miR-29c通过下调TNFR1信号通路减轻TNF-α诱导的小鼠海马神经元HT22细胞损伤*
2022-10-13李勃鲁莹万琪梁璇杨沂文韩勇曾俊伟
李勃, 鲁莹, 万琪, 梁璇, 杨沂文, 韩勇, 曾俊伟
· 论著 ·
miR-29c通过下调TNFR1信号通路减轻TNF-α诱导的小鼠海马神经元HT22细胞损伤*
李勃, 鲁莹, 万琪, 梁璇, 杨沂文, 韩勇, 曾俊伟△
(遵义医科大学生理学教研室,贵州 遵义 563000)
探讨微小RNA-29c(microRNA-29c, miR-29c)对肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)诱导小鼠海马神经元HT22细胞损伤效应的影响及机制。构建慢病毒(lentivirus, LV)介导的过表达/低表达miR-29c的小鼠海马神经元HT22,分为空载体细胞株(LV-vector)组、LV-vector+TNF-α组、miR-29c过表达细胞株(LV-miR-29c)+TNF-α组和miR-29c低表达细胞株(LV-miR-29c sponge)+TNF-α组。采用CCK-8法和乳酸脱氢酶(lactate dehydrogenase, LDH)释放实验评价TNF-α对HT22细胞的毒性作用;免疫荧光染色观察肿瘤坏死因子受体1(tumor necrosis factor receptor 1, TNFR1)的表达;RT-qPCR检测miR-29c和TNFR1 mRNA的表达;Western blot检测TNFR1、TNFR相关死亡域蛋白(TNFR-associated death domain protein, TRADD)、Fas相关死亡结构域蛋白(Fas-associated death domain protein, FADD)、pro-caspase-8、cleaved caspase-8、pro-caspase-3和cleaved caspase-3的蛋白水平;Hoechst和TUNEL染色检测细胞凋亡率。(1)CCK-8和LDH释放实验结果显示,与LV-vector组相比,LV-vector+TNF-α组细胞活力显著下降(<0.05),且LDH释放显著增多(<0.05);与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组细胞活力显著上升(<0.05),且LDH释放显著减少(<0.05),而LV-miR-29c sponge+TNF-α组细胞活力显著下降(<0.05),且LDH释放显著增多(<0.05)。(2)RT-qPCR和免疫荧光结果显示,与LV-vector组相比,LV-vector+TNF-α组TNFR1表达显著增多(<0.05);与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组TNFR1表达显著减少(<0.05),而LV-miR-29c sponge+TNF-α组TNFR1表达显著增多(<0.05)。(3)Western blot结果显示,与LV-vector组相比,LV-vector+TNF-α组细胞中TNFR1、TRADD、FADD、cleaved caspase-8和cleaved caspase-3蛋白水平显著上调(<0.05);与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组TNFR1、TRADD、FADD、cleaved caspase-8和cleaved caspase-3蛋白水平表达显著下调(<0.05),而LV-miR-29c sponge+TNF-α组TNFR1、TRADD、FADD、cleaved caspase-8和cleaved caspase-3蛋白水平显著上调(<0.05)。(4)Hoechst和TUNEL染色结果显示,与LV-vector组相比,LV-vector+TNF-α组凋亡细胞数目显著增加(<0.05);与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组凋亡细胞数目显著减少(<0.05),而LV-miR-29c+sponge+TNF-α组凋亡细胞数显著增加(<0.05)。miR-29c减轻TNF-α诱导的小鼠海马神经元HT22细胞损伤,其机制可能与抑制TNFR1信号通路激活导致的细胞凋亡有关。
微小RNA-29c;肿瘤坏死因子α;TNFR1信号通路;细胞凋亡;海马神经元
海马脑区是边缘系统的重要组成部分之一,在痛觉感受、情绪体验以及学习记忆等功能中发挥重要作用。在阿尔茨海默病(Alzheimer disease, AD)、脑缺血再灌注损伤和创伤后应激障碍等许多神经系统疾病的病变进展过程中,均可见到在海马脑区炎症因子的积聚,这可诱发神经元的损伤甚至凋亡[1-4]。在这些疾病的发生发展过程中,往往伴随海马脑区微小RNA(microRNA, miRNA, miR)表达谱及多种炎症因子的表达发生改变,提示这些miRNA可能通过调节海马炎症,促进神经元损伤,参与病变进展[5-6]。
miR-29c是miR-29s家族成员之一,作为重要的基因表达调控因子,在心脏、肝脏、子宫及肌肉组织中表达较低,而在大脑和脊髓处于高水平表达[7]。在缺血再灌注小鼠海马组织和氧糖剥夺损伤的PC12细胞中均可见miR-29c表达下降,但肿瘤坏死因子受体1(tumor necrosis factor receptor 1, TNFR1)及炎症因子白细胞介素1β(interleukin-1β, IL-1β)、IL-6和TNF-α表达增加[8]。在坐骨神经损伤性疼痛的大鼠,海马组织中miR-29c的表达下降近70%;在脊神经损伤小鼠,海马小胶质细胞释放肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)增多,作用于神经元TNFR1,导致神经元损伤,可见神经元树突分支减少,树突棘密度降低,但这些变化在基因敲除后显著减轻[9-10]。双萤光素酶报告基因实验证实miR-29c可与TNFR1 mRNA的3'非翻译区(3'-untranslated region, 3'-UTR)结合,导致TNFR1表达下调[11]。因此,有必要深入研究miR-29c是否可以通过下调TNFR1表达,抑制TNFR1下游通路激活导致的海马神经元损伤。
