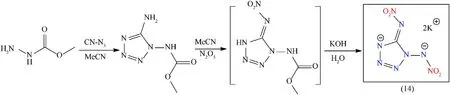
Fig.9.Synthesis scheme of K2DNAT(14).
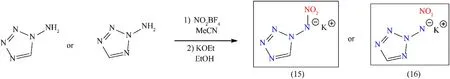
Fig.10.Synthesis scheme of compound(15)and(16).

Fig.11.Synthetic pathway towards K2DNABT(17).

Fig.12.Synthesis scheme of K2ABNAT(18).
Energetic compounds based on furazan have relatively enhanced thermal stability,good oxygen balance,high detonation heat,and high density.The combination of nitroamino and furazan[62]is responsible for high density,good thermal stability,good oxygen balance,and high heat of formation;it also confirms the molecule"s ability to pair with potassium metal by salt formulation.Additionally,the introduction of explosophoric groups(-NNO2,-C(NO2)2,1,4,2,5-dioxadiazine,and 1H-tetrazole,etc.)on furazan ring can tune the performance of this kind of material,which is a potential candidate for a novel class of energetic green materials[63]with promising properties to be recruited as environmental-friendly primary explosives.For the synthesis of 3-chlorodinitromethyl-4-nitraminofurazan,3-amino-4-chloroximinofurazan was treated with N2O5(prepared by the distillation of 95%HNO3with P2O5on a 10 g scale)in the presence of chloroform.Following the removal of the nitrogen oxides and solvent under vacuum,this reaction mixture was contacted with a potassium iodide in methanol solution,yielding dipotassium 3-dinitromethyl-4-nitraminofurazan(K2DNMNAF)(19)as a yellow solid(46.3% yield).Dipotassium 3,4-dinitraminofurazan(K2DNAF)(20)was prepared in high yield(91.4%)through metathesis reactions of its corresponding silver salt(20a)with an equimolar ratio of K2SO4and KCl in water(Fig.13).K2DNMNAF(19)and K2DNAF(20)possess high-density and good thermal stabilities(281°C and 291°C,respectively)as compared to LA.Attractive energetic and physicochemical properties of salt(19)include good thermal stabilities(Td:281°C)and better detonation properties(Pd:27.3 GPa,Dc:7759 m/s)(Table 4).These promising properties are responsible for making them competitive applicants for the substitution of lead-based primary explosives[64].
Dipotassium 3-nitramino-4-tetrazolefurazan(K2NATF)(21)was prepared in moderate yield(60%)by the reaction of its corresponding barium salt(21a)with the equimolar ratio of K2SO4and KCl in water(Fig.14).A well-known compound of nitraminofurazan with potassium was prepared by easy method and this salt exhibits excellent properties and is considered as a green alternative to lead-based primary explosives.K2NATF(21)possesses good thermal stability(311°C)comparable to lead-based primary explosives(Table 4).Despite all these properties,the initiation test was not performed concerning compounds(19)-(21)[64].
The heat of formation can be improved by the addition of an azo-linkage to the furazan moieties.Furazan has two nitrogenoxygen(N-O)and carbon-nitrogen(C=N)bonds that are responsible for goodOBand better density[53].Sensitivity to detonation and stability are significant aspects in designing of primary explosives.A brilliant choice is a combination of energetic groups(i.e.,NO2,N3,or NHNO2)with a comparatively compact molecule.The trinitromethyl group is not considered an appropriate moiety due to its low decomposition temperatures compared to dinitromethyl groups.These groups are higher energetic candidates with improved thermal stabilities.The combination of dinitromethyl and azofurazan groups with potassium should be suitable for primary explosives[65,66].Potassium 4,4’-bis(dinitromethyl)-3,3’-azofurazanate(22)consists of two furazan rings that are responsible for good oxygen balance and improved density,these rings are connected by an azo-bridge and they can enhance the heat of formation.Compound(22)was obtained in low yield(33.5%)in a three-step method:(1)oxidation by using KMnO4/HCl,(2)diazotization by using NH2OH/HCl/NaNO2as reagents,and(3)nitration followed by KI reduction(Fig.15).Nitrogen-rich energetic MOF(22)is considered as promising primary explosive due to its excellent detonation performance,good oxygen balance,and better thermal stability(229°C)with high impact(2 J)and friction(20 N)sensitivities(Table 4)[32].However,no data is reported in this article regarding the initiation test of compound(22).
