叉头框蛋白O1介导的自噬途径在抗阻训练缓解低氧诱导大鼠肌萎缩中的作用*
2022-10-13付鹏宇于晶晶龚丽景2
付鹏宇, 于晶晶, 龚丽景2,△
叉头框蛋白O1介导的自噬途径在抗阻训练缓解低氧诱导大鼠肌萎缩中的作用*
付鹏宇1,2, 于晶晶3, 龚丽景2,3△
(1西北工业大学体育部,陕西 西安 710072;2北京体育大学中国运动与健康研究院,北京 100084;3北京体育大学运动与体质健康教育部重点实验室,北京 100084)
探究抗阻训练对缓解低氧诱导大鼠肌萎缩的作用,以及该过程中叉头框蛋白O1(forkhead box protein O1, FoxO1)介导的自噬途径的调节机制。40只SD大鼠随机分为常氧安静(normoxic control, N)组、常氧训练(normoxic resistance training, R)组、低氧安静(hypoxic control, H)组和低氧训练(hypoxic resistance training, HR)组,每组10只。低氧各组生活在氧浓度为12.4%的低氧房中,训练各组隔天进行递增负重爬梯训练,干预4周。期间记录大鼠的摄食量与体重;干预结束后,测量大鼠体成分;称量趾长伸肌(extensor digitorum longus, EDL)湿重;HE染色观察肌纤维形态并测量纤维横截面积(fiber cross-sectional area, FCSA);自噬PCR芯片检测自噬相关基因(autophagy-related genes, ATGs)的表达,分析差异基因的功能富集,并与FoxO1进行互作分析,筛选差异基因进行芯片准确性验证;Western blot测试转录因子FoxO1和乙酰化FoxO1(acetylated FoxO1, Ac-FoxO1)的相对蛋白表达。(1)各组摄食量在干预后期趋于接近;干预4周后,HR组体重高于H组;与N组相比,R组大鼠瘦体重百分率和EDL湿重百分率显著升高,H组显著降低,而HR组的都显著高于H组(<0.05)。H组FCSA较N组显著降低(<0.05)。(2)与N组相比,H组自噬差异基因表达以上调为主,功能主要富集于自噬囊泡形成等过程;与H组相比,HR组自噬差异基因表达下调,功能富集于自噬与凋亡共调节过程;将差异基因与FoxO1进行互作分析,结果显示与有一级调控关系的基因主要富集于自噬与凋亡共调节过程。筛选在R/N组和HR/H组均下调的差异基因组蛋白脱乙酰酶1(histone deacetylase 1,),在R/N组和HR/H组均上调、在H/N组下调的差异基因哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,),和H/N组上调的一级调控关系的差异基因微管相关蛋白1轻链3A(microtubule-associated protein 1 light chain 3A,)进行蛋白表达验证,结果与芯片基本一致;(3)H组FoxO1和Ac-FoxO1蛋白水平及HDAC1/Ac-FoxO1比值显著高于N组,HR组FoxO1蛋白水平和HDAC1/Ac-FoxO1比值显著低于H组(<0.05)。抗阻训练可通过抑制自噬过程而缓解低氧诱导的大鼠EDL萎缩,FoxO1及其乙酰化修饰在该过程中可能发挥重要作用。
抗阻训练;低氧暴露;骨骼肌萎缩;自噬;叉头框蛋白O1
在多种运动形式中,抗阻训练对骨骼肌的刺激最为强烈,可以有效地增加骨骼肌质量,且这种作用具有肌纤维选择性,快肌纤维比慢肌纤维发生肥大的可能性高约50%[1]。抗阻训练已被证明是抵抗或缓解增龄性、废用性、胰岛素抵抗或糖尿病等所致肌萎缩的非药物治疗手段[2-3]。
竞技体育中高原/低氧训练,大众健身中的高原徒步旅行或低氧减肥使得更多世居平原者进入低氧环境,这会对代谢系统产生一系列不良影响,其中之一是骨骼肌萎缩[4]。实验室前期研究显示,在海拔高度3 650 m的拉萨暴露10天,可显著降低男性大学生体重、全身和腿部瘦体重、肌纤维横截面积(fiber cross-sectional area, FCSA)。而采用负重深蹲练习及其他辅助性抗阻练习则可有效缓解上述指标的下降[5],证明抗阻训练是缓解低氧诱导肌萎缩的有效措施。但该过程中的具体分子生物学机制尚不清楚。
抗阻训练促进肌肉肥大的作用机制通常被认为与增加骨骼肌蛋白质的合成有关。但在肌萎缩模型中,抗阻训练对蛋白分解的抑制效果可能在维持或增加骨骼肌质量中发挥着更关键的作用[6]。自噬是蛋白质分解的重要途径。在多种肌萎缩和肌营养不良症等肌病皆出现自噬的积累[7]。许多自噬相关基因(autophagy associated genes, ATGs)及其复合物参与自噬过程的各步骤[8]。转录因子叉头框蛋白O1(forkhead box protein O1, FoxO1)是FoxOs家族中一个重要的诱导自噬的成员,可参与自噬起始、囊泡成核和囊泡延伸等过程。siRNA基因敲除或抑制FoxO1的表达,可明显阻断自噬过程[9]。因此,我们推测FoxO1所介导的自噬途径可能在抗阻训练缓解低氧诱导的肌萎缩中起重要作用。
鉴于自噬过程是一个复杂的蛋白网络协同工作的过程,涉及众多ATGs。我们应用包含84个ATGs的PCR芯片作为自噬的研究工具[10],探讨抗阻训练缓解低氧诱导大鼠趾长伸肌(extensor digitorum longus, EDL;属于快肌)萎缩过程中所涉及的自噬阶段,及FoxO1的调节作用。
材料和方法
1 主要试剂
哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)抗体、组蛋白脱乙酰酶1(histone deacetylase 1, HDAC1)抗体和FoxO1抗体购于Abcam;微管相关蛋白1轻链3(microtubule-associated protein 1 light chain 3, LC3)抗体购于Novusbio;乙酰化FoxO1(acetylated FoxO1, Ac-FoxO1)抗体购于Biorbyt;α-tubulin抗体购于Sigma;荧光标记山羊抗鼠IgG、荧光标记山羊抗兔IgG和封闭液购自LI-COR;FITC标记山羊抗鼠IgG(GB22301)购于赛维尔公司;4%~12% Bis-Tris梯度胶、MES电泳缓冲液、NC膜等均购自Invitrogen。RNA提取、纯化所用试剂由沃吉基因提供并完成自噬PCR芯片(WC-MRNA0268-R)测试。
2 动物和分组干预
40只健康8周龄SPF级的雄性SD大鼠[起始体重为(236.40±10.69) g]购自北京维通利华实验动物技术有限公司,许可证号为SCXK(京)2015-0001。将大鼠随机分为4组(=10):常氧安静组(N组)、常氧训练组(R组)、低氧安静组(H组)和低氧训练组(HR组)。N组和R组大鼠置于常氧环境中,H组和HR组大鼠置于氧浓度为12.4%的低氧环境中(相当于海拔4 000 m高度)。低氧设备:制氮机(北京创文气体有限公司)、冻干机(杭州超滤净化设备有限公司)和空气压缩机(Ingersoll-Rand)。R组和HR组大鼠进行隔天一次递增负重的抗阻爬梯训练,具体方案为:爬梯长度为1.2 m,与地面呈85°放置,有效攀爬高度为1 m,将大鼠置于梯子底部,在其尾部给予适当的刺激,使大鼠从梯子底部爬到顶部,即为一次训练,控制每次攀爬时间在10 s内完成。负重通过调节大鼠尾部悬挂的离心管中钢珠数量来调整(以50%体重的负重适应性训练1周,正式训练开始从50%体重开始递增负重,每次增加10%,直至增加至130%体重,而后保持该负重,直至训练结束[11]),所有干预共持续4周。动物饲养和训练均在北京体育大学动物实验室进行,许可证号为SYXK(京)2016-0034。所有实验操作过程符合北京体育大学运动科学伦理委员会实验动物伦理要求。
干预期间每天记录各组大鼠的摄食量和体重,最后一次干预24 h后,3%戊巴比妥钠麻醉,双能X射线吸收测量法体成分仪(XR-46,Norland)测试体成分计算瘦体重百分率(肌肉质量/体重×100%)。麻醉后腹主动脉取血处死,分离大鼠两侧的EDL,一侧称量湿重,计算EDL湿重百分率(EDL湿重/肌肉总量×100%),后置于4%多聚甲醛固定液中固定;另一侧分为两份,一份置于RNAstore中保存,另一份置于-80 ℃冰箱保存。
3 主要方法
3.1苏木精-伊红(hematoxylin⁃eosin, HE)染色固定24 h后,经过浸蜡、包埋、切片后,进行二甲苯脱蜡、梯度乙醇浸泡、Harris苏木素和0.5%伊红分别染色、梯度乙醇浸泡、封片,显微镜下观察并拍照。采用Image-Pro Plus 6.0软件统计FCSA。
3.2自噬PCR芯片Trizol法提取RNA,紫外吸收测定法质检后,进行琼脂糖凝胶电泳,合成cDNA,高通量荧光定量qPCR检测84个ATGs表达。筛选差异表达基因,对差异基因进行相互作用网络和功能分析。
3.3Western blot检测蛋白相对含量组织中加入裂解液,匀浆仪研磨,离心取上清液得到蛋白原液。BCA法检测蛋白浓度,制备样品。梯度胶电泳分离蛋白,转膜后根据Marker条带及目的蛋白的分子量裁膜,封闭,4 °C孵育Ⅰ抗过夜,TBST洗涤,室温孵育Ⅱ抗1 h,用近红外光谱检测系统(Odyssey CLX,LI-COR)检测条带信号值,Image Studio 5.2软件进行相对定量分析。
4 统计学处理
PCR芯片结果用2-ΔΔCt法计算基因的差异表达倍数,检验计算值,以<0.05为差异有统计学意义,以差异倍数(fold change)>1.5为差异判断标准。其他结果使用SPSS 19.0软件分析,数据以均数±标准差(mean±SD)表示。各组间比较使用双因素方差分析,若两因素间有交互作用,使用简单效应检验;若无则采用最小显著性差异法(LSD法)进行组间检验。<0.05表示差异具有统计学意义。
结果
1 干预期间大鼠摄食量、体重和瘦体重百分率的变化结果
干预初期低氧组较常氧组摄食量显著下降,干预后期各组摄食量趋于接近,见图1A。干预期间,低氧各组大鼠平均体重显著低于常氧组;干预结束后,HR组体重高于H组,见图1B。4周干预后,R组瘦体重百分率显著高于N组,H组显著低于N组,HR组显著高于H组(<0.05),见图1C。
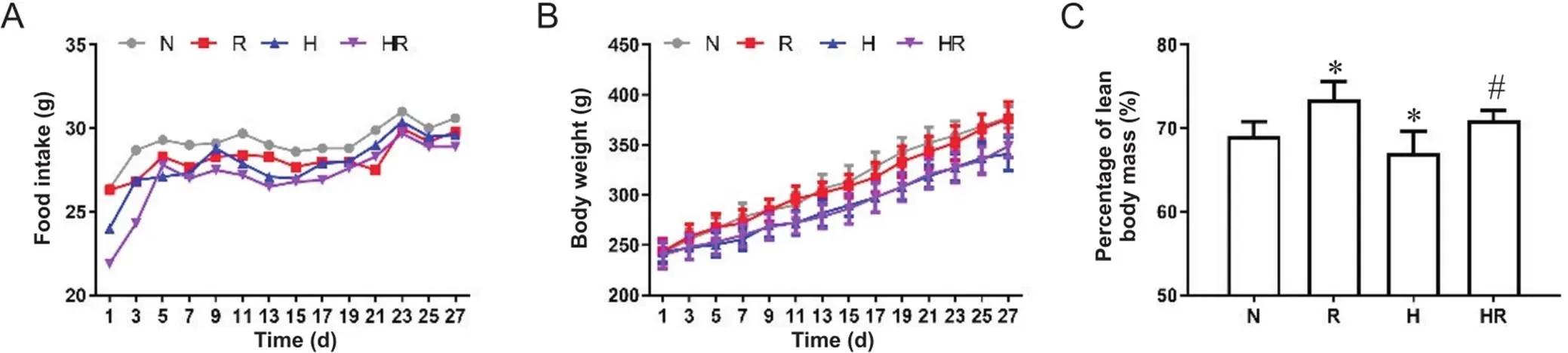
Figure 1. The changes of food intake (A) and weight (B) of rats during intervention, and the percentage of lean body mass after intervention (C). N: normoxic control group; R:normoxic resistance training group; H: hypoxic control group; HR: hypoxic resistance training group. Mean±SD. n=10. *P<0.05 vs Ngroup; #P<0.05 vs H hroup.