HT22细胞是一种源于小鼠的永生化海马神经元细胞系,广泛应用于多种神经系统疾病的体外研究实验中[12]。有研究报道,TNF-α作用于HT22细胞可以导致其细胞活力下降且凋亡率上升[13]。因此,本实验采用TNF-α诱发HT22细胞损伤,以慢病毒(lentivirus, LV)转染技术构建miR-29c过表达或低表达的稳转细胞株,观察miR-29c过表达或低表达是否影响TNF-α诱发的HT22细胞损伤,探讨miR-29c是否可以抑制TNFR1的表达及后续的细胞凋亡,以期在细胞水平上明确miR-29c对TNF-α/TNFR1通路激活导致的海马神经元损伤的抑制作用。
材料和方法
1 主要材料与试剂
小鼠海马神经元HT22细胞购自美国模式培养物集存库(American Type Culture Collection, ATCC);胎牛血清(fetal bovine serum, FBS)购自MRC;DMEM高糖培养液和胰蛋白酶均购自HyClone;兔抗Fas相关死亡结构域蛋白(Fas-associated death domain protein, FADD)多克隆抗体、兔抗TNFR1多克隆抗体、兔抗β-actin多克隆抗体和山羊抗兔Cy3荧光Ⅱ抗均购自Proteintech;兔抗cleaved caspase-3单克隆抗体和兔抗cleaved caspase-8单克隆抗体购自Cell Signaling Technology;兔抗TNFR相关死亡域蛋白(TNFR-associated death domain protein, TRADD)多克隆抗体和兔抗GAPDH单克隆抗体均购自HuaBio;兔抗pro-caspase-3单克隆抗体、兔抗pro-caspase-8单克隆抗体和小鼠TNF-α重组蛋白均购自Abcam;引物设计与合成和慢病毒包装与合成均由上海生工有限公司提供;CCK-8试剂盒、乳酸脱氢酶(lactate dehydrogenase, LDH)检测试剂盒和一步法TUNEL细胞凋亡检测试剂盒(Cy3-dUTP)均购自上海碧云天生物科技公司;Hoechst 33258染色液购自北京索莱宝公司;Triozl试剂、逆转录试剂盒和SYBR Green荧光染料试剂盒购自TaKaRa。
2 主要仪器与设备
激光扫描共聚焦显微镜Airyscan 2购自Zeiss;NanoDrop 2000/2000C、低温高速离心机、QuantStudioTM6 Flex PCR仪和FORM Series ll Water Jacket CO2细胞培养箱购自Thermo Fisher Scientific;荧光显微镜和倒置相差显微镜购自Leica;电泳槽、电泳仪和半干电转移系统购自Bio-Rad;全能型凝胶成像系统购自Syngene。
3 方法
3.1构建慢病毒介导的miR-29c过表达/低表达的HT22细胞株取状态良好的HT22细胞进行胰酶消化,细胞计数后,按照每孔1×104个细胞接种至24孔板中,放入37 ℃、体积分数5% CO2的培养箱中过夜培养。待细胞密度达到30%~50%后,按照感染复数(multiplicity of infect, MOI)=120配制含有病毒原液的培养液进行病毒感染,24 h后吸弃含有病毒的培养液,换含10% FBS的DMEM高糖培养基后放入培养箱继续培养。3 d后,通过荧光显微镜观察细胞的增强型绿色荧光蛋白(enhanced green fluorescent protein, EGFP)的表达效率。将适量嘌呤霉素加入新鲜培养基进行筛选,直到存活细胞的EGFP表达效率达到90%以上。本实验中,miR-29c sponge的序列含有8段重复序列(5'-TAACCGATTTTTTTGGTGCTA-3'),可以与miR-29c(5'-TAGCACCATTTGAAATCGGTTA-3')结合。
3.2实验分组将慢病毒转染构建的空载体(LV-vector)、miR-29c过表达(LV-miR-29c)及miR-29c低表达(LV-miR-29c sponge)HT22细胞分为以下4组:LV-vector组、LV-vector+TNF-α组、LV-miR-29c+TNF-α组和LV-miR-29c sponge+TNF-α组。
3.3CCK-8法检测细胞活力将HT22细胞接种到96孔细胞培养板中,密度为每孔7 000个。各组细胞处理完毕后,根据试剂盒说明书,向每孔加入10 μL CCK-8溶液,将培养板放入孵箱中孵育2 h后,用酶标仪在450 nm处测定各孔吸光度(absorbance,)。按照下述公式进行计算:细胞活力(%)=(加药组-空白组)/(对照组-空白组)×100%。
3.4LDH释放检测将HT22细胞接种到96孔细胞培养板中,密度为每孔7 000个。将培养孔进行以下分组:(1)无细胞的培养液孔;(2)未经药物处理的对照细胞孔;(3)未经药物处理的用于后续裂解的细胞孔;(4)药物处理的细胞孔。标记好后常规培养。检测前1 h,从孵箱中取出细胞培养板,在(3)中加入LDH释放试剂后,反复吹打数次混匀,然后继续在细胞培养箱中孵育。1 h过后开始检测,离心(400×, 5 min),取120 μL各孔的上清液并加入到新的96孔板相应孔中,随即在各孔分别加入LDH检测工作液50 μL混匀(乳酸溶液∶INT溶液∶酶溶液=1∶1∶1),室温避光孵育30 min,酶标仪在490 nm处测定各组值,并将得到的值[(1)、(2)、(3)和(4)组的值分别为1、2、3和4]按照下述公式进行计算:LDH释放率(%)=(4-2)/(3-2)×100%。
3.5细胞免疫荧光实验HT22细胞接种于盖玻片上(每孔1×105)并放于孵箱培养24 h后给予TNF-α(400 μg/L,24 h)。弃去上清液后用PBS漂洗3次,每次5 min,4%多聚甲醛固定20 min,PBS漂洗3~4次,每次5 min;加入兔抗TNFR1单克隆抗体(1∶350)覆盖于玻片上,37 ℃避光孵育1 h后置于4 ℃冰箱过夜。次日,用PBS漂洗3~4次,每次5 min,随后加入Cy3标记的山羊抗兔IgG(1∶350),37 ℃避光孵育30 min,室温避光孵育1 h后取出;加入DAPI(1∶500,5 min)标记细胞核;甘油+PBS(体积比1∶1)封片。在激光共聚焦显微镜下观察并拍照,使用ZEN软件处理得到单细胞荧光强度数值(每组不少于100个细胞)。
