黄芪甲苷通过调控线粒体功能抑制H2O2诱导的SH-SY5Y细胞凋亡*
2022-10-13于婧文郭敏芳李苏垚孟涛张海飞杨德斌宋丽娟马存根尉杰忠
于婧文, 郭敏芳, 李苏垚, 孟涛, 张海飞, 杨德斌,宋丽娟,3, 马存根,△, 尉杰忠,,4△
黄芪甲苷通过调控线粒体功能抑制H2O2诱导的SH-SY5Y细胞凋亡*
于婧文1, 郭敏芳1, 李苏垚2, 孟涛1, 张海飞1, 杨德斌1,宋丽娟2,3, 马存根1,2△, 尉杰忠1,2,4△
(1山西大同大学脑科学研究所/附属第一医院神经科,山西 大同 037009;2山西中医药大学国家中医药管理局多发性硬化益气活血重点研究室/神经生物学研究中心,山西 晋中 030619;3山西医科大学生理学系,山西 太原 030001;4山西大同市第四人民医院,山西 大同 037009)
探讨黄芪甲苷(astragaloside IV, AST IV)对由氧化应激损伤引起的线粒体功能障碍以及细胞凋亡的作用及机制。体外培养人神经母细胞瘤SH-SY5Y细胞,用过氧化氢(hydrogen peroxide, H2O2)诱导建立氧化应激模型,分为PBS组、模型组(H2O2组)和H2O2+AST IV组。应用ATP检测试剂盒检测细胞内ATP水平;用线粒体膜电位检测试剂盒JC-1检测细胞线粒体膜电位变化;应用Western blot法检测线粒体呼吸链上的complex I~V,分别为NADH:泛醌氧化还原酶亚基B8(NADH:ubiquinone oxidoreductase subunit B8, NDUFB8; complex I)、琥珀酸脱氢酶B(succinate dehydrogenase B, SDHB; complex II)、泛醇-细胞色素C还原酶核心蛋白2(ubiquinol-cytochrome C reductase core protein 2, UQCRC2; complex III)、细胞色素C氧化酶I(mitochondrially encoded cytochrome C oxidase I, MTCO1; complex IV)和ATP合酶F1亚基α(ATP synthase F1 subunit alpha, ATP5A; complex V),检测线粒体动力学分裂蛋白——磷酸化发动蛋白相关蛋白1(phosporylated dynamin-related protein 1, p-Drp1)和线粒体分裂蛋白1(mitochondrial fission protein 1, Fis1),以及融合蛋白——线粒体融合蛋白1(mitofusin 1, Mfn1)、Mfn2和视神经萎缩症蛋白1(optic atrophy protein 1, OPA1),检测凋亡相关蛋白Bcl-2、Bax和cleaved caspase-3;采用免疫荧光染色法检测NDUFB8和MTCO1表达;采用TUNEL染色检测细胞凋亡。在200 μmol/L H2O2诱导的SY5Y细胞氧化应激模型中,线粒体膜电位(<0.01)和ATP水平(<0.05)显著下调,呼吸链氧化磷酸化过程中NDUFB8、SDHB、ATP5A和MTCO1的表达均显著减少(<0.05或<0.01),线粒体分裂蛋白Fis1(<0.05)和p-Drp1(<0.01)蛋白水平显著升高,融合蛋白Mfn1、Mfn2和OPA1表达则显著降低(<0.05或<0.01),促凋亡蛋白Bax和cleaved caspase-3蛋白水平显著升高(<0.01),抗凋亡蛋白Bcl-2表达量显著降低(<0.01)。AST IV能够显著提高细胞线粒体膜电位(<0.01)和ATP水平(<0.01),显著促进呼吸链氧化磷酸化过程中NDUFB8、SDHB、MTCO1、ATP5A及UQCRC2的表达(<0.05或<0.01),显著降低p-Drp1和Fis1蛋白水平(<0.01),显著增加OPA1、Mfn1和Mfn2的表达(<0.05或<0.01),显著增加Bcl-2表达(<0.05),显著降低Bax和cleaved caspase-3蛋白水平(<0.01)。AST IV能够保护神经元,其可能的机制是通过改善线粒体功能,调控线粒体动力学分裂/融合平衡,从而减轻氧化应激损伤导致的神经元凋亡。
黄芪甲苷;氧化应激;线粒体;SH-SY5Y细胞
线粒体是一个动态的细胞器,不断进行分裂融合维持自身的形态、功能以及整个细胞的功能,分裂和融合的异常是导致线粒体功能障碍的重要因素。细胞氧化损伤和活性氧(reactive oxygen species, ROS)的增加,使线粒体成为容易被攻击的靶点[1]。线粒体形态结构和功能变化与内源性的神经元凋亡有密切的关系[2-4]。在神经元受损时,线粒体形态由管状变为颗粒状, 线粒体外膜通透性发生改变,同时释放凋亡因子,激活凋亡信号通路,促进细胞凋亡[2],而在凋亡过程中会发生过度的线粒体分裂。在帕金森病(Parkinson disease, PD)、阿尔茨海默病(Alzheimer dissease, AD)、肌萎缩侧索硬化症(amyotraphic lateral sclerosis, ALS)及亨廷顿病(Huntington disease, HD)等疾病的早期,会发生线粒体功能障碍[3-6]。通过各种细胞系中线粒体分裂和融合、氧化磷酸化和能量代谢的研究证实,线粒体动力学会影响神经的可塑性,线粒体动力学基因改变,能量代谢异常可能是AD和PD发病的病理机制[3-4]。如果能在疾病早期发现并改善线粒体功能障碍,则可以为治疗提供时间窗,以推迟或逆转症状[2]。