2 EDL湿重百分率和FCSA
4周干预后,R组EDL湿重百分率显著高于N组,H组显著低于N组,HR组显著高于H组(<0.05),见图2A、B;HE染色可见,H组肌纤维的肌间隔增加,呈现不规则的多边形,同一视野的肌纤维形状大小不一,肌纤维开始出现分裂,且细胞核出现内移(异常处如图2A中黄色箭头所示),H组FCSA较N组显著降低(<0.05),见图2C。
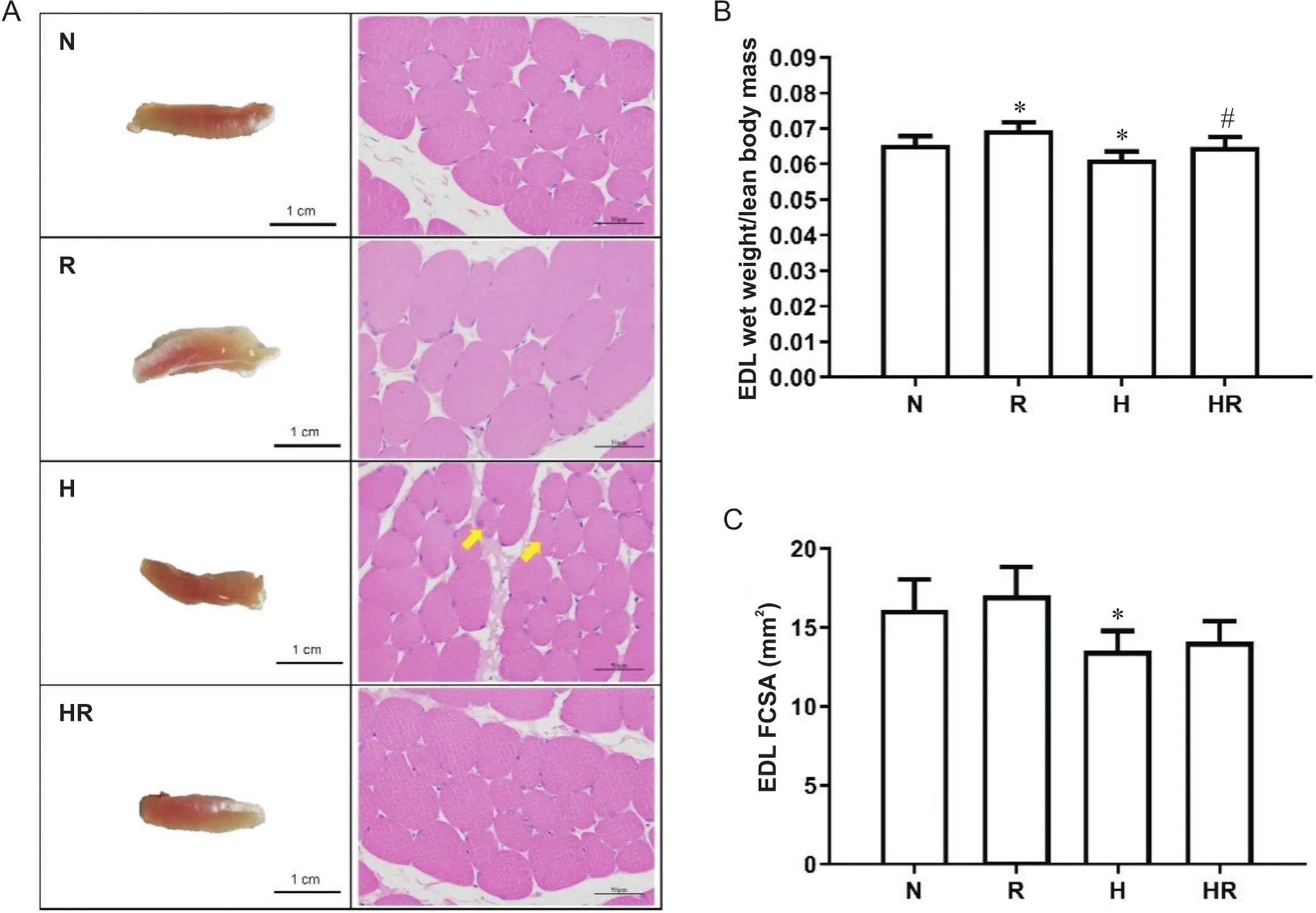
Figure 2. The percentage of wet weight and FCSA of EDL in rats after intervention. A: the macroscopic observation (scale bar=1 cm) and HE staining (scale bar=50 μm) of EDL; B: the ratio of EDL wet weight to lean body mass; C: the FCSA of EDL. Abnormal myofibers and nuclear inward migration were indicated by yellow arrows. N: normoxic control group; R: normoxic resistance training group; H: hypoxic control group; HR: hypoxic resistance training group. Mean±SD. n=10. *P<0.05 vs N group; #P<0.05 vs H group.
3 自噬PCR芯片结果
3.1自噬差异基因的表达R组与N组相比,自噬差异表达基因共有32个,其中上调基因有11个,下调21个,差异基因表达以下调为主,见图3A;H组与N组相比,差异基因共有29个,上调基因21个,下调基因8个,以上调为主,见图3B;HR组与H组相比,差异基因共有55个,上调基因5个,下调基因50个,以下调为主,见图3C。
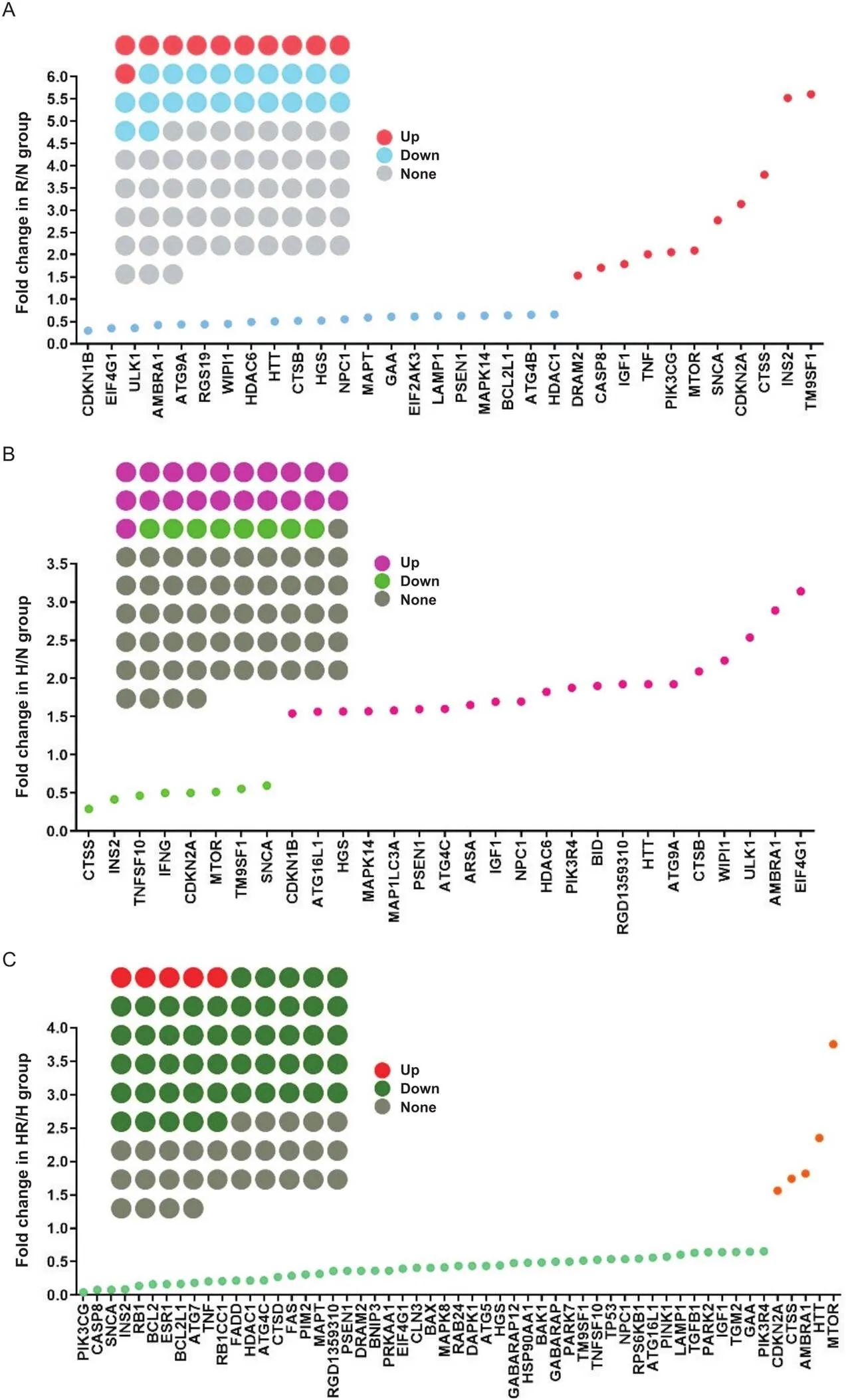
Figure 3. The number of EDL differential genes and their differential multiples in groups R/N (A), H/N (B) and HR/H (C) after intervention. The gray dots indicate genes whose fold difference is less than 1.5 or 0.67 (the same below). A: the blue dots indicate the down-regulated genes of group R/N, and the red ones indicate the up-regulated genes; B: the light green dots indicate the down-regulated genes of group H/N, and the pink ones indicate the up-regulated genes; C: the green dots indicate the down-regulated genes of group HR/H, and the orange ones indicate the up-regulated genes. n=3.