3.6Western blot各组细胞弃去上清,按照BCA试剂盒说明书进行操作。步骤如下:预冷的PBS(PH 7.4)清洗后加入裂解液,将细胞刮下。4 ℃静置半小时,低温高速(4 ℃,4 500×)离心5 min,提取上清后加入上样缓冲液,热变性。每孔30 μg蛋白样品,SDS-PAGE分离,膜转移,用含5%脱脂奶粉的 TBST 缓冲液封闭2 h。分别孵育兔抗TNFR1(1∶500)、TRADD(1∶500)、FADD(1∶500)、pro-caspase-3(1∶1 000)、cleaved caspase-3(1∶1 000)、pro-caspase-8(1∶1 000)、cleaved caspase-8(1∶1 000)、β-actin(1:4 000)和GAPDH(1∶8 000)抗体,4 ℃过夜。膜清洗15 min,加入HRP标记的山羊抗兔IgG(1∶4 000),室温孵育1 h,洗膜、显色曝光。所有数据采取ImageJ 1.48软件处理。
3.7RT-qPCR使用Trizol试剂提取各组细胞总RNA,按照逆转录试剂盒和荧光染料试剂盒说明书进行操作。逆转录反应条件为:37 ℃ 15 min,85 ℃ 5 s。扩增反应条件为:95 ℃ 30 s;95 ℃ 5 s,60 ℃ 30 s,40个循环。miR-29c的上游引物序列为5′-CGCGTAGCACCATTTGAAAT-3′,下游引物序列5′-AGTGCAGGGTCCGAGGTATT-3′;U6的上游引物序列为5′-CTCGCTTCGGCAGCACATATACT-3′,下游引物序列为5′-ACGCTTCACGAATTTGCGTGTC-3′;TNFR1的上游引物序列为5′-CCGGGAGAAGAGGGATAGCTT-3′,下游引物序列为5′-TCGGACAGTCACTCACCAAGT-3′;β-actin的上游引物序列为5′-GGCTGTATTCCCCTCCATCG-3′,下游引物序列为5′-CCAGTTGGTAACAATGCCATGT-3′。分别以β-actin和U6为内参照,统计各组Ct值,采用2-ΔΔCt法进行数据分析。
3.8Hoechst染色HT22细胞正常消化,接种于盖玻片上(每孔1×105)并放于孵箱培养24 h后给予TNF-α(400 μg/L,24 h)处理。弃去上清液后用PBS漂洗3次,每次5 min,4%多聚甲醛固定15 min,PBS漂洗3次,每次5 min,加入Hoechst 33258 (10 mg/L)染色液在室温下避光孵育5 min,PBS漂洗后封片(甘油∶PBS=1∶1),倒置荧光显微镜下观察胞核形态变化并拍照,细胞核呈现淡蓝色椭圆形为正常细胞,细胞核呈现高亮蓝色皱缩为凋亡细胞,采用以下公式计算凋亡率:凋亡率(%)=凋亡细胞数量/细胞总数×100%。
3.9TUNEL染色HT22细胞正常消化,接种于盖玻片上(每孔1×105)并放于孵箱培养24 h后给予TNF-α(400 μg/L,24 h)处理。弃去上清液后用PBS漂洗3次,每次5 min,4%多聚甲醛固定30 min,PBS漂洗3次,每次5 min;之后选用0.3% Triton X-100处理5 min,PBS漂洗3次,每次5 min;然后按照试剂盒说明书进行染色。每张玻片滴加 TUNEL反应混合液30 μL(TdT酶∶荧光标记液=1∶9),然后置于暗湿盒中,37 ℃避光孵育1 h,PBS漂洗3次,每次5 min;然后用DAPI溶液对细胞核进行避光染色5 min,PBS漂洗3次,每次5 min,在激光共聚焦显微镜下拍照并保存好图像资料。细胞发生凋亡后,染色体DNA双链断裂或单链断裂而产生大量的黏性3'-OH末端,可以在TdT酶的催化下加上红色荧光探针Cy3标记的dUTP,进行凋亡细胞检测,采用以下公式计算凋亡率:凋亡率(%)=凋亡阳性细胞数目/细胞总数×100%。
4 统计学处理
采用SPSS 17.0软件统计实验结果。数据表示为均数±标准差(mean±SD)。组间均数比较选用单因素方差分析,方差齐选用Dunnett检验进行两两比较。以<0.05为差异有统计学意义。
结果
1 构建慢病毒介导的过表达/低表达miR-29c的HT22细胞株
在荧光显微镜下可见慢病毒感染后的HT22细胞呈现绿色荧光;RT-qPCR结果显示,与LV-vector组相比,LV-miR-29c组中的miR-29c表达显著上调(<0.05),而LV-miR-29c sponge组中的miR-29c表达显著下调(<0.05),见图1。这提示空载体慢病毒转染(LV-vector)、miR-29c过表达(LV-miR-29c)及miR-29c低表达(LV-miR-29c sponge)的小鼠海马神经元HT22细胞株均已构建。

Figure 1. Establishment of stable HT22 cell line with overexpression or low expression of miR-29c. A: the HT22 cells in LV-vector, LV-miR-29c and LV-miR-29c sponge groups were detected by fluorescence microscopy after lentiviral transfection (top row: observation under green fluorescence; bottom row: observation under white light; scale bar=100 µm); B: RT-qPCR analysis of miR-29c expression in the HT22 cells transfected with LV-vector, LV-miR-29c or LV-miR-29c sponge. Mean±SD. n=4. *P<0.05 vs LV-vector group; #P<0.05 vs LV-miR-29c group.