在祖国传统医学中,黄芪有“十方八芪”之美称。黄芪甲苷(astragaloside IV, AST IV)是黄芪的主要活性成分之一,具有较强的抗氧化作用。研究表明,其可以有效减轻妊娠期糖尿病小鼠胎盘氧化应激[7]和子痫前期大鼠氧化应激[8],改善氧化应激介导的心血管疾病内皮功能障碍[9]和氨诱导的牛乳腺上皮细胞氧化应激[10]等。我们的前期研究也证实,AST IV可以通过抑制炎性小胶质细胞极化抑制其介导的神经元凋亡,但其是否可以通过调节线粒体功能、线粒体动力学与氧化磷酸化来改善氧化应激造成的神经元损伤尚不清楚。
本研究应用过氧化氢(hydrogen peroxide, H2O2)诱导的人神经母细胞瘤SH-SY5Y细胞作为神经细胞氧化应激模型,研究AST IV对线粒体功能、代谢及动力学的影响,探讨其保护神经元的作用机制,为AST IV保护线粒体提供参考资料。
材料和方法
1 材料
SH-SY5Y细胞株购自国家生物医学实验细胞资源库。AST IV(纯度>98%)和羧甲纤维素钠购自上海阿拉丁生化公司;细胞蛋白提取试剂盒、ATP检测试剂盒、线粒体膜电位检测试剂盒JC-1、TUNEL细胞凋亡检测试剂盒均购自上海碧云天生物技术有限公司;兔抗磷酸化发动蛋白相关蛋白1(phosporylated dynamin-related protein 1, p-Drp1)、线粒体分裂蛋白1(mitochondrial fission protein 1, Fis1)、线粒体融合蛋白1(mitofusin 1, Mfn1)、Mfn2和视神经萎缩症蛋白1(optic atrophy protein 1, OPA1)单克隆抗体,以及小鼠抗NADH:泛醌氧化还原酶亚基B8(NADH:ubiquinone oxidoreductase subunit B8, NDUFB8; complex I)、琥珀酸脱氢酶B(succinate dehydrogenase B, SDHB; complex II)、泛醇-细胞色素C还原酶核心蛋白2(ubiquinol-cytochrome C reductase core protein 2, UQCRC2; complex III)、细胞色素C氧化酶I(mitochondrially encoded cytochrome C oxidase I, MTCO1; complex IV)和ATP合酶F1亚基α(ATP synthase F1 subunit alpha, ATP5A; complex V)单克隆抗体均购自Abcam;兔抗Bcl-2单克隆抗体、兔抗Bax单克隆抗体、兔抗cleaved caspase-3和兔抗GAPDH单克隆抗体均购自Cell Signaling Technology;辣根过氧化物酶(horseradish peroxidase, HRP)标记的山羊抗兔IgG、HRP标记的山羊抗小鼠IgG和DyLight®594标记的山羊抗小鼠IgG均购自ArthOx;高糖DMEM培养液和胎牛血清(fetal bovine serum, FBS)购自Gibco;青霉素、链霉素和谷氨酰胺均购自HyClone;脱脂奶粉和牛血清白蛋白(bovine serum albumin, BSA)购自Thermo。
2 方法
2.1细胞培养将SH-SY5Y细胞培养于8 cm培养皿中,用含10% FBS、1×105U/L青霉素、100 mg/L链霉素和10 g/L谷氨酰胺的DMEM培养液培养,置于37 ℃、5% CO2细胞培养箱中培养。细胞培养条件参照细胞资源库培养说明。隔天换液1次,细胞汇合度至约80%,用0.125%的胰蛋白酶消化并传代,选取对数生长期细胞进行以下实验。
2.2细胞分组及给药实验分为PBS组、模型组(H2O2组)和H2O2+AST IV组。H2O2组加入200 μmol/L H2O2刺激24 h[11-12],建立氧化应激损伤模型,H2O2+AST IV组在加入H2O2之前2 h加入25 μmol/L AST IV共孵育24 h。
2.3线粒体膜电位检测6孔板细胞加药培养24 h后,吸除培养液,按照线粒体膜电位检测产品说明书(C2006),加入1 mL细胞培养液,再加入1 mL提前配制好的JC-1染色工作液,充分混匀,细胞培养箱中37 ℃孵育20 min。孵育结束后,吸除上清,用JC-1染色缓冲液洗涤2次。加入2 mL细胞培养液,在激光共聚焦显微镜下观察。检测JC-1单体时,把激发光设置为488 nm;检测JC-1聚合物时,把激发光设置为594 nm。出现绿色荧光说明线粒体膜电位下降,并且该细胞很可能处于细胞凋亡早期。出现红色荧光说明线粒体膜电位比较正常,细胞的状态也比较正常。常用红绿荧光的相对比例来衡量线粒体去极化的比例。