3.2各组共同差异基因韦恩图分析R/N组上调和HR/H组上调、R/N组下调和HR/H组下调、H/N组上调和HR/H组上调、H/N组上调和HR/H组下调、H/N组下调和HR/H组下调、H/N组下调和HR/H组上调之间的共同差异基因,基因数目见图4,详细信息见表1。
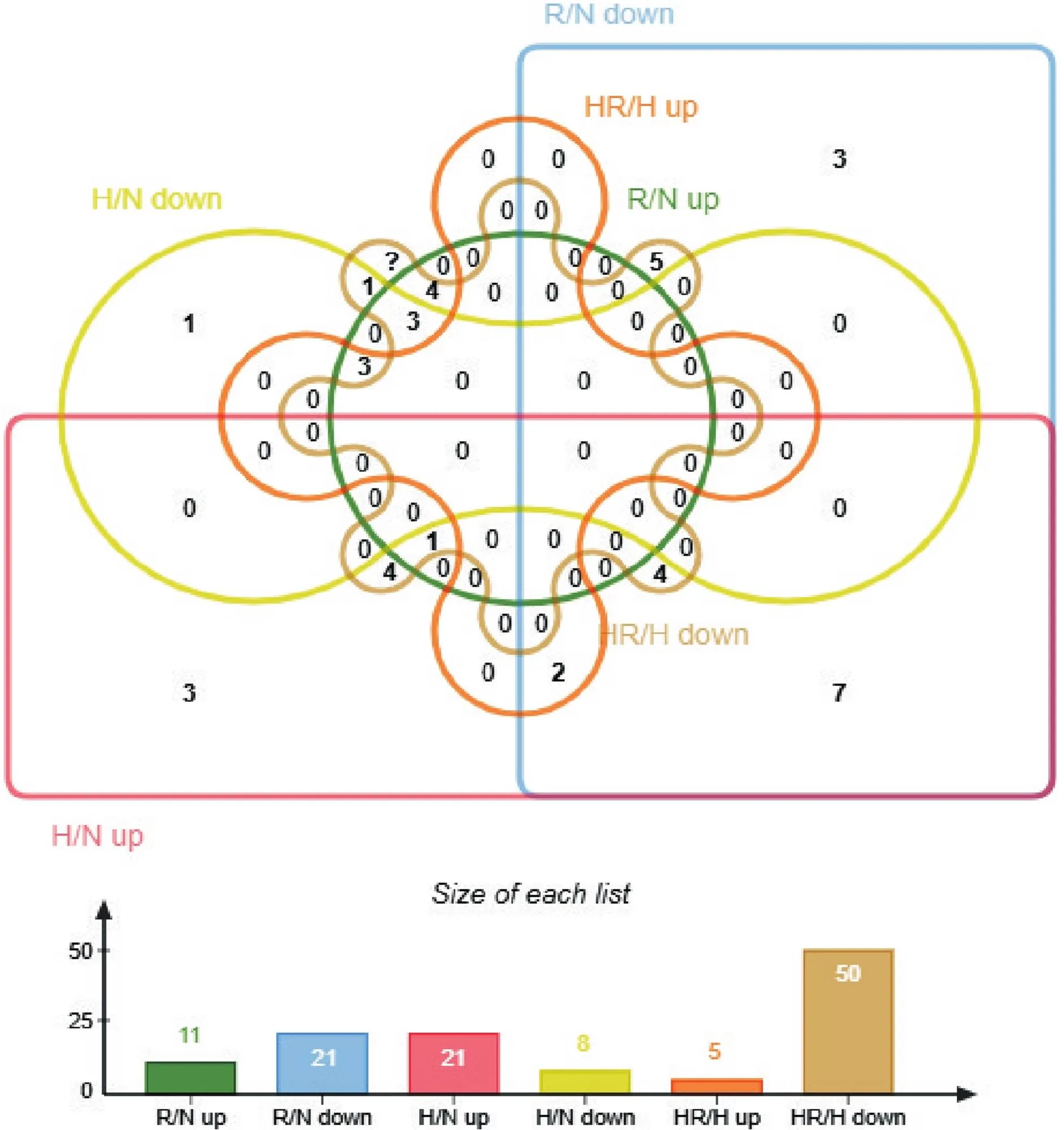
Figure 4. The number of common differential genes of EDL in groups R/N, H/N and HR/H after intervention. n=3.
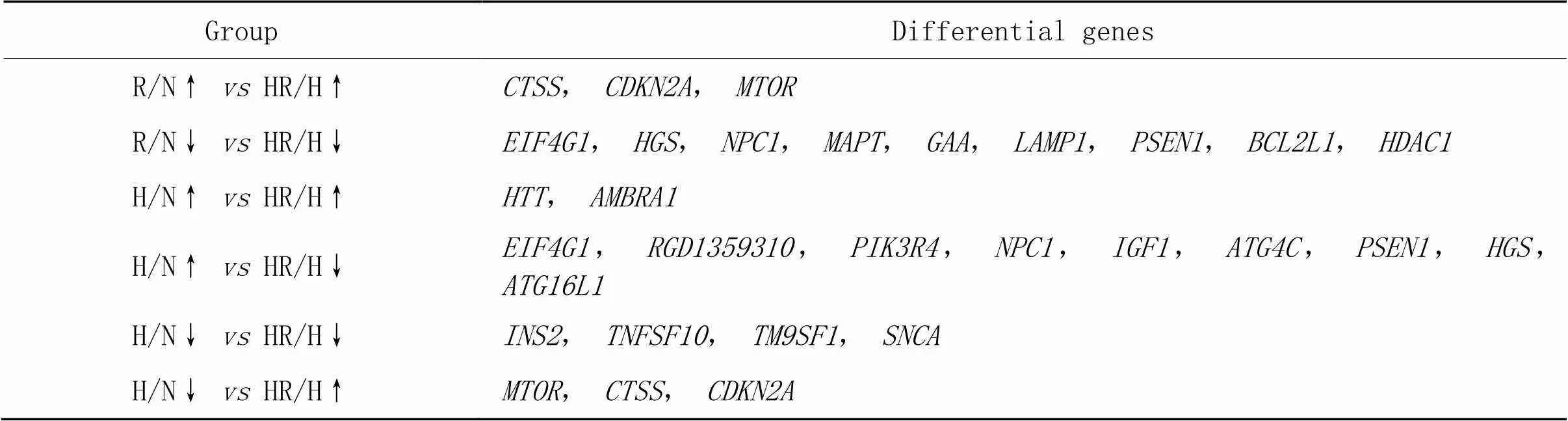
表1 R/N组、H/N组和HR/N组EDL共同差异基因
↑ indicates up-regulated genes; ↓ indicates down-regulated genes.