2 miR-29c减轻TNF-α导致的HT22细胞损伤
CCK-8法检测各组细胞的活力,结果显示,与LV-vector组相比,LV-vector+TNF-α组细胞活力显著下降,且当浓度为400 µg/L的TNF-α作用24 h后,细胞活力下降近50%(<0.05),见图2A。于是后续实验选用该浓度检测miR-29c对TNF-α诱导的HT22细胞活力下降的影响。与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组的细胞活力显著上升(<0.05),而LV-miR-29c sponge+TNF-α组的细胞活力显著下降(<0.05),见图2B。
同时,LDH释放检测结果显示,与LV-vector组相比,LV-vector+TNF-α组LDH释放显著增多(<0.05);与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组LDH释放显著减少,而LV-miR-29c sponge+TNF-α组细胞LDH释放显著增多(<0.05),见图2C。
倒置相差显微镜下观察,LV-vector组细胞生长状态良好,呈贴壁性生长;而LV-vector+TNF-α组部分细胞皱缩变圆,贴壁不紧有脱落现象;与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组上述表现有所缓解;与LV-vector+TNF-α组相比,LV-miR-29c sponge+TNF-α组细胞损伤现象进一步加重,见图2D。
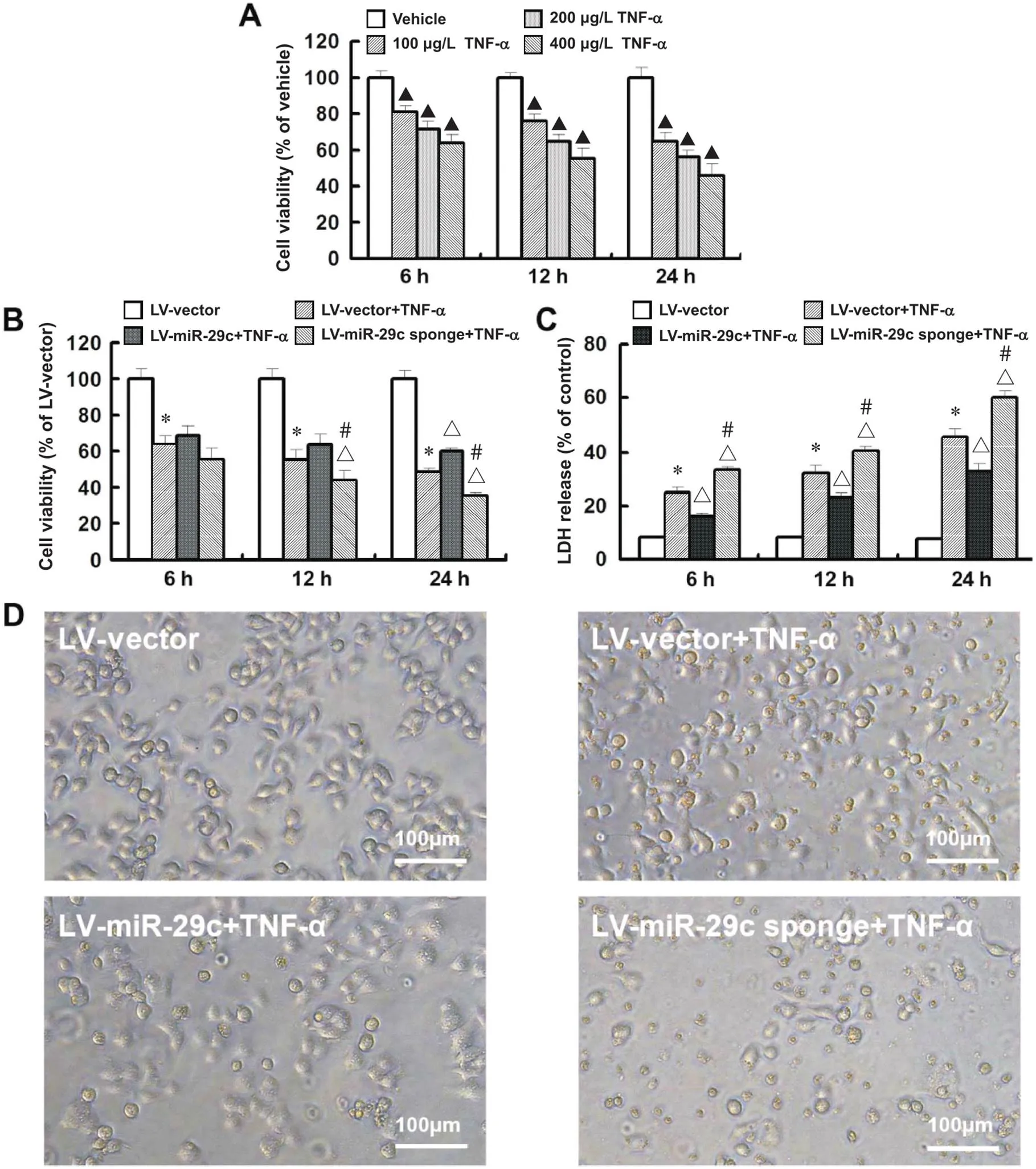
Figure 2. miR-29c overexpression protected HT22 cells from TNF-α-induced injury. A and B: cell viability was measured by CCK-8 assay; C: cytotoxicity was measured by LDH release assay; D: morphological changes of HT22 cells were observed(scale bar=100 µm). Mean±SD. n=6. ▲P<0.05 vs vehicle group; *P<0.05 vs LV-vector group; △P<0.05 vs LV-vector+TNF-α group; #P<0.05 vs LV-miR-29c+TNF-α group.