2.4细胞内ATP水平的测定6孔板细胞加药培养24 h后,吸除培养液,加入RIPA裂解液,使细胞充分裂解,4 ℃、12 000×离心5 min,取上清,用于ATP的测定。按试剂盒说明书(S2006)配置ATP检测工作液,同时用ATP标准溶液制备标准曲线。先在检测孔内加100 μL ATP检测工作液,室温放置3~5 min,消除本底影响。再分别加适量样品或标准品,混匀,用化学发光仪测相对光单位RLU值。
2.5Western blot法检测呼吸链氧化磷酸化复合体蛋白、线粒体分裂融合蛋白和细胞凋亡蛋白的表达6孔板细胞加药培养24 h后,吸除培养液,加入RIPA裂解液,使细胞充分裂解,4 ℃、12 000×离心5 min,取上清液,用Nanodrop测定蛋白质含量,并调整蛋白浓度。配制10%聚丙烯酰胺凝胶,上样蛋白量为30 μg,电泳分离蛋白质后,湿式转移法转移到PVDF膜。5%脱脂奶粉溶液封闭2 h,分别加入1∶1 000稀释的兔抗p-Drp1、Fis1、Mfn1、Mfn2、OPA1、Bcl-2、Bax和cleaved caspase-3单克隆抗体,以及小鼠抗NDUFB8、SDHB、UQCRC2、MTCO1和ATP5A单克隆抗体,并选取兔抗GAPDH(内参照)单克隆抗体(1∶5 000),4 ℃孵育过夜;洗涤后,加入HRP标记的山羊抗兔IgG或HRP标记的山羊抗小鼠IgG(1∶10 000),室温孵育2 h。洗膜后进行化学发光反应,用凝胶成像分析仪检测条带,用目的蛋白条带吸光度与内参照GAPDH吸光度的比值表示蛋白质相对表达量。
2.6免疫荧光细胞染色法检测呼吸链氧化磷酸化复合体蛋白NDUFB8和MTCO1的表达将SH-SY5Y细胞接种于放有爬片的24孔板,加药培养24 h后,弃上清液,用冷的PBS洗细胞3次,每次5 min;用4%的多聚甲醛固定30 min,PBS洗3次,用含0.3% Triton X-100的PBS室温孵育10 min。用0.1% BSA封闭30 min,分别加小鼠抗NDUFB8单克隆抗体(1∶1 000)和小鼠抗MTCO1单克隆抗体(1∶1 000),4 ℃孵育过夜;PBS洗3次,加DyLight®594标记的山羊抗小鼠IgG(1∶500),室温避光孵育2 h;PBS洗3次,用Hoechst 33342染料的抗荧光淬灭封片液封片。显微镜下观察NDUFB8和MTCO1的表达。
2.7TUNEL法检测SH-SY5Y细胞凋亡将SH-SY5Y细胞接种于放有细胞爬片的24孔板,加药处理24 h 后,操作同2.6。含0.3% Triton X-100的PBS室温孵育完成后,按照TUNEL试剂盒说明书(C1089)配制TUNEL检测工作液,每孔加入适量TUNEL工作液,37 ℃避光孵育60 min,PBS洗3次并用含Hoechst 33342染料的抗荧光淬灭封片液封片。在荧光显微镜下观察,Cy3的激发波长为550 nm,发射波长为570 nm。统计视野下阳性细胞的数量(红色荧光)和细胞总数量(蓝色荧光),计算细胞凋亡率,细胞凋亡率(%)=阳性细胞数/细胞总数×100%。
3 统计学处理
采用GraphPad Prism 5.0软件对实验数据进行分析处理。计量资料以均数±标准差(mean±SD)表示。多组比较采用单因素方差(one-way ANOVA)分析,组间两两比较用LSD-检验。以<0.05为差异有统计学意义。
结果
1 AST IV抑制H2O2诱导的SH-SY5Y细胞线粒体膜电位降低
由图1可见,三组中H2O2组绿色荧光最强,说明细胞处于细胞凋亡早期,线粒体膜电位下降;H2O2+AST IV组与H2O2组相比,绿色荧光减少而红色荧光增加,说明线粒体膜电上升。进一步用线粒体检测试剂盒进行定量分析,结果显示,H2O2组的线粒体膜电位显著低于PBS组(<0.01);H2O2+AST IV组与H2O2组相比,线粒体膜电位显著升高(<0.01)。
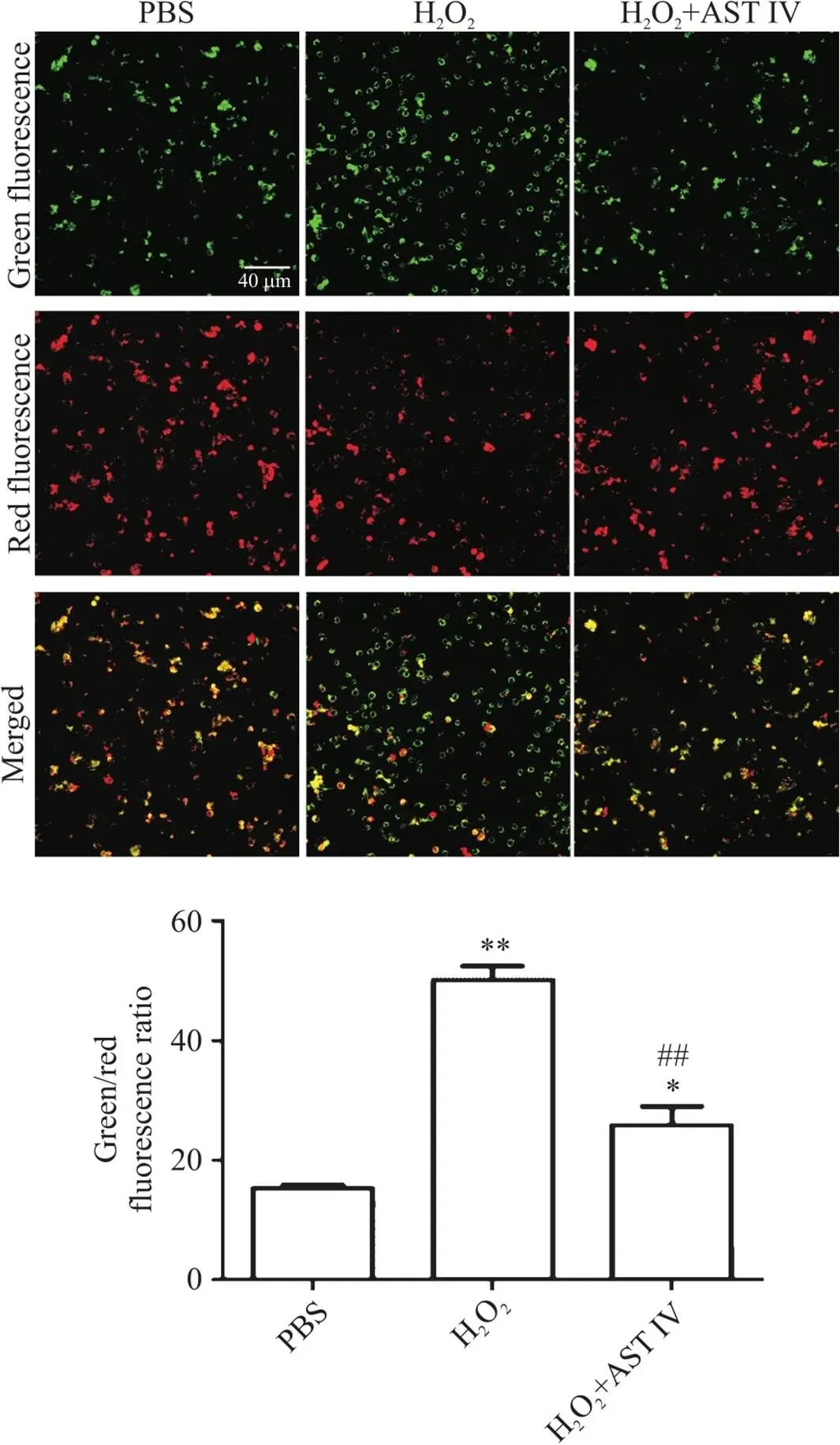
Figure 1. Astragaloside IV (AST IV) inhibited H2O2-induced decrease in mitochondrial membrane potential in SH-SY5Y cells. The SH-SY5Y cells were stained with JC-1 probe, and the staining was observed by confocal laser microscopy (scale bar=40 μm). Average single red/green fluorescence intensity was shown as normal/decreased mitochondrial membrane potential. The ratio of green fluorescence to red fluorescence was used to measure the degree of mitochondrial depolarization. Mean±SD. n=5. **P<0.01 vs PBS group; ##P<0.01 vs H2O2 group.