3.3各组差异基因数目及其所富集的自噬功能R/N组下调基因的功能主要集中在自噬囊泡的形成、自噬和凋亡共调节因子及自噬对其他细胞内信号的应答;H/N组上调基因的功能与R/N组下调基因功能一致;HR/H组下调基因的功能主要集中在自噬囊泡的形成、蛋白转运、自噬和凋亡共调节因子(自噬调节组分)、自噬和细胞周期共调节因子和自噬对其他细胞内信号的应答,见表2。
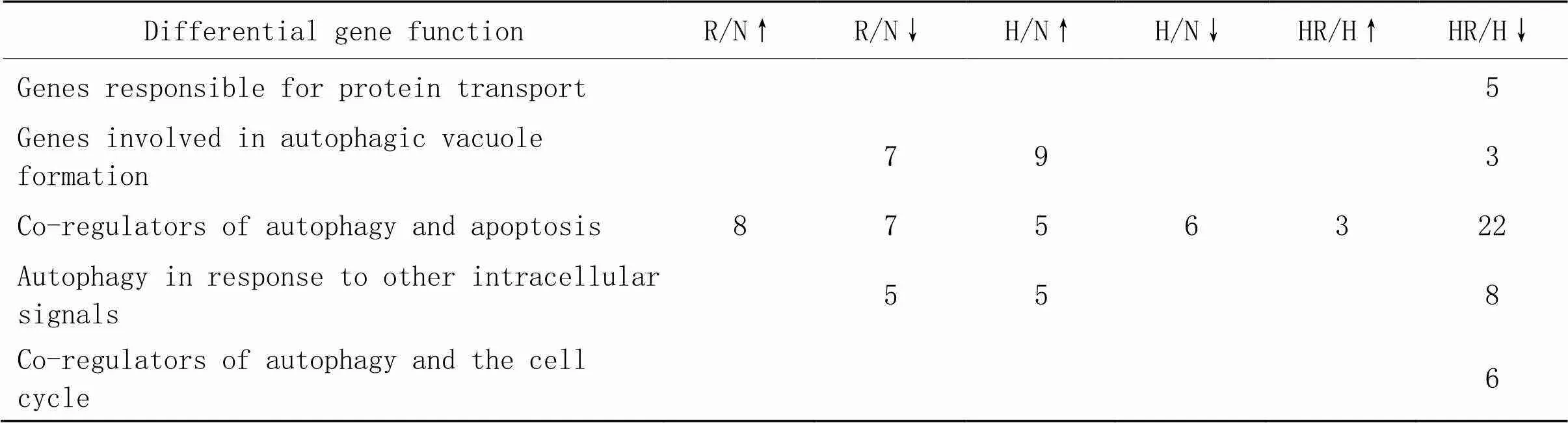
表2 R/N组、H/N组和HR/N组EDL差异基因所富集的自噬功能及基因数目
↑ indicates up-regulated genes; ↓ indicates down-regulated genes.
3.4各组差异基因与FoxO1相互作用网络分析STRING数据库分析R/N组下调(图5A)、H/N组上调(图5B)和HR/H组下调(图5C)差异基因与间的相互作用网络;表3为与存在一级互作关系的基因(的直接调控基因)。
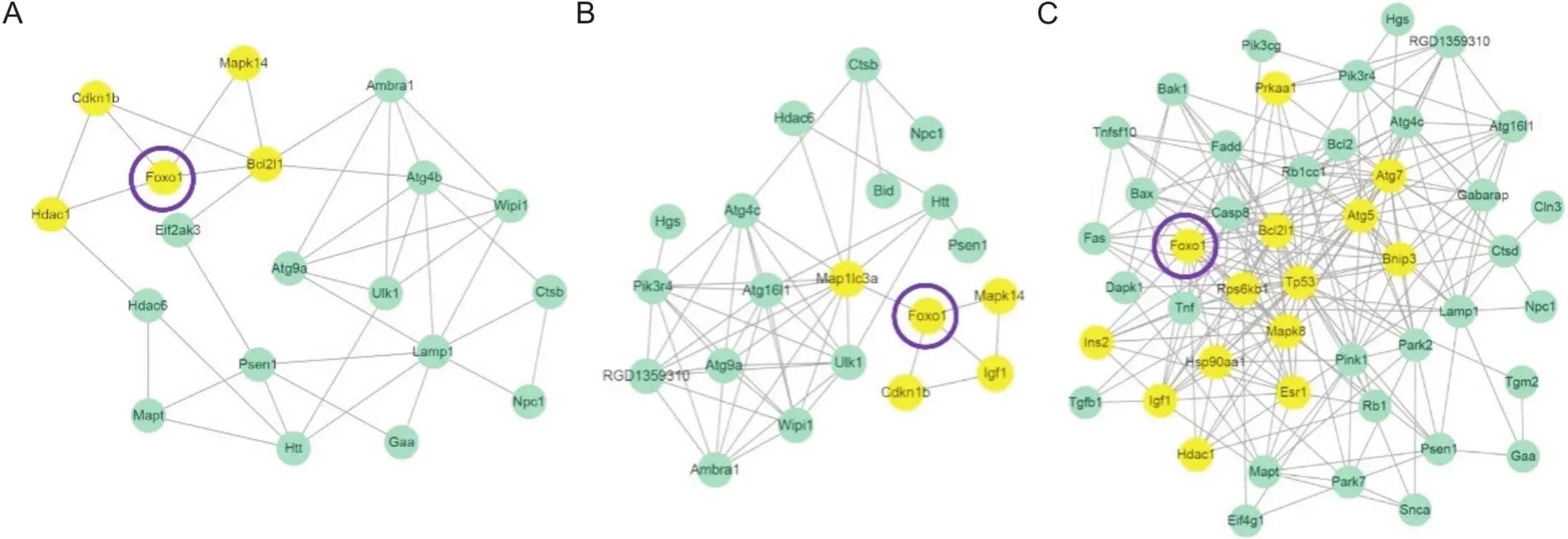
Figure 5. The network diagram of interaction between differential genes and FoxO1 in EDL of groups R/N (A), H/N (B) and HR/H (C) after intervention. The yellow circles are the primary interacting genes with FoxO1. n=3.
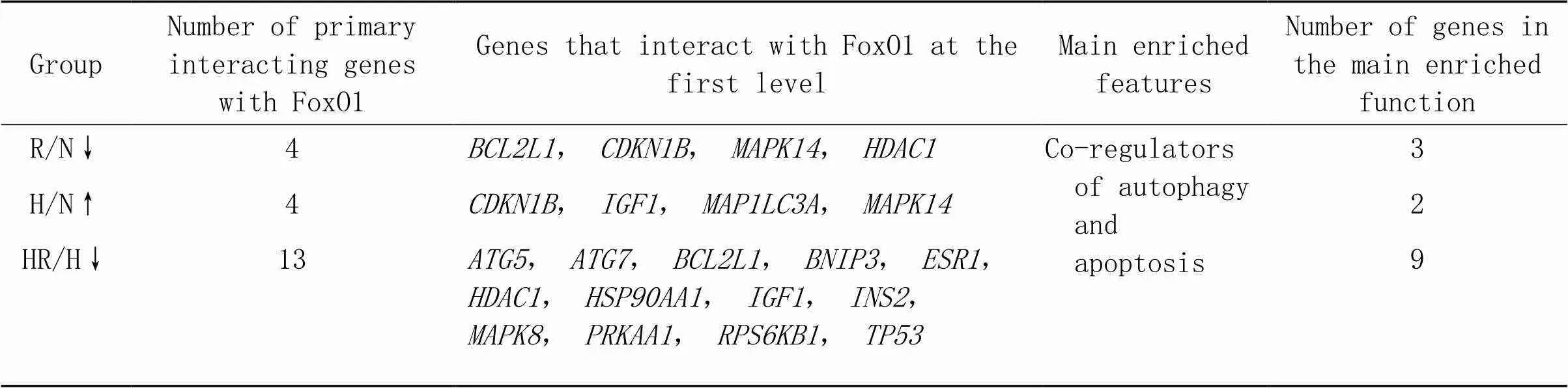
表3 R/N组、H/N组和HR/H组与FoxO1的一级互作基因及其功能
↑ indicates up-regulated genes; ↓ indicates down-regulated genes.