3 miR-29c抑制TNF-α诱导的HT22细胞中TNFR1表达
免疫荧光染色结果所示,与LV-vector组相比,LV-vector+TNF-α组细胞中TNFR1荧光强度显著升高(<0.05);与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组细胞TNFR1荧光强度显著下降(<0.05),而LV-miR-29c sponge+TNF-α组细胞TNFR1荧光强度显著升高(<0.05),见图3A。
RT-qPCR和Western blot结果表明,与LV-vector组相比,LV-vector+TNF-α组的TNFR1在mRNA和蛋白水平的表达均显著上调(<0.05);与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组细胞TNFR1在mRNA和蛋白水平的表达均显著下调(<0.05),而LV-miR-29c sponge+TNF-α组TNFR1在mRNA和蛋白水平的表达显著上调(<0.05),见图3B、C。

Figure 3. miR-29c overexpression inhibited TNF-α-induced the expression of TNFR1 in HT22 cells.A: immunofluorescence staining of TNFR1 (scale bar=50 μm) and the mean fluorescence intensity per cell (≥100 cells); B: RT-qPCR analysis of TNFR1 mRNA level; C: Western blot was applied to detect the protein expression of TNFR1. Mea±SD. n=4. *P<0.05 vs LV-vector group; △P<0.05 vs LV-vector+TNF-α group; #P<0.05 vs LV-miR-29c+TNF-α group.
4 miR-29c抑制TNF-α诱导的HT22细胞中TNFR1信号通路的蛋白表达
Western blot结果显示,在4组细胞中,pro-caspase-8的变化无显著差异。与LV-vector组相比,LV-vector+TNF-α组中TRADD、FADD和cleaved caspase-8的蛋白水平均显著上调(<0.05);与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组中TRADD、FADD和cleaved caspase-8的蛋白水平显著下调(<0.05);而LV-miR-29c sponge+TNF-α组中TRADD、FADD和cleaved caspase-8的蛋白水平显著上调(<0.05)。见图4。
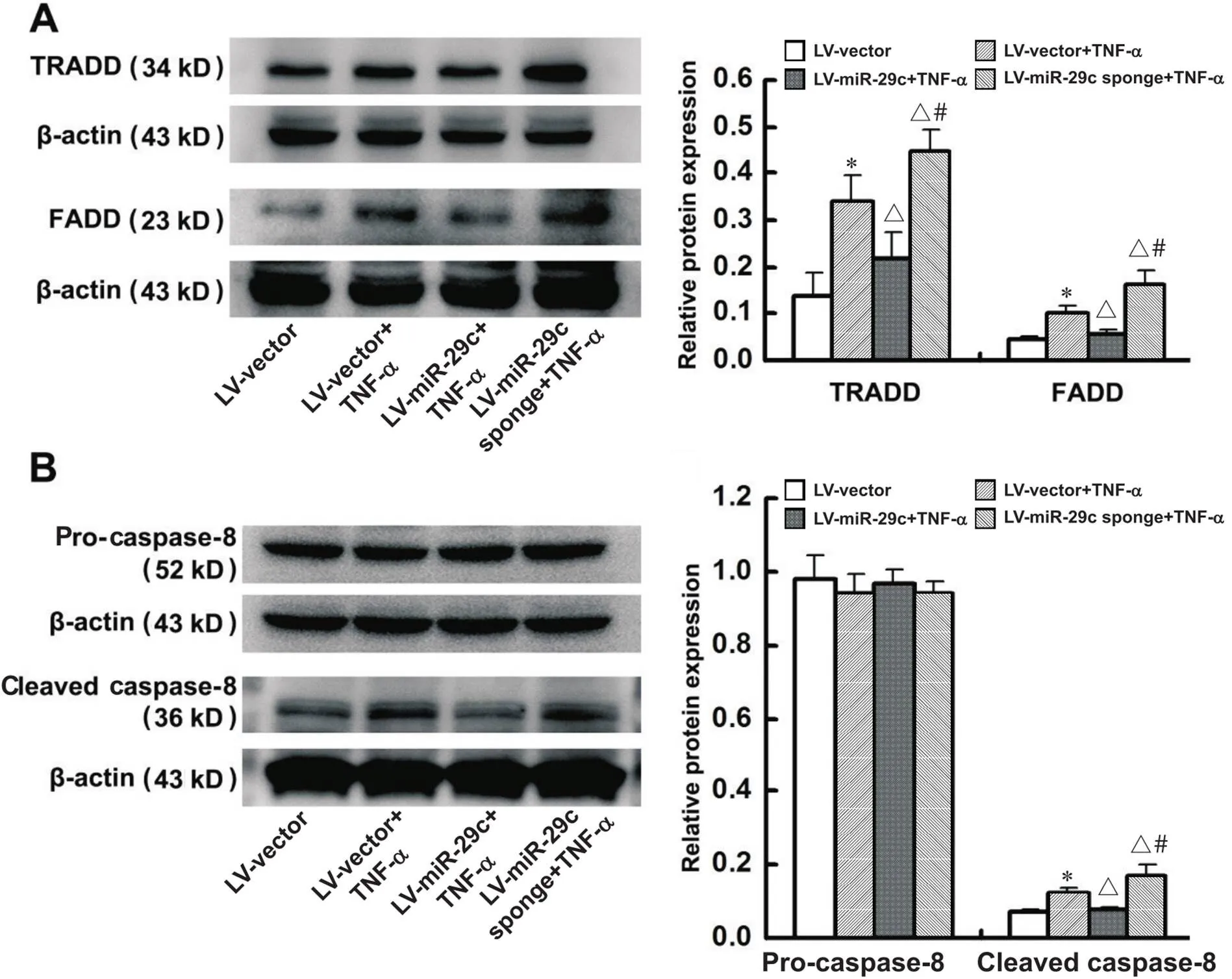
Figure 4. miR-29c overexpression inhibited TNF-α-induced expression of TNFR1 signaling pathway-related proteins in HT22 cells. A: Western blot was applied to detect the protein levels of TRADD and FADD; B: Western blot was applied to detect the protein levels of pro-caspase-8 and cleaved caspase-8. Mean±SD. n=4. *P<0.05 vs LV-vector group; △P<0.05 vs LV-vector+TNF-α group; #P<0.05 vs LV-miR-29c+TNF-α group.
5 miR-29c减轻TNF-α导致的HT22细胞凋亡
Western blot结果显示,在4组细胞中,pro-caspase-3变化无显著差异。与LV-vector组相比,LV-vector+TNF-α组中cleaved caspase-3的蛋白水平显著上调(<0.05);与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组中cleaved caspase-3的蛋白水平显著下调(<0.05);而LV-miR-29c sponge+TNF-α组中cleaved caspase-3的蛋白水平显著上调(<0.05)。见图5A。
Hoechst染色观察到,在荧光显微镜下,LV-vector组细胞核大多呈现弥散均匀荧光;与LV-vector组相比,LV-vector+TNF-α组细胞核内可见浓染致密的颗粒块状荧光,且光亮度增加,凋亡细胞数显著增加(<0.05);与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组虽然也有部分高亮蓝色皱缩形的凋亡细胞,但数目显著减少(<0.05),LV-miR-29c sponge+TNF-α组高亮蓝色皱缩形的凋亡细胞数目显著增多(<0.05),见图5B。