2 AST IV通过促进H2O2诱导的SH-SY5Y细胞呼吸链氧化磷酸化恢复ATP水平
图2A结果显示,H2O2组的ATP水平与PBS组相比显著降低(<0.05),而H2O2+AST IV组与H2O2组相比ATP水平显著增加(<0.01)。因此,我们进一步采用Western blot法检测细胞线粒体呼吸链氧化磷酸化相关蛋白的表达水平。结果显示,H2O2组NDUFB8(complex I)、SDHB(complex II)、MTCO1(complex IV)与ATP5A(complex V)蛋白水平较PBS组显著降低(<0.05或<0.01),UQCRC2(complex III)与PBS组相比无显著差异;H2O2+AST IV组与H2O2组相比,上述蛋白的表达均显著增加(<0.01或<0.05),见图2B。免疫荧光染色法检测了NDUFB8和MTCO1,结果显示:H2O2组NDUFB8和MTCO1的荧光强度与PBS组相比减弱,而H2O2+AST IV组NDUFB8和MTCO1荧光强度增加(图2C)。
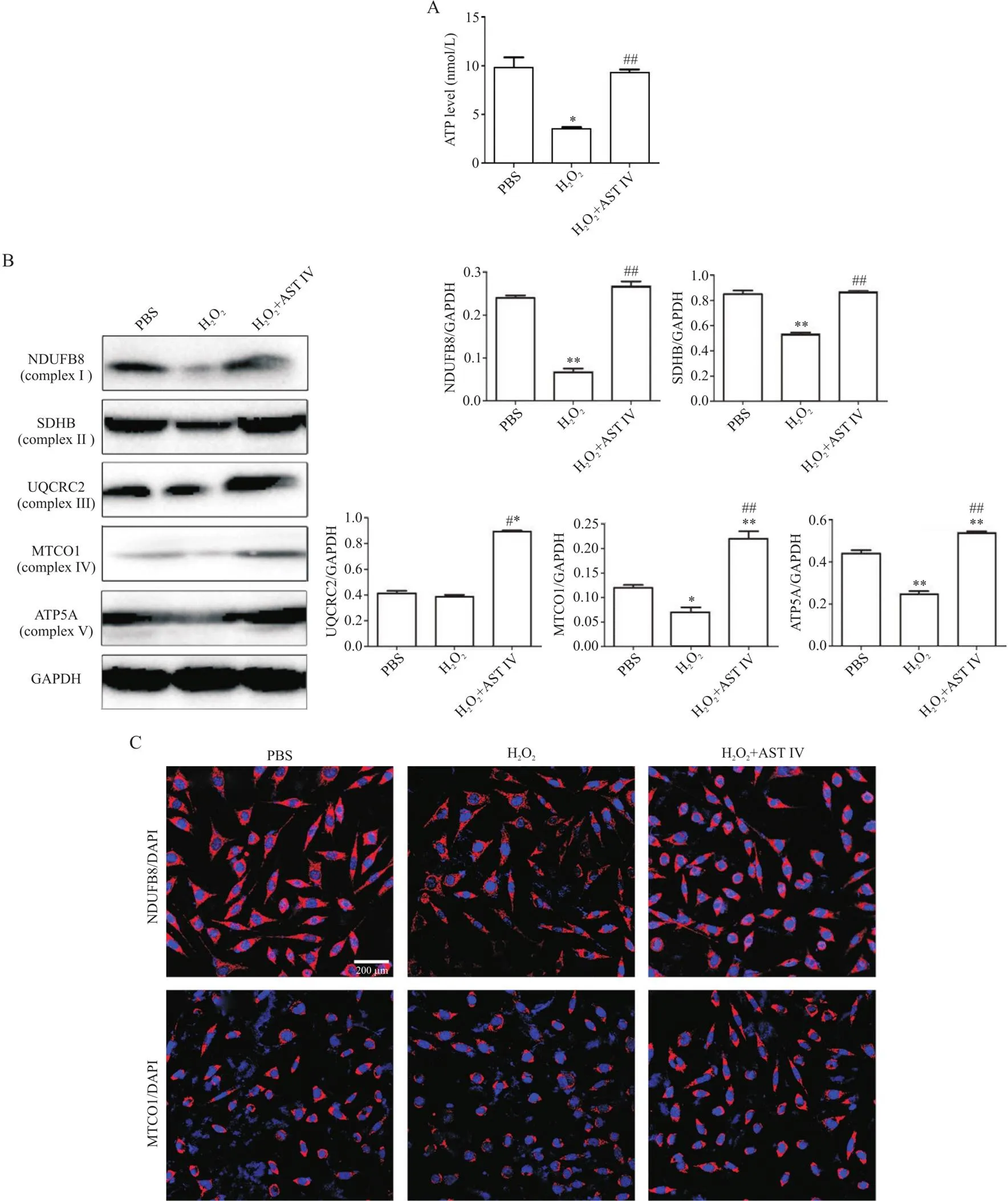
Figure 2. Astragaloside IV (AST IV) restored ATP levels by promoting oxidative phosphorylation of the respiratory chain in H2O2-induced SH-SY5Y cells. A: ATP level was detected by ATP assay kit (n=5); B: the expression of respiratory chain oxidative phosphorylation complex I~V proteins was detected by Western blot (n=3); C: the expression of NDUFB8 and MTCO1 (red) was detected by immunofluorescence staining (scale bar=200 μm). Mean±SD. *P<0.05, **P<0.01 vs PBS group; #P<0.05, ##P<0.01 vs H2O2 group.