As discussed above,furazan-containing energetic compounds possess high heat of formation and better thermal stabilities than other oxygen-nitrogen bearing moieties.Explosive properties and the density of any energetic compound can be tuned with the introduction of different explosophoric functionalities(CH(NO2)2,-NO2,and-N3)to the furazan ring[67].Azide group(-N3)decreases the thermal stability,whereas-NO2exhibits limited coordination positions.Dinitromethyl group is definitively an excellent choice to achieve balance among coordination sites,thermal stability,and low toxicity.Meanwhile,the carbon in the dinitromethyl functionality can treat with metal hydroxides and alkaline ammonium to produce various energetic compounds via a simple acid-base reaction[68].Additionally,a combination of two dinitromethyl-functionalized furazan moieties via bisnitraminomethylene linkage can enhance the oxygen balance and density.4,4’-Bis(dinitromethyl)-3,3’-bisnitramide-methylene-furazanate (DBMF2-)is a powerful energetic ligand that consists of higher contents of nitrogen and oxygen,and combining of this ligand with low toxic alkali metals may lead to stimulating the development of new primary explosive dipotassium 4,4’-bis(dinitromethyl)-3,3’-bisnitramide-methylene-furazanate K2DBMF(23)(yield 39.6%)(Fig.16).Compound(23)possesses positive OB,high nitrogen and oxygen contents(N+O:70.47%),and theoretically predicated detonation solid is K2CO3[69].Furthermore,compound(23)shows improved detonation performance(Dc:8227 m/s;Pd:32.5 GPa)as compared to LA,but despite all these good properties,it exhibits low synthetic yield and low decomposition temperature(133°C)(Table 4).In the detonation test,90 mg(MPC)of compound(23)was used as an initiator to detonate 450 mg RDX with an electric firing igniter.Its initiation performance was determined by examining the hole caused by an explosion.The experiment indicates that(23)can be treated as primary explosive,showing strong initiating performance comparable to LA.
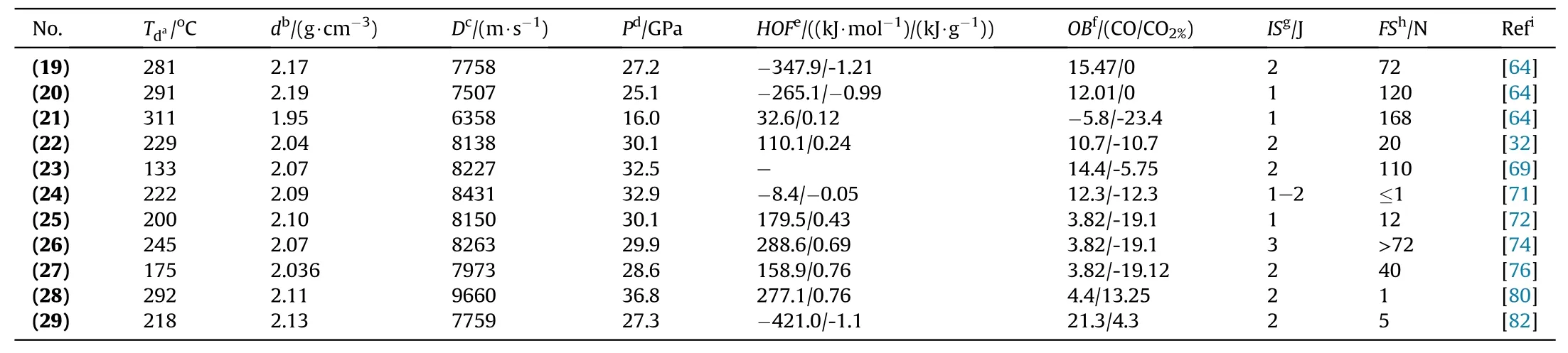
Table 4Energetic and physicochemical properties of compounds(19)to(29).
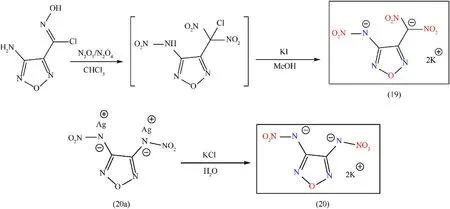
Fig.13.Synthesis scheme of(19)and(20).
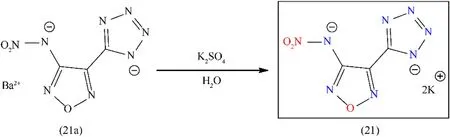
Fig.14.Synthesis scheme of(21).