TUNEL染色结果显示,与LV-vector组相比,LV-vector+TNF-α组TUNEL阳性细胞数目显著增加(<0.05);与LV-vector+TNF-α组相比,LV-miR-29c+TNF-α组TUNEL阳性细胞数目显著减少(<0.05),LV-miR-29c sponge+TNF-α组TUNEL阳性细胞数目显著增多(<0.05),见图5C。
讨论
在海马脑区,TNF-α/TNFR1信号通路的异常激活促进了神经炎症和和神经元的凋亡过程,参与了如脑外伤、脑卒中、阿尔兹海默症和帕金森症等神经系统疾病的病变进程[14-16]。因此,如何抑制TNF-α/TNFR1信号通路过度激活已成为神经系统疾病治疗的一个关键问题。鉴于miR-29c作为一个重要的基因表达调控因子,高表达于人类和啮齿类动物的海马组织,因此,有必要深入探讨miR-29c对受损海马神经元是否具有保护作用并探讨其机制。
本实验结果表明,TNF-α刺激小鼠海马神经元HT22细胞,导致细胞活力下降,LDH释放增加,甚至出现细胞凋亡。然而,在HT22细胞过表达miR-29c后可以显著减轻TNF-α诱导的上述细胞损伤;相反,在HT22细胞转染miR-29c sponge之后,miR-29c表达下降,而且由于miR-29c sponge与miR-29c的结合,导致miR-29c的保护作用大大减弱,因此TNF-α诱导的HT22细胞损伤进一步加重。由此可见,miR-29c对TNF-α诱导的小鼠HT22海马神经元损伤具有保护作用。虽然细胞实验不同于在体神经元所处的细胞环境,研究结果具有一定的局限性,但这些结果还是提示miR-29c具有神经元保护效应,有望成为治疗炎症和凋亡相关神经系统疾病的一个潜在靶点。
以往研究报道,TNF-α通过TNFR1途径介导HT22细胞凋亡[17]。而且,在U251人胶质母细胞瘤细胞和SH-SY5Y人神经母细胞瘤细胞中,miR-29c和TNFR1之间的调控关系通过双萤光素酶报告基因实验得到验证[11]。因此,本研究检测miR-29c是否通过影响TNFR1的表达及下游途径的激活发挥神经元保护效应。通过免疫荧光染色、RT-qPCR和Western blot技术观察到,TNF-α作用于HT22细胞,导致TNFR1表达升高,而miR-29c过表达可显著抑制TNFR1的表达,但在HT22细胞转染miR-29c sponge后,miR-29c表达下降,TNFR1的表达处于高水平,这充分说明miR-29c可以负向调节TNFR1的表达。
TNFR1具有一个可以募集TRADD和FADD的胞质死亡域,通过活化caspase-8从而与位于caspase级联反应下游的caspase-3结合并使其激活,最终诱导细胞凋亡的产生[18-19]。另外,有研究报道,在酗酒模型大鼠的海马组织,TNFR1、TRADD和FADD表达同时上调,而且三者形成复合物,这对caspase-8激活至关重要;在乌头碱诱导的HT22细胞凋亡中也观察到FADD和caspase-8表达同步增加[20-22]。因此,本实验检测miR-29c对TNF-α诱导的HT22细胞凋亡的影响。结果表明,TNF-α处理HT22细胞后,凋亡细胞数目增多,TNFR1、TRADD、FADD、cleaved caspase-8和cleaved caspase-3蛋白水平显著升高。过表达miR-29c后,TNF-α作用于HT22细胞,凋亡细胞数目减少,TNFR1、TRADD和FADD的表达降低,活化型的caspase-8和caspase-3表达下降;相反,当miR-29c的表达被抑制后,TNF-α诱导的上述凋亡相关蛋白表达更加明显,凋亡细胞数目增多。虽然本实验没有直接探讨miR-29c对TNFR1-FADD-TRADD复合物形成的影响,但目前结果仍然提示miR-29c可能通过抑制TNFR1信号通路从而减轻TNF-α诱导的HT22细胞凋亡。这也是本实验的一个局限之处。miR-29c对TNFR1-FADD-TRADD复合物结合的影响有待后续研究。
miRNAs通过与靶基因特异性结合,导致mRNA降解或翻译阻滞,在多种生理病理过程中发挥重要调控作用[23]。miRNA可与多个mRNA下游靶点结合,并且多个miRNA也可调控同一个mRNA下游靶点。此外,氧糖剥夺/复氧处理的HT22细胞中miR-29c水平降低,导致其直接靶点Map2k6的表达增加,促进凋亡发生[8]。在本研究中,miR-29c对TNF-α诱导HT22细胞凋亡的保护作用有可能是通过调控多种mRNA下游靶点产生的。未来还需要通过数据库检索、RNA测序与双萤光素酶报告基因实验等进一步探索与确认miR-29c可以影响哪些mRNA下游靶点的表达,进而产生神经元保护效应。
综上所述,TNF-α诱导HT22细胞损伤后,TNFR1信号通路被激活,TRADD、FADD及活化型cleaved caspase-8/-3蛋白水平升高,细胞凋亡率上升;通过慢病毒转染技术,使HT22细胞中miR-29c表达上调后,TNF-α诱导的上述效应显著减轻,提示miR-29c的神经元保护效应可能是抑制TNFR1信号通路过度激活,从而缓解TNF-α诱导的细胞损伤。
[1] Song Q, Feng YB, Wang L, et al. COX-2 inhibition rescues depression-like behaviors via suppressing glial activation, oxidative stress and neuronal apoptosis in rats[J]. Neuropharmacology, 2019, 160:107779.