3 AST IV抑制H2O2诱导的SH-SY5Y细胞线粒体分裂而促进融合
为进一步明确细胞线粒体动力学机制,通过Western blot法检测各组细胞线粒体分裂蛋白Fis1和p-DRP1,以及线粒体融合蛋白Mfn1、Mfn2和OPA1的表达水平。如图3所示,与PBS组相比,H2O2组Fis1和p-Drp1蛋白水平均显著升高(<0.05或<0.01),而Mfn1、Mfn2和OPA1表达水平均显著降低(<0.05或<0.01);与H2O2组相比,H2O2+AST IV组Fis1和p-Drp1蛋白水平均显著降低(<0.05或<0.01),而Mfn1、Mfn2和OPA1表达水平均显著升高(<0.05或<0.01)。
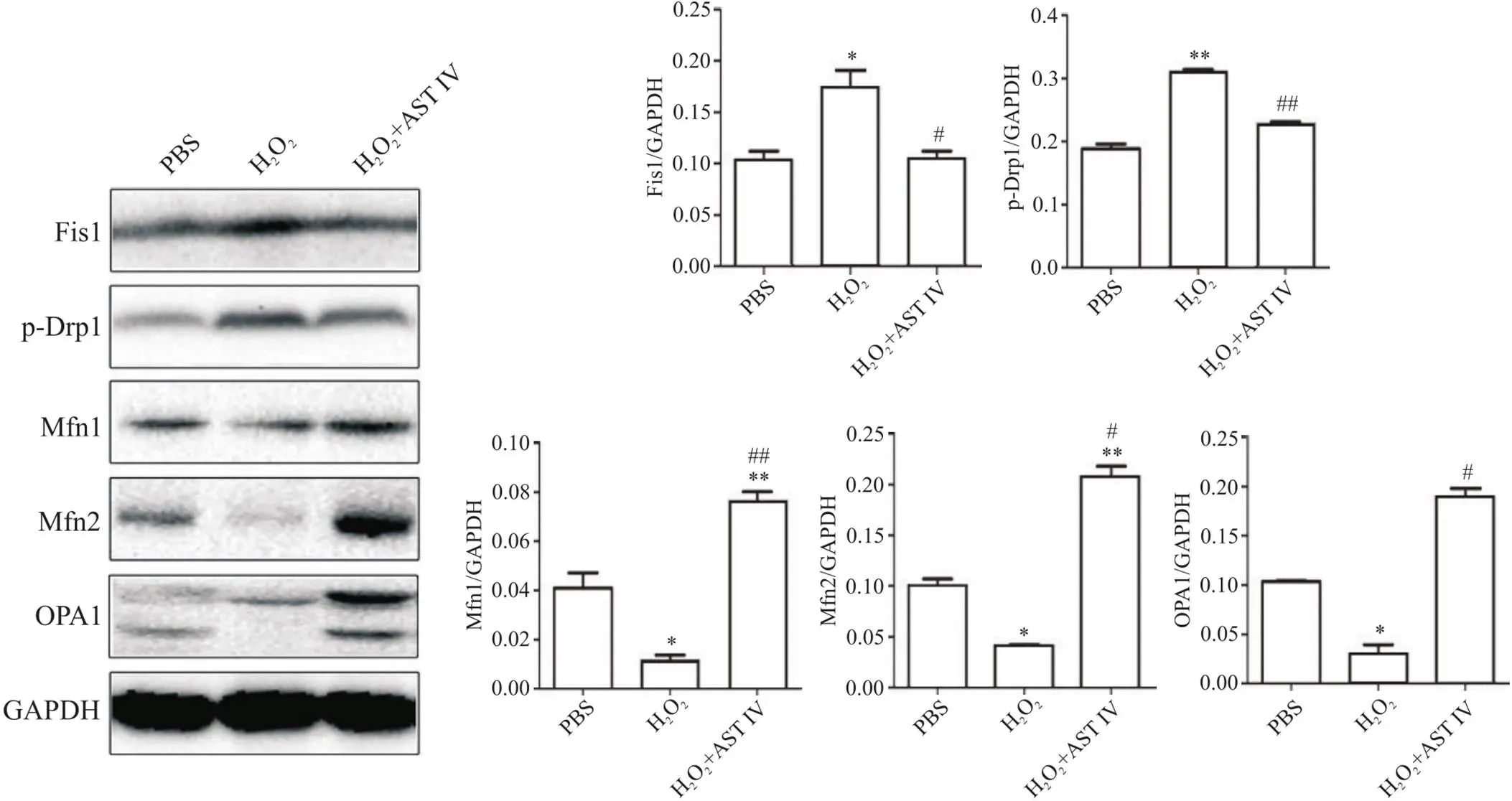
Figure 3. Astragaloside IV (AST IV) inhibited mitochondrial fission and promoted fusion in H2O2-induced SH-SY5Y cells. The protein levels of p-Drp1, Fis1, OPA1, Mfn1 and Mfn2 were detected by Western blot (normalized to GAPDH). Mean±SD. n=3. *P<0.05, **P<0.01 vs PBS group; #P<0.05, ##P<0.01 vs H2O2 group.