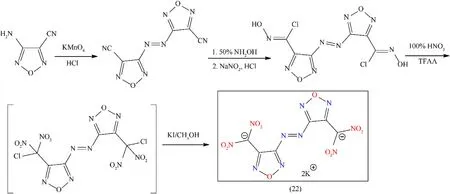
Fig.15.Synthesis scheme of(22).
Many energetic properties of a molecule can be significantly improved by the introduction of high-energy furazanyl ethers.When bridged oxygen atoms are periodically attached with the furazan rings,it can incredibly improve both the thermal stabilities and densities of the molecules[70].Over the last couple of years,furazanyl ether compounds have been studying extensively due to their promising properties and numerous compounds are reported,i.e.,oxy-bridged bis(1H-tetrazol-5-yl)furazan,3,3’-dinitrodifurazanyl ether,3,3′-bis(nitro-NNO-azoxy)-difurazanyl ether,etc.Furthermore,the introduction of furazanyl ether as a backbone into dinitromethide groups are considered as an efficient way due to its superiority,such as its high oxygen contents,explosive properties,and different mode of co-ordination.Dipotassium 3,4-bis(3-dinitromethylfurazan-4-oxy)furazan(K2BDFOF)(24)was prepared in high yield(88.9%)by simply four steps,including addition,diazotization,nitration,and reduction(Fig.17).Compound(24)is entitled as a greener primary explosive due to its higher density,superior thermostability 221°C,and its impact(1 J)and friction(1 N)sensitivities are comparable to LA and K2DNABT.Additionally,compound(24)shows suitable properties,particularly its non-toxic nature,but it is very sensitive and should be handled with care(Table 4).According to the literature,the most important criteria of a high-initiation-power primary explosive are its detonation velocity V,and its detonation pressure P,but experimentally no detonation test was carried out in favor of compound(24)[71].
The preparation of dipotassium 3,6-bis(4-nitramino-1,2,5-oxadiazol-3-yl)-1,2,4,5-dioxadiazine(K2BNOD)(25)is carried out in one equivalent of K2CO3with the aqueous solution of(25a)(Fig.18).A tricyclic compound was prepared by the facile route and following compound own excellent properties and it can be suggested as a green alternative of heavy-metal-based primary explosives.K2BNOD(25)displays superior detonation performance(Dc:8150 m/s;Pd:30.1 GPa),but this salt has higher impact and friction sensitivities(1 J and12 N)and no initiation test was performed concerning it(Table 4)[72].

Fig.16.Synthesis scheme of(23).
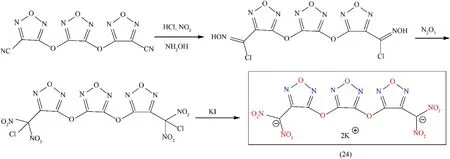
Fig.17.Synthetic pathway towards K2BDFOF(24).

Fig.18.Synthesis scheme of(25).
In the synthesis of modern nitrogen-rich explosives,a combination of a nitro or nitramino group with a furoxan and furazan ring in one molecule,is considered a legitimate scheme[73].The preparation of novel energetic materials with better thermal stabilities and their properties depends on furoxan and furazan rings.In the present study,3,4-bis(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,5-furoxan(BNFF,also known as DNTF)was prepared by oxidation of 3,4-bis(4-amino-1,2,5-oxadiazol-3-yl)-1,2,5-furoxan(BAFF)with trifluoroacetic acid and 50%hydrogen peroxide.BNFF exhibits better heat of formation(657 kJ/mol),high decomposition temperature(292°C),and excellent energetic performance.To improve the detonation properties of BAFF,amino groups are replaced by nitro groups.Dipotassium 3,4-bis(4-nitramino-1,2,5-oxadiazol-3-yl)-1,2,5-furoxan(K2BNAFF)(26)was prepared at high yield(90%)through nitration of 3,4-bis(4-amino-1,2,5-oxadiazol-3-yl)-1,2,5-furoxan(BAFF)with 100% HNO3and subsequently treated with KOH(Fig.19)[74].This nitrogen-rich energetic salt(26)is considered a green primary explosive due to its better detonation performance,good thermal stability(245°C),reasonable impact(3 J),and friction(>72 N)sensitivities(Table 4)but no data is reported in this article regarding initiation test.