3.5差异基因蛋白表达的验证选择(R/N组上调、H/N组下调、HR/H组上调的差异基因),(R/N组下调和HR/H组下调的差异基因),(LC3; H/N组上调的差异基因)进行自噬PCR芯片结果的蛋白表达验证。R组mTOR蛋白相对表达量和LC3-II/LC3-I比值显著高于N组(<0.05);H组mTOR表达量显著低于N组,HDAC1表达量和LC3-II/LC3-I比值显著高于N组;HR组mTOR表达量显著高于H组,HDAC1表达量和LC3-II/LC3-I比值显著低于H组(<0.05),见图6。以上结果与芯片结果基本符合,证明了芯片结果的可靠性。
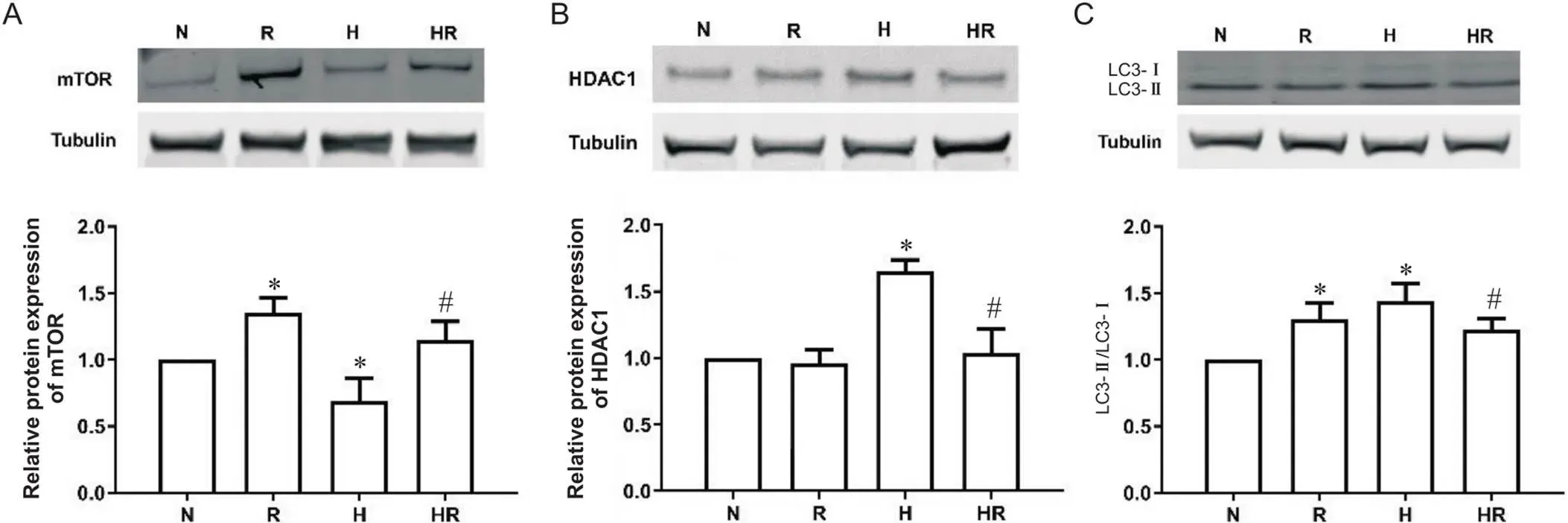
Figure 6. The relative protein expression levels of mTOR (A), HDAC1 (B) and LC3 (C) in EDL after intervention. N: normoxic control group; R: normoxic resistance training group; H: hypoxic control group; HR: hypoxic resistance training group. Mean±SD. n=10. *P<0.05 vs N group; #P<0.05 vs H group.
4 FoxO1及Ac-FoxO1蛋白水平
H组FoxO1和Ac-FoxO1蛋白水平显著高于N组,HR组FoxO1表达水平显著低于H组(<0.05);结合图6中HDAC1蛋白表达的结果,用HDAC1/Ac-FoxO1比值反映脱乙酰酶活性[12],H组HDAC1/Ac-FoxO1比值显著高于N组,HR组HDAC1/Ac-FoxO1比值显著低于H组(<0.05),见图7。
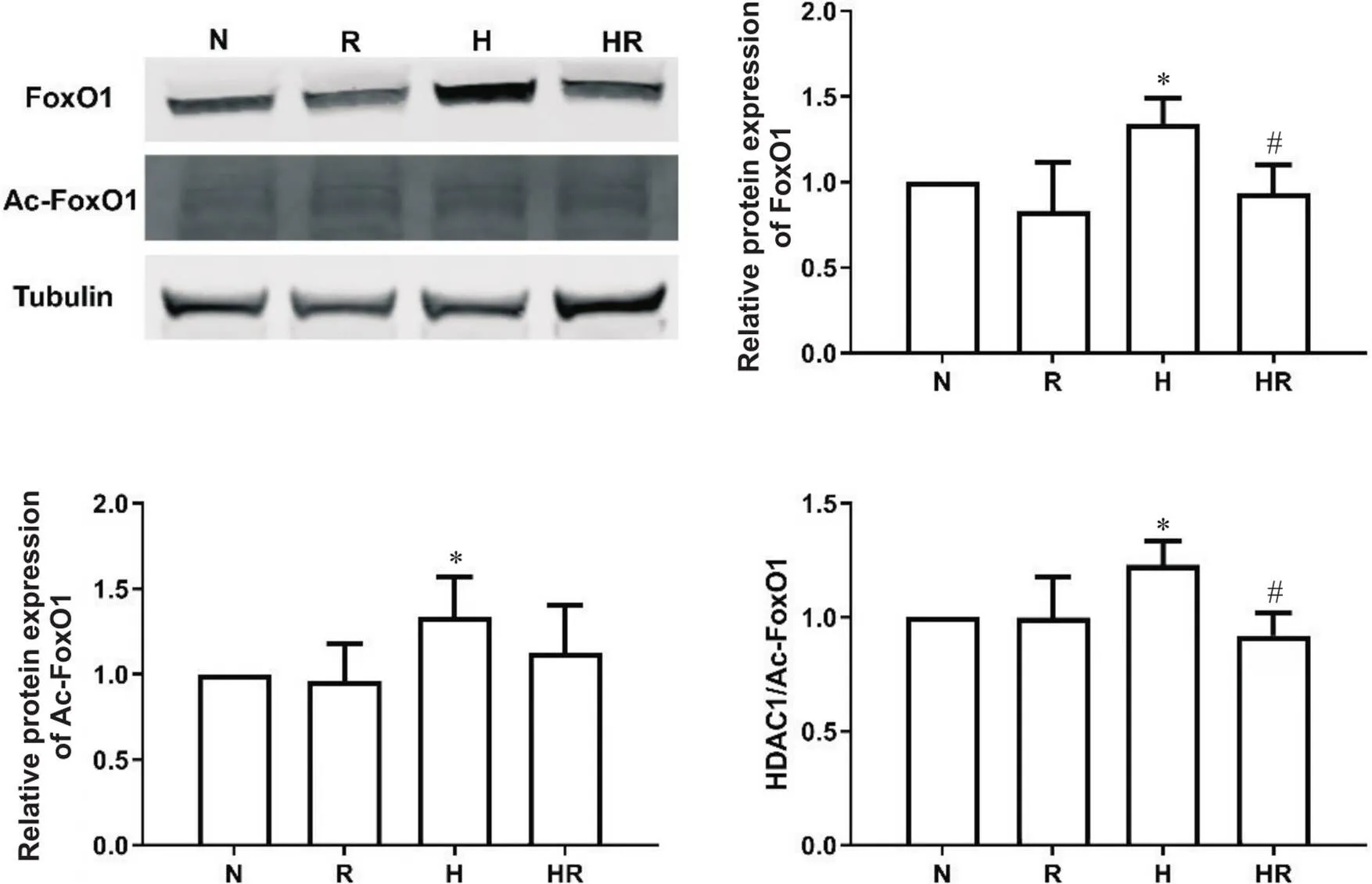
Figure 7. The relative protein levels of FoxO1 and Ac-FoxO1 in EDL after intervention. N: normoxic control group; R: normoxic resistance training group; H: hypoxic control group; HR: hypoxic resistance training group. Mean±SD. n=10. *P<0.05 vs N group; #P<0.05 vs H group.