4 AST IV促进H2O2诱导的SH-SY5Y细胞Bcl-2表达,抑制Bax和cleaved caspase-3的表达
Western blot法检测细胞凋亡情况,图4A显示:与PBS组相比,H2O2组Bcl-2蛋白表达水平显著降低(<0.01),而Bax和cleaved caspase-3蛋白水平显著升高(<0.01);与H2O2处理组相比,H2O2+AST IV组Bcl-2蛋白表达水平显著升高(<0.05),Bax和cleaved caspase-3蛋白水平显著降低(<0.01)。
TUNEL法对凋亡细胞进行检测,结果显示:H2O2组细胞凋亡率显著高于PBS组(<0.01),而H2O2+AST IV组细胞凋亡率则显著低于H2O2组(<0.01),见图4B。
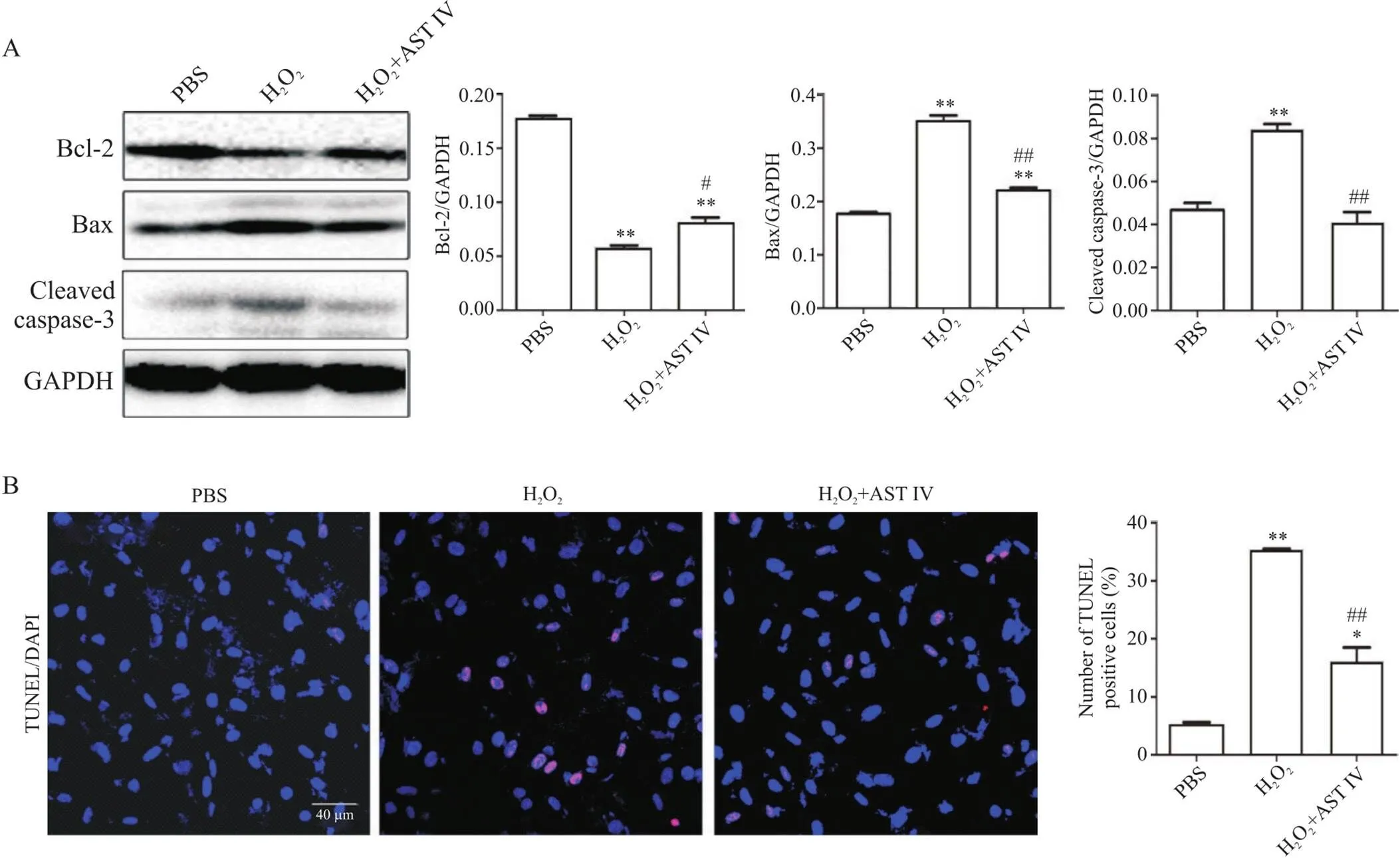
Figure 4. Astragaloside IV (AST IV) inhibited apoptosis of H2O2-induced SH-SY5Y cells. A: the expression of apoptosis-related proteins Bcl-2, Bax and cleaved caspase-3 was detected by Western blot (n=3); B: the apoptosis of SH-SY5Y cells was detected by TUNEL (scale bar=40 μm; n=5). Positive cells (red) were observed by fluorescence microscope. The number of positive cells (red fluorescence) and total number of cells (blue fluorescence) were counted. Mean±SD. *P<0.05, **P<0.01 vs PBS group; #P<0.05, ##P<0.01 vs H2O2 group.