Fused heterocyclic nitrogen-rich compounds are a novel and attractive family of high HEDMs.These compounds are interesting due to their conjugated and coplanar structure,which is responsible for the enhancement of thermal stability and heat of formation[74].In the area of energetic materials,only a few fused-heterocyclic compounds are used for the synthesis of unusual and different molecules with long-hindered extension.As a consequence,it is of great interest to consider novel methods for constructing fused cyclic skeletons and their derivatives.To the development of new HEDMs,the introduction of the energetic group(NO2,ONO2,NHNO2,C(NO2)2H,C(NO2)3,etc.)to fused heterocyclic ring is a judicious choice,which would improve the density,upgraded the detonation properties and improve the oxygen balance[75].However,the preparation of a fused compound is still a challenge.A new high energy salt potassium 6-nitro-pyrazolo[3,4-c]furazanate 5-oxide(27),was prepared by unusual intramolecular cyclization reaction,nitration of chlorohydroximoyl and amino groups by using a mixture of 100% nitric acid and trifluoroacetic anhydride followed by reduction with potassium iodide in methanol(Fig.20)[76].This novel synthetic strategy can be widely applied in various fields of science and technology.Compound(27)is a fused heterocyclic ring based primary explosive with a conjugated system and coplanar structure which exhibits high heats of formation(0.76 kJ/g),efficient detonation properties(Dc:7973 m/s;Pd:28.6 GPa),and satisfactory sensitivity towards impact(2 J)and friction(40 N)(Table 4).Low yield(22%),thermal stability below 180°C,and six steps synthesis are its major drawbacks.The initiation test of compound(27)is not carried out yet[76].
Nitramino-based energetic materials always exhibit high sensitivities to obtain a balance between low sensitivities and highenergy nitramino energetic materials,one well-known strategy is the introduction of nitramino groups into stable nitrogen-rich heterocycles like tetrazine,tetrazole,furazan,and triazole[77].A well-known fused furazan compound,4,8-dihydrodifurazano[3,4-b,e]pyrazine(DFP),contains two furazans in its backbone.Many energetic bonds(C-N,N-O,and C=N)are responsible for the high enthalpy of formation.DFP possesses a high crystal density of 2.01 g/cm3due to its symmetric planar structure,with comparatively higher N/O contents(ca.70 wt%).The following structural properties indicate that DFP is a favorable backbone for designing promising HEDMs[78]:(1)limited synthetic methodology due to only two reaction sites(-NH group);(2)Easy removal of piperazine ring in the DFP molecule and the generation of a free radical.So,the preparation of novel DFP-based energetic compounds remains a challenge.In the area of energetic materials,energetic metalorganic frameworks(E-MOFs)have been pursued because their sensitivities and energy levels can be controlled by explosive ligands and assembly of reasonable metal ions[79].In the work below,3D E-MOF was prepared by using nitroamine furazans ligand and potassium ions as the metal center.This energetic ligand consists of two furazan rings with many energetic bonds,such as C-N,N-O,and C=N,which are responsible for the enhanced energy of the final 3D E-MOF.Dipotassium 4,8-dinitraminodifurazano[3,4-b,e]-Pyrazine(28)was synthesized from potassium ions and energetic ligands,which are attached via 6,8-connection mode to form a 3D framework with pcu topology(yield 30.2%)(Fig.21)[80].Compound(28)has better thermal stability(292°C),excellent detonation velocity(9660 m/s),and reasonable friction and impact sensitivities(1 N and 2 J,respectively)(Table 4).Better thermal stability and excellent detonation performance associated with reasonable mechanical sensitivities are responsible for making(28)as a potential candidate for primary explosives.Despite these excellent properties the compound(28)still has not gone through the initiation test.
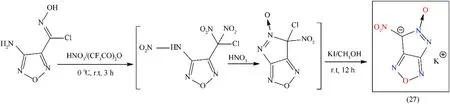
Fig.20.Synthesis scheme of(27).