讨论
为了进一步探究抗阻训练缓解低氧诱导肌萎缩的机制,本研究对低氧环境下大鼠施加负重爬梯训练干预,观察肌萎缩程度;以EDL作为快肌的典型代表,采用自噬PCR芯片,检测ATGs的变化,与FoxO1进行互作分析,并检测FoxO1及重要ATGs的相互关系,以探究FoxO1介导的自噬途径在其中的作用。负重爬梯训练是一种通过诱导刺激使大鼠在尾部负重的情况下,自觉完成爬梯以促进骨骼肌肥大的训练模型,是目前所知的与人类抗阻运动最接近的训练方式[13]。本研究结果显示,低氧下进行抗阻训练可提高大鼠体重,增加瘦体重百分含量和EDL湿重。证明已成功建立抗阻训练环境低氧诱导肌萎缩的动物模型。
在低氧环境下,骨骼肌蛋白质代谢增加,表现为合成代谢和分解代谢均增加,但分解代谢率远高于合成,是诱导肌萎缩的关键。作为重要的蛋白质分解途径,自噬过程受到许多细胞途径和进化保守的ATGs调控,主要过程包括:自噬的启动、自噬体的形成、自噬体和溶酶体的融合与降解[14]。本研究PCR芯片结果显示,低氧暴露下,EDL中的自噬差异基因表达以上调为主,说明低氧可激活自噬过程;分析差异基因功能可知,上调基因的功能主要富集于ALP前期的自噬囊泡形成阶段。LC3前体可被加工为可溶性的LC3-I,而后在Atg3和Atg7的作用下与磷脂酰乙醇胺(phosphatidylethanolamine, PE)结合形成脂溶性的LC3-II-PE,调节自噬过程,LC3-II/LC3-I是衡量自噬通量的关键指标。本研究中低氧暴露增加了LC3-II/LC3-I比值。以上结果均提示低氧可能通过激活自噬前期过程而促进蛋白质分解,以降低骨骼肌质量。
研究指出规律性的重复运动可提高自噬的基线水平,从而降低应激刺激对自噬的激活的阈值,使机体免受自噬的负面影响[15]。常氧环境下,8周的抗阻爬梯训练可显著降低LC3-II/LC3-I比值,增加p62蛋白表达,提示长期规律性的抗阻训练具有调节骨骼肌自噬水平的作用[16]。本研究中,低氧下进行抗阻训练,EDL中的差异基因以下调为主,差异基因的功能主要富集于自噬调节组分中的自噬与凋亡的共调节过程,且降低LC3-II/LC3-I比值。提示抗阻训练可通过降低自噬与凋亡的共调节作用而抑制低氧诱导肌萎缩。在应激条件下,细胞自噬和细胞凋亡之间存在交叉抑制和激活的相互作用,但某些特定的应激也会共同激活自噬和凋亡,两种机制的相互关系在调节蛋白稳态、骨骼肌萎缩细胞死亡中起重要作用[17-18]。
FoxO1在骨骼肌质量的调控中处于中心位置,也是FoxOs家族中重要的诱导细胞自噬成员,FoxO1可参与自噬起始、囊泡成核和囊泡延伸的过程。作为自噬的重要媒介,FoxO1可以直接上调ATGs的表达;还可以通过调节sestrin 3 (SESN3),以刺激结节性硬化症蛋白1-2(tuberous sclerosis 1-2,TSC1-2)复合物抑制mTOR途径,促进自噬过程[19]。本研究结果中与各组自噬差异基因间均存在相互作用,说明FoxO1可能在自噬介导的肌肉质量调节中发挥着重要作用。常氧环境下的抗阻训练可以通过抑制骨骼肌中FoxO1表达以抵抗增龄性肌萎缩的发生[20]。本研究中,HR/H组下调的差异基因中约26%与存在一级互作关系。我们对在R/N组和HR/H组均上调、在H/N组下调的差异基因进行了蛋白表达的验证,结果显示低氧下抗阻训练可增加mTOR的蛋白表达,mTOR表达增加可抑制自噬过程。说明抗阻训练可能通过FoxO1对自噬过程的直接和间接调节过程而缓解低氧所诱导的肌萎缩过程。
分析各组的差异基因结果显示,在R/N组和HR/H组均下调表达。HDAC1不仅是可促进自噬囊泡的形成以增加自噬通量[21],还可增加FoxO1在mRNA和蛋白质水平上的表达,且可增强FoxO1的转录活性[19]。因此,我们检测了FoxO1及其乙酰化水平,以及HDAC1的活性,结果显示,低氧暴露下,FoxO1和Ac-FoxO1蛋白水平及HDAC1/Ac-FoxO1比值均显著增加,而低氧下抗阻训练可降低FoxO1表达量和HDAC1/Ac-FoxO1比值。这提示抗阻训练介导的FoxO1及其乙酰化修饰可能在调控低氧诱导骨骼肌萎缩中发挥了重要的作用。
综上所述,低氧暴露4周可导致大鼠瘦体重下降,EDL发生萎缩,自噬囊泡形成阶段基因表达增加,该过程伴随着FoxO1表达及其乙酰化水平升高;递增负重的爬梯抗阻训练可有效缓解低氧所致的大鼠肌萎缩,其作用机制可能为:FoxO1调控下自噬与凋亡共调节过程相关基因表达降低,抑制骨骼肌蛋白质的分解。
[1] Ogborn D, Schoenfeld BJ. The role of fiber types in muscle hypertrophy: implications for loading strategies[J]. StrengthCondJ, 2014, 36(2):20-25.
[2] Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart[J]. Circ Res, 2007, 100(10):1512-1521.
[3] Mark R, Hannah L, Lawrence H, et al. Potential cellular and biochemical mechanisms of exercise and physical activity on the ageing process[J]. Subcell Biochem, 2019, 91:311-338.
[4] Edwards LM, Murray AJ, Tyler DJ, et al. The effect of high-altitude on human skeletal muscle energetics:31P-MRS results from the caudwell xtreme everest expedition[J]. PloS One, 2010, 5(5):e10681.
[5]王宁琦, 包大鹏, 龚丽景, 等. 短期高原抗阻练习对人骨骼肌的影响及基因芯片分析[J]. 中国运动医学杂志, 2021, 40(2):98-108.
Wang NQ, Bao DP, Gong LJ, et al. The effect of short-term high-altitude resistance exercise on skeletal muscle and its mRNA microarray analysis[J]. Chin J Sports Med, 2021, 40(2):98-108.