[2] Yang Y, Li X, Zhang L, et al. Ginsenoside Rg1 suppressed inflammation and neuron apoptosis by activating PPARɤ/HO-1 in hippocampus in rat model of cerebral ischemia-reperfusion injury[J]. Int J Clin Exp Pathol, 2015, 8(3):2484-2494.
[3] Zong Y, Yu P, Cheng H, et al. miR-29c regulates NAV3 protein expression in a transgenic mouse model of Alzheimer's disease[J]. Brain Res, 2015, 1624:95-102.
[4]古春青, 张运克, 杨广华, 等. 虾青素预处理通过调控Sirt1/miR-134信号通路改善脑缺血再灌注大鼠认知功能[J]. 中国病理生理杂志, 2021, 37(9):1620-1627.
Gu CQ, Zhang YK, Yang GH, et al. Astaxanthin preconditioning ameliorates cognitive function in rats with cerebral ischemia/reperfusion by regulating Sirt1/miR-134 signaling pathway[J]. Chin J Pathophysiol, 2021, 37(9):1620-1627.
[5] Liu Y, Liu D, Xu J, et al. Early adolescent stress-induced changes in prefrontal cortex miRNA-135a and hippocampal miRNA-16 in male rats[J]. Dev Psychobiol, 2017, 59(8):958-969.
[6] Yang Y, Ye Y, Kong C, et al. MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway[J]. Neurochem Res, 2019, 44(4):811-828.
[7] Li C, Wang X, Zhang G, et al. Downregulation of microRNA29c reduces pain after child delivery by activating the oxytocin-GABA pathway[J]. Mol Med Rep, 2020, 22(3):1921-1931.
[8] Tang C, Ou J, Kou L, et al. Circ_016719 plays a critical role in neuron cell apoptosis induced by I/R via targeting miR-29c/Map2k6[J]. Mol Cell Probes, 2020, 49:101478.
[9] Hori Y, Goto G, Arai-Iwasaki M, et al. Differential expression of rat hippocampal microRNAs in two rat models of chronic pain[J]. Int J Mol Med, 2013, 32(6):1287-1292.
[10] Liu Y, Zhou LJ, Wang J, et al. TNF-α differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury[J]. J Neurosci, 2017, 37(4):871-881.
[11] Wang M, Guo J, Dong LN, et al. Cerebellar fastigial nucleus stimulation in a chronic unpredictable mild stress rat model reduces post-stroke depression by suppressing brain inflammation via the microRNA-29c/TNFRSF1A signaling pathway[J]. Med Sci Monit, 2019, 25:5594-5605.
[12] Koh EJ, Seo YJ, Choi J, et al. Spirulina maxima extract prevents neurotoxicity via promoting activation of BDNF/CREB signaling pathways in neuronal cells and mice[J]. Molecules, 2017, 22(8):1363.