讨论
AD、PD和ALS的病理均涉及神经组织的氧化损伤[13-15]。H2O2诱导的SH-SY5Y细胞是构建神经细胞氧化应激损伤体外模型的常用方法[11-12]。本文探讨了AST IV对H2O2诱导的SH-SY5Y细胞中线粒体相关功能的调控作用和机制,结果显示,AST IV干预能显著提高ATP水平,增加线粒体膜电位。已有研究表明,AST IV不仅可以调控线粒体动态稳定,保护缺氧复氧损伤大鼠的心肌细胞[16],而且可以改善肾血管性高血压大鼠主动脉内皮线粒体损伤[17]。以上结论说明,AST IV可以保护线粒体,与本文研究结果一致。
大部分ATP是通过氧化磷酸化产生的。研究表明,线粒体功能下降,线粒体DNA(mtDNA)点突变和缺失增加,会损伤氧化磷酸化复合物,增加ROS,导致进一步破坏线粒体蛋白、脂质和mtDNA的恶性循环[18]。在缺氧大鼠模型中,氧化苦参碱和人参皂苷均能提高氧化磷酸化效率,增加线粒体膜电位[19]。AST IV与人参皂苷均属于四环三萜皂苷元,我们的研究结果表明,AST IV可以通过增加呼吸链氧化磷酸化过程中相关蛋白的表达来增加ATP的产生,从而逆转氧化应激造成的能量不足。TrkB1受体激动剂R13可促进线粒体氧化磷酸化,增加complex I、complex II、complex III和complex IV,增强线粒体的生物发生和代谢而用于治疗AD[20]。依替福辛通过恢复脑外伤氧化磷酸化能力,发挥神经保护作用,改善行为和认知[21]。因此,恢复线粒体氧化磷酸化能力,维护线粒体功能正常,对防治神经退行性疾病具有重要意义。
线粒体分裂/融合失衡是神经退行性疾病的关键诱因[22]。研究表明,在AD患者的脑皮质样本研究中,线粒体分裂蛋白Drp1和Fis1的mRNA水平升高,融合蛋白Opa1、Mfn1和Mfn2的mRNA水平降低[23]。用Aβ25-35处理后的SH-SY5Y细胞,线粒体融合基因表达水平下调,而分裂基因表达水平上调[24]。本实验结果也证实,氧化应激损伤后线粒体分裂蛋白p-Drp1和Fis1蛋白水平显著升高,融合蛋白Mfn1、Mfn2和OPA1表达显著减少;而AST IV处理抑制了线粒体分裂的同时促进了融合,表明AST IV可以调节线粒体动力学,修复氧化应激损伤后的线粒体功能。
线粒体分裂/融合可以调节细胞凋亡。研究表明,Fis1过表达化引起细胞色素C的释放和细胞凋亡[25]。Mfn1和Mfn2融合蛋白的过表达,可以促进线粒体之间的交流,延迟细胞色素C的释放和凋亡通路中Bax和Bak的激活[26-27]。敲除、或的线粒体对凋亡刺激的敏感性增加[27-28]。本研究也证实,AST IV可以通过调控线粒体动力学,抑制凋亡信号通路Bax/cleaved caspase-3的激活,从而减少神经元的凋亡。
综上所述,AST IV可以保护神经元,其可能的机制是通过修复线粒体功能,调控线粒体动力学来减轻氧化应激损伤导致的神经元凋亡。
[1] Peoples JN, Saraf A, Ghazal N, et al. Mitochondrial dysfunction and oxidative stress in heart disease[J]. Exp Mol Med, 2019, 51(12):1-13.
[2] Bertholet AM, Delerue T, Millet AM, et al. Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity[J]. Neurobiol Dis, 2016, 90:3-19.
[3] Swerdlow RH. Mitochondria and mitochondrial cascades in Alzheimer's disease[J]. J Alzheimers Dis, 2018, 62(3):1403-1416.
[4] Bose A, Beal MF. Mitochondrial dysfunction in Parkinson's disease[J]. J Neurochem, 2016, 139(Suppl 1):216-231.
[5] Zuo X, Zhou J, Li Y, et al. TDP-43 aggregation induced by oxidative stress causes global mitochondrial imbalance in ALS[J]. Nat Struct Mol Biol, 2021, 28(2):132-142.
[6] Fão L, Rego AC. Mitochondrial and redox-based therapeutic strategies in Huntington's disease[J]. Antioxid Redox Signal, 2021, 34(8):650-673.
[7] Zhou L, Zhang R, Yang S, et al. Astragaloside IV alleviates placental oxidative stress and inflammation in GDM mice[J]. Endocr Connect, 2020, 9(9):939-945.
[8] Yang S, Zhang R, Xing B, et al. Astragaloside IV ameliorates preeclampsia-induced oxidative stress through the Nrf2/HO-1 pathway in a rat model[J]. Am J Physiol Endocrinol Metab, 2020, 319(5):E904-E911.
[9] Meng P, Yang R, Jiang F, et al. Molecular mechanism of Astragaloside IV in improving endothelial dysfunction of cardiovascular diseases mediated by oxidative stress[J].Oxid Med Cell Longev, 2021, 2021:1481236.
[10] Wang F, Zhao Y, Chen S, et al. Astragaloside IV alleviates ammonia-induced apoptosis and oxidative stress in bovine mammary epithelial cells[J]. Int J Mol Sci, 2019, 20(3):600.
[11] 宋哲, 薛超, 张小曼等. 脂联素通过激活PP2A减轻H2O2诱导的SH-SY5Y细胞损伤及tau蛋白过度磷酸化[J]. 中国病理生理杂志, 2015, 31(2):207-212.
Song Z, Xue C, Zhang X, et al. Adiponectin alleviates H2O2-induced SH-SY5Y cell injury and tau hyperphosphorylation via activating PP2A[J]. Chin J Pathophysiol, 2015, 31(2):207-212.
[12] 颜明, 刘洁婷, 李洪志, 等. 黄芩苷对H2O2诱导的SH-SY5Y细胞损伤的保护作用及其对Trx表达的影响[J]. 中国生化药物杂志, 2012, 33(5):574-577.
Yan M, Liu J, Li H, et al. Effect of baicalin on H2O2-induced SH-SY5Y cell injury and its influence on Trx expression[J]. Chin J Biochem Pharm, 2012, 33(5):574-577.
[13] Simunkova M, Alwasel SH, Alhazza IM, et al. Management of oxidative stress and other pathologies in Alzheimer's disease[J]. Arch Toxicol, 2019, 93(9):2491-2513.
[14] Baroli B, Loi E, Solari P, et al. Evaluation of oxidative stress mechanisms and the effects of phytotherapic extracts on Parkinson's diseasePINK1B9 model[J]. FASEB J, 2019, 33(10):11028-11034.
[15] Pollari E, GoldsteinsG, Bart G, et al. The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis[J]. Front Cell Neurosci, 2014, 8:131.
[16] 刘啊敏, 牟幼灵, 徐紫薇, 等. 黄芪甲苷通过调节线粒体稳态减轻大鼠心肌细胞缺氧复氧损伤[J]. 药学学报, 2020, 55(10):2398-2404.
Liu A, Mou Y, Xu Z, et al. Astragaloside IVameliorates hypoxia/reoxygenation injury viaregulating mitochondrial homeostasis in rat cardiomyocytes[J]. Acta Pharm Sin, 2020, 55(10):2398-2404.
[17] 张少君, 吴恒芳, 陈相健, 等. 黄芪甲苷对肾血管性高血压大鼠主动脉内皮细胞线粒体损伤的保护作用[J]. 南京医科大学学报(自然科学版), 2014, 34(7):889-893.
Zhang S, Wu H, Chen X, et al. Effect of astragaloside IV on mitochondrial injury of aortic endothelial cells from renovascular hypertensive rats[J]. J Nanjing Med Univ (Nat Sci), 2014, 34(7):889-893.