[6] Yoo SZ, No MH, Heo JW, et al. Role of exercise in age-related sarcopenia[J]. J Exerc Rehabil, 2018, 14(4):551-558.
[7] Dikic I. Proteasomal and autophagic degradation systems[J]. Annu Rev Biochem, 2017, 86:193-224.
[8] Klionsky DJ, Codogno P. The mechanism and physiological function of macroautophagy[J]. JInnate Immun, 2013, 5(5):427-433.
[9] He W, Zhang A, Qi L, et al. FoxO1, a potential therapeutic target, regulates autophagic flux, oxidative stress, mitochondrial dysfunction, and apoptosis in human cholangiocarcinoma QBC939 cells[J]. CellPhysiolBiochem, 2018, 45(4):1506-1514.
[10] Chen X, Chen X, Huang Y, et al. TCP1 increases drug resistance in acute myeloid leukemia by suppressing autophagy via activating AKT/mTOR signaling[J]. Cell Death Dis, 2021, 12(11):1-11.
[11] Lee S, Kim K, Lambrecht N J, et al. Interaction of resistance training, electroacupuncture and Huang Qi supplementation on skeletal muscle function and GLUT4 protein concentration in rats[J]. AcupunctMed, 2016, 34(5):380-385.
[12] 舒晴. 电针调控下丘脑弓状核SIRT1/FoxO1抑制摄食改善胰岛素抵抗肥胖的机制研究[D]. 武汉: 湖北中医药大学, 2017:20-22.
Shu Q. The mechanism of electroacupuncture inhibiting feeding to improve obesity with insulin resistance via regulating SIRT/FoxO1 signaling pathway in hypothalamic arcuate nucleus[D]. Wuhan: Hubei University of Chinese Medicine, 2017:20-22.
[13] Hellyer NJ, Nokleby JJ, Thicke BM, et al. Reduced ribosomal protein s6 phosphorylation after progressive resistance exercise in growing adolescent rats[J]. J Strength Cond Res, 2012, 26(6):1657-1666.
[14] Neel BA, Lin Y, Pessin JE. Skeletal muscle autophagy: a new metabolic regulator[J]. Trends Endocrin Met, 2013, 24(12):635-643.
[15] Lira VA, Okutsu M, Zhang M, et al. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance[J]. FASEB J, 2013, 27(10):4184-4193.
[16] Kwon I, Jang YC, Cho JY, et al. Autophagy is inhibited in hypertrophied skeletal muscle in response to 8 weeks of progressive resistance exercise[J]. FASEB J, 2016, 30(Suppl 1):1245.30.
[17] Bargiela A, Cerro-Herreros E, Fernandez-Costa JM, et al. Increased autophagy and apoptosis contribute to muscle atrophy in a myotonic dystrophy type 1 Drosophila model[J]. Dis Model Mech, 2015, 8(7):679-690.
[18]龚丽景, 贾杰, 孙民康, 等. 短期间歇和急性低氧对大鼠快慢肌萎缩的影响及其作用机制[J]. 中国病理生理杂志, 2022, 38(2):238-249.
Gong LJ, Jia J, Sun MK, et al. Effects of short-term intermittent and acute hypoxia on fast- and slowtwitch muscle atrophy in rats and its mechanism[J]. Clin J Pathophysiol, 2022, 38(2):238-249.
[19] Zhang J, Ng S, Wang J, et al. Histone deacetylase inhibitors induce autophagy through FOXO1-dependent pathways[J]. Autophagy, 2015, 11(4):629-642.
[20] Mbt R, Guzzoni V, Hord JM, et al. Resistance training regulates gene expression of molecules associated with intramyocellular lipids, glucose signaling and fiber size in old rats[J]. Sci Rep, 2017, 7(1):8593.
[21] Körholz K, Ridinger J, Krunic D, et al. Broad-spectrum HDAC inhibitors promote autophagy through FOXO transcription factors in neuroblastoma[J]. Cells, 2021, 10(5):1001.
Effects of forkhead box protein O1-mediated autophagy pathway on resistance training alleviating hypoxia-induced muscle atrophy in rats
FU Peng-yu1,2, YU Jing-jing3, GONG Li-jing2,3△
(1,,710072,;2,,100084,;3,,,100084,)
To explore the effects of resistance training on alleviating hypoxia-induced muscle atrophy in rats, and the regulatory mechanism of forkhead box protein O1 (FoxO1)-mediated autophagy pathway during this process.Forty SD rats were randomly divided into 4 groups: normoxic control group (N group), normoxic resistance training group (R group), hypoxic control group (H group) and hypoxic resistance training group (HR group). The rats in the H and HR groups were placed in 12.4% O2chamber, and the rats in the R and HR groups
incremental weight-bearing ladder training every other day. In 4 weeks, the food intake and body weight were recorded every day. The body composition and the wet weight of the extensor digitorum longus (EDL) of rats in four groups were measured after intervention. The morphological changes of muscle fibers were observed by HE staining and the fiber cross-sectional area (FCSA) of EDL was measured. The expression level of autophagy-related genes (ATGs) was tested by autophagy PCR array, and the functional enrichment and the interactions between differentially expressed ATGs and FoxO1 were analyzed. To validate the accuracy of PCR array, the protein expression of several differential genes were detected. And the expression of FoxO1 and acetylated FoxO1 (Ac-FoxO1) were detected by Western blot.(1) The body weight of rats in the HR group was higher than that in the H group after intervention. The food intake of rats in four groups showed no significant difference in the late period of intervention. Compared with the N group, the percentages of lean body mass and EDL wet weight of rats in the R group increased significantly, and decreased in the H group significantly (<0.05), while the percentages of lean body mass and EDL wet weight of rats in the HR group were both significantly higher than the H group after intervention (<0.05). The FCSA of rat EDL in the H group was significantly lower than that in the N group (<0.05). (2) Compared with the N group, the expression of autophagy differential genes in EDL was mainly up-regulated in the H group, and the function was mainly enriched in the process of autophagic vacuole formation. Compared with the H group, the expression of autophagy differential genes in EDL was mainly down-regulated in the HR group, and the function was mainly enriched in the co-regulators of autophagy and apoptosis. The interaction within the primary regulatory relationship showed that, the differential genes were mainly enriched in the co-regulators process of autophagy and apoptosis. The differential gene histone deacetylase 1 () was both down-regulated in groups R/N and HR/H, mammalian target of rapamycin () was both up-regulated in groups R/N and HR/H, but down-regulated in groups H/N, and microtubule-associated protein 1 light chain 3A () was down-regulated in group H/N. The expression levels of these proteins were basically consistent with the results of PCR array. (3) The protein levels of FoxO1 and Ac-FoxO1 and the ratio of HDAC1/Ac-FoxO1 in the H group were significantly higher than those in the N group, while the protein expression of FoxO1 and the ratio of HDAC1/Ac-FoxO1 in the HR group were significantly lower than those in the H group (<0.05).Resistance training alleviates EDL atrophy in rats caused by hypoxiainhibiting autophagy, and FoxO1 and its acetylation may play an important role in this process.
Resistance training; Hypoxic exposure; Skeletal muscle atrophy; Autophagy; Forkhead box protein O1
1000-4718(2022)09-1667-10
2022-05-06
2022-08-05
010-62989303; E-mail: lijing.gong@bsu.edu.cn
Q445; R363.2+1
A
10.3969/j.issn.1000-4718.2022.09.017
[基金项目]中央高校基本科研业务费专项资金资助课题(No. D5000220377; No. 2021TD012)
(责任编辑:李淑媛,罗森)