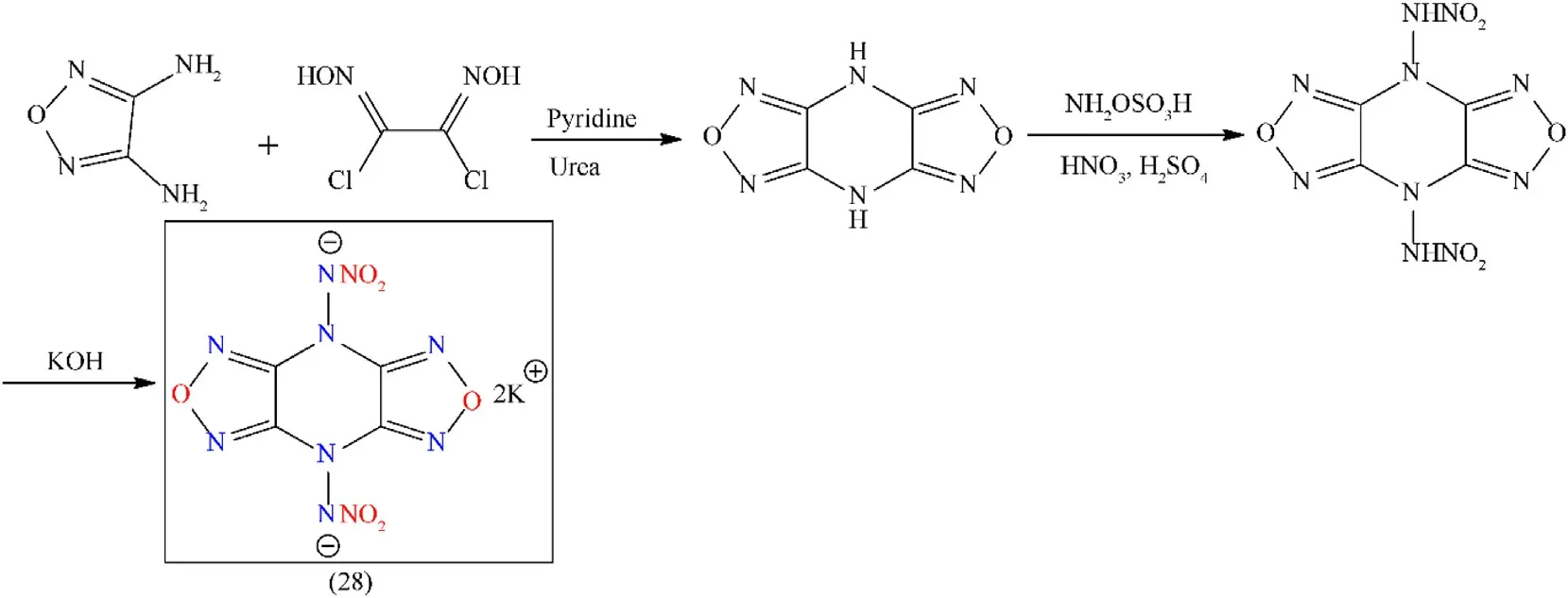
Fig.21.Synthesis scheme of(28).
Recently,many satisfactory substitutes for lead-based primary explosives have been reported,but few of them possess good oxygen balance.By turning all carbon into carbon dioxide and all hydrogen into water,energetic compounds with a positive or zero oxygen balance(OB)releases small number of toxic gases.Consequently,compounds with a positiveOBare considered greener than negativeOBcompounds[81].The majority of candidates synthesized as primary green explosives having negativeOB.Nitro groups could be introduced in a compound to improve theOBefficiently.Dipotassium 4,5-bis(dinitromethyl)furoxanate(29)was obtained in 30.2% yield by nitrating the starting material(with a mixture of 100% HNO3and trifluoroacetic acid anhydride)followed by potassium-iodide reduction(Fig.22)[82].Compound(29)consist of dinitromethyl groups and it is responsible for improved oxygen balance,enhanced detonation properties,and good density.Additionally,the planarity of this group makes(29)more stable as compared to trinitromethyl-containing molecules.Due to the positive oxygen balance,it liberates the minimum amount of toxic products.N2,CO2,and H2O are the theoretically calculated main detonation products.Compound(29)is a reasonable candidate as a greener primary explosive due to reasonable sensitivity,remarkable detonation performance,improved decomposition temperature(218°C)(Table 4),and theoretically calculated green detonation products(CO%+NO%=0.29%)[82].Initiating efficiency of compound(29)has not been evaluated yet through a detonation test.In Table 5 oxygen balance(ΩCO% and ΩCO2%)of the different compounds(K2DNABT,K2BDFOF,and K2DNAT)are reported and drawn a comparison with(29).