[18] Nissanka N, Moraes CT. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease[J]. FEBS Lett, 2018, 592(5):728-742.
[19] Mazat J, Devin A, Ransac S. Modelling mitochondrial ROS production by the respiratory chain[J]. Cell Mol Life Sci, 2020, 77(3):455-465.
[20] LiT, LiX, HuangX, et al. Mitochondriomics reveals the underlying neuroprotective mechanism of TrkB receptor agonist R13 in the 5×FAD mice[J]. Neuropharmacology, 2022, 204:108899.
[21] Palzur E, Edelman D, Sakas R, et al. Etifoxine restores mitochondrial oxidative phosphorylation and improves cognitive recovery following traumatic brain injury[J]. Int J Mol Sci, 2021, 22(23):12881.
[22] Chan D. Mitochondrial dynamics and its involvement in disease[J]. Annu Rev Pathol, 2020, 15:235-259.
[23] Reddy P. Inhibitors of mitochondrial fisson as a therapetutic strategy for diseases with oxidative stress and mitochondria dysfunction[J]. J Alzhelmers Dis, 2014, 40(2):245-256.
[24] Manczak M, Mao P, Calkins M, et al. Mitochondria targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons[J]. J Alzhelmers Dis, 2010, 20(Suppl 2):S609-S631.
[25] Horbay R, Bilyy R. Mitochondrial dynamics during cell cycling[J]. Apoptosis, 2016, 21(12):1327-1335.
[26] Neuspiel M, Zunino R, Gangaraju S, et al. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization[J]. J Biol Chem, 2005, 280(26):25060-25070.
[27] Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis[J]. J Biol Chem, 2004, 279(50):52726-52734.
[28] Yapa N, Lisnyak V, Reljic B, et al. Mitochondrial dynamics in health and disease[J]. FEBS Lett, 2021, 595(8):1184-1204.
Astragaloside IV inhibits H2O2-induced apoptosis of SH-SY5Y cells by regulating mitochondrial function
YU Jing-wen1, GUO Min-fang1, LI Su-yao2, MENG Tao1, ZHANG Hai-fei1, YANG De-bin1, SONG Li-juan2,3, MA Cun-gen1,2△, YU Jie-zhong1,2,4△
(1,,037009,;2,,,030619,;3,,030001,;4,037009,)
To investigate the effects of astragaloside IV (AST IV) on mitochondrial damage and cell apoptosis in human neuroblastoma SH-SY5Y cells induced by hydrogen peroxide (H2O2).The SH-SY5Y cells were treated with PBS, H2O2or H2O2+AST IV. Intracellular ATP level was measured by ATP detection kit. Mitochondrial membrane potential was measured by mitochondrial membrane potential assay kit with JC-1. Mitochondrial respiratory chain-related proteins NADH:ubiquinone oxidoreductase subunit B8 (NDUFB8; complex I), succinate dehydrogenase B (SDHB; complex II), ubiquinol-cytochrome C reductase core protein 2 (UQCRC2; complex III), mitochondrially encoded cytochrome C oxidase I (MTCO1; complex IV) and ATP synthase F1 subunit alpha (ATP5A; complex V), mitochondrial dynamic fission proteins phosphorylated dynamin-related protein 1 (p-Drp1) and mitochondrial fission protein 1 (Fis1), fusion proteins mitofusin 1 (Mfn1), Mfn2 and optic atrophy protein 1 (OPA1), and apoptosis-related proteins cleaved caspase-3, Bcl-2 and Bax were detected by Western blot. The expression of NDUFB8 and MTCO1 was detected by immunofluorescence staining. TUNEL staining was applied to observe SH-SY5Y cell apoptosis.The SH-SY5Y cells were treated with H2O2at the dose of 200 μmol/L to establish the oxidative stress cell model. In oxidative stress model, mitochondrial membrane potential (<0.01) and ATP level (<0.05) were down-regulated significantly, and the expression of NDUFB8, SDHB, ATP5A and MTCO1 were significantly decreased (<0.05 or<0.01). Mitochondrial fission proteins Fis1 (<0.05) and p-Drp1 (<0.01) were significantly increased, fusion proteins Mfn1, Mfn2 and OPA1 were significantly decreased, the expression levels of pro-apoptotic proteins Bax and cleaved caspase-3 were significantly increased (<0.01), and Bcl-2 was significantly decreased (<0.01) after treatment with H2O2. Treatment with AST IV significantly increased mitochondrial membrane potential (<0.01) and ATP level (<0.01), significantly up-regulated the complex proteins NDUFB8, SDHB, MTCO1, ATP5A and UQCRC2 (<0.05 or<0.01), significantly down-regulated the expression of mitochondrial fission proteins p-DRP1 and Fis1 (<0.01), and remarkably up-regulated the expression of mitochondrial fusion proteins OPA1, Mfn1 and Mfn2 (<0.05 or<0.01). Meanwhile, AST IV significantly increased the expression of Bcl-2 (<0.05), significantly decreased the expression of Bax and cleaved caspase-3 (<0.01), and inhibited SH-SY5Y cell apoptosis.Astragaloside IV attenuates the apoptosis of SH-SY5Y cells induced by H2O2through regulating mitochondrial function.
Astragaloside IV; Oxidative stress; Mitochondria; SH-SY5Y cells
1000-4718(2022)09-1553-08
2022-04-01
2022-07-07
马存根 Tel: 18203515288; E-mail: macungen@sxtcm.edu.cn; 尉杰忠 Tel: 13834129435; E-mail: sxdtyjz@qq.com
R338.2; R741.02
A
10.3969/j.issn.1000-4718.2022.09.003
[基金项目]山西省基础研究计划资助项目(No. 20210302123337); 大同市应用基础研究计划项目资助(No. 2020145); 国家中医药管理局多发性硬化益气活血重点研究室开放课题(No. 2021-KF-08T); 山西省教育厅高等学校科技创新项目(No. 2019L0734); 神经炎症和变性疾病基础与应用研究山西省重点实验室开放课题(No. KF-2019002)
(责任编辑:李淑媛,罗森)