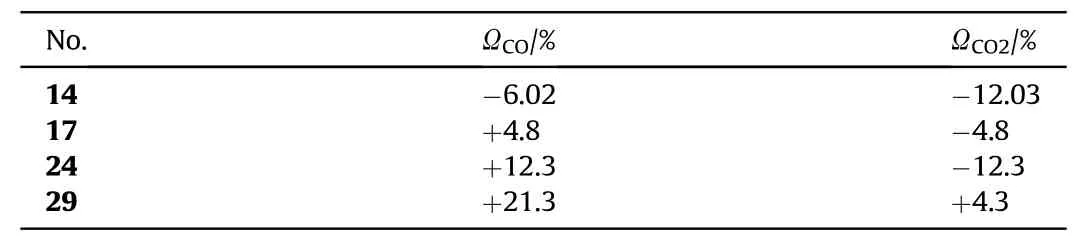
Table 5Oxygen balance of some potassium-based primary explosives.
These(8)-(29)newly synthesized primary explosives are considered to be“greener”energetic materials than the traditional materials.This type of greenness is limited to a single property,free from heavy metal pollution in the environment,which is either directly or indirectly hazardous to living organisms.However,it is a misconception that a green primary explosive should have numerous green attributes,such as low toxicity and biodegradability[83].
4.Conclusions and future trends
Heavy-metal-based primary explosives(MF,LA,and LS)were ruled in the area of primaries for a very old time.Long-term exposure to these heavy metals causes many health effects,environmental contamination,and the threat from higher sensitivities,so it is necessary to find efficient and green substitutes.Given thesafety of production and transportation,sensitivity to external stimuli is a critical component.
The synthesis process and energetic features of numerous potassium-based primary explosives,as well as their currently accessible practical possibilities,are covered in this paper.Many potassium-based primaries were reported in the past,but these compounds are seldom used because of their complex synthesis and in terms of priming capability and initiating ability,they are comparatively weaker than heavy-metals-based primary explosives.In recent years(since 2014),many potassium-based primary explosives(8)-(29)have been synthesized.As compared to traditional primary explosives,these compounds were prepared from easily available and cheap material.They are thermally very stable except(13),(16),(23),and(27),whereas(8),(13),(19),(20),(28)and(29),own good oxygen balance.The compounds’sensitivities((8)-(13),(19)-(23),(25)-(27),and(29))are better than traditional primary explosives,but they are still quite sensitive,and higher sensitivities cause a premature or accidental explosions.Additionally,there exist complex synthesis processes and a low yield,which appears to limit future research.Only compounds(8),(14),(15),(16),(17),(23),and(24)go through an initiation test,which are possibly the most important criteria for primary explosives.

Fig.22.Synthesis scheme of(29).
Three stages may be required to obtain a green primary explosive with the desired attributes in a more acceptable manner.The first step is to choose greener cations and anions that will ensure the required properties of the target material.The second step is estimating attributes for the energetic salts chosen and selecting the most promising compounds as potential targets for further synthesis.Third,green primaries should next be synthesized via shorter,safer,and cost-effective routes,and their physical properties,detonation performance,and initiating ability should all be studied.
From the ongoing research,it is clearly indicated that appreciable efforts have been devoted over the past several years for the replacement of toxic metals from primary explosives.Many publications on potassium-based primary explosives exist,indicating that this is a fascinating field that merits more investigation because it is crucial to the safe production of primary explosives with discriminatory features.
5.Cautions
It is to remind the readers that this review is about the current progress of potassium-based(they are the combination of various energetic ligands with potassium metal)and metal-free green primary explosives.Many of the compounds included in this review are very energetic and more sensitive,they have the probability to explode under certain conditions.Therefore,the proper protective measures such as the use of leather gloves,fume hoods,face shields,and careful handling are highly recommended throughout the preparation of targeted compounds.Moreover,the original references should be referred for detailed synthesis method and safety information.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are thankful to the NSAF(U1830134),NSFC(22175025),for their generous financial support.This job was supported by a project of State Key Laboratory of Explosion Science and Technology(Beijing Institute of Technology).The project number is YBKT21-02.
杂志排行
Defence Technology的其它文章
- Defence Technology
- The SSA-BP-based potential threat prediction for aerial target considering commander emotion
- Visual-simulation region proposal and generative adversarial network based ground military target recognition
- Preparation and characterization of HMX/NH2-GO composite with enhanced thermal safety and desensitization
- Interception probability simulation and analysis of salvo of two electromagnetic coil launched anti-torpedo torpedoes
- Damage behavior of the KKV direct hit against fluid-filled submunition payload