[13] Xu Z, Lu Y, Wang J, et al. The protective effect of propofol against TNF-α-induced apoptosis was mediated via inhibiting iNOS/NO production and maintaining intracellular Ca2+homeostasis in mouse hippocampal HT22 cells[J]. Biomed Pharmacother, 2017, 91:664-672.
[14] Wang SS, Jia J, Wang Z. Mesenchymal stem cell-derived extracellular vesicles suppresses iNOS expression and ameliorates neural impairment in Alzheimer's disease mice[J]. J Alzheimers Dis, 2018, 61(3):1005-1013.
[15] Zhao N, Xu X, Jiang Y, et al. Lipocalin-2 may produce damaging effect after cerebral ischemia by inducing astrocytes classical activation[J]. J Neuroinflammation, 2019, 16(1):168.
[16] Yang G, Song Y, Zhou X, et al. DNA methyltransferase 3, a target of microRNA-29c, contributes to neuronal proliferation by regulating the expression of brain-derived neurotrophic factor[J]. Mol Med Rep, 2015, 12(1):1435-1442.
[17] Wang L, Chang X, Feng J, et al. TRADD mediates RIPK1-independent necroptosis induced by tumor necrosis factor[J]. Front Cell Dev Biol, 2019, 7:393.
[18] 商华, 任宪辉, 杨红欣, 等. 姜黄素对Aβ25-35诱导的PC12细胞caspase-3、caspase-8和caspase-9表达的影响[J]. 中国病理生理杂志, 2018, 34 (1):168-172, 182.
Shang H, Ren XH, Yang HX, et al. Effects of curcumin on expression of caspase-3, caspase-8 and caspase-9 in PC12 cells induced by Aβ25-35[J]. Chin J Pathophysiol, 2018, 34(1):168-172, 182.
[19] Zelová H, Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances[J]. Inflamm Res, 2013, 62(7):641-651.
[20] Liu W, Vetreno RP, Crews FT. Hippocampal TNF-death receptors, caspase cell death cascades, and IL-8 in alcohol use disorder[J]. Mol Psychiatry, 2021, 26(6):2254-2262.
[21] Thompson SJ, Ashley MD, Stöhr S, et al. Suppression of TNF receptor-1 signaling in anmodel of epileptic tolerance[J]. Int J Physiol Pathophysiol Pharmacol, 2011, 3(2):120-132.
[22] Wang H, Liu Y, Guo Z, et al. Aconitine induces cell apoptosis via mitochondria and death receptor signaling pathways in hippocampus cell line[J]. Res Vet Sci, 2022, 143:124-133.
[23] D'Amato G, Luxán G, del Monte-Nieto G, et al. Sequential Notch activation regulates ventricular chamber development[J]. Nat Cell Biol, 2016, 18(1):7-20.
miR-29c attenuates TNF-α-induced injury of mouse hippocampal neuronal HT22 cells via inhibition of TNFR1 signaling pathway
LI Bo, LU Ying, WAN Qi, LIANG Xuan, YANG Yi-wen, HAN Yong, ZENG Jun-wei△
(,,563000,)
To investigate the effect and mechanism of microRNA-29c (miR-29c) on tumor necrosis factor-α (TNF-α)-induced mouse hippocampal neuronal HT22 cell injury.Overexpression and low expression of miR-29c were performed by lentivirus (LV) transfection in mouse hippocampal neuronal HT22 cells. The HT22 cells were divided into LV-vector (cell line with empty vector) group, LV-vector+TNF-α group, LV-miR-29c (cell line with overexpression of miR-29c)+TNF-α group and LV-miR-29c sponge (cell line with low expression of miR-29c)+TNF-α group. The toxicity of TNF-α to HT22 cells was evaluated by CCK-8 and lactate dehydrogenase (LDH) release assays. The expression of tumor necrosis factor receptor 1 (TNFR1) was observed by immunofluorescence staining. The expression of miR-29c and TNFR1 mRNA was detected by RT-qPCR. The protein levels of TNFR1, TNFR-associated death domain protein (TRADD), Fas-associated death domain protein (FADD), pro-caspase-8, cleaved caspase-8, pro-caspase-3 and cleaved caspase-3 were determined by Western blot. Apoptosis was observed via TUNEL staining and Hoechst 33258 staining.(1) The results of CCK-8 and LDH release assays showed that compared with LV-vector group, the cell viability was significantly decreased in LV-vector+TNF-α group (<0.05), and the release of LDH was significantly increased (<0.05). Compared with LV-vector+TNF-α group, the cell viability was significantly increased in LV-miR-29c+TNF-α group (<0.05), and the release of LDH was significantly decreased (<0.05). However, decreased cell viability induced by TNF-α was substantially deteriorated in LV-miR-29c sponge+TNF-α group. (2) The results of RT-qPCR and immunofluorescence staining showed that compared with LV-vector group, the expression of TNFR1 in LV-vector+TNF-α group was significantly increased (<0.05). Compared with LV-vector+TNF-α group, the expression of TNFR1 in LV-miR-29c+TNF-α group was significantly decreased (<0.05), while that in LV-miR-29c sponge+TNF-α group was significantly increased (<0.05). (3) The results of Western blot showed that compared with LV-vector group, the protein levels of TNFR1, TRADD, FADD, cleaved caspase-8 and cleaved caspase-3 in LV-vector+TNF-α group were significantly increased (<0.05). Compared with LV-vector+TNF-α group, the protein levels of TNFR1, TRADD, FADD, cleaved caspase-8 and cleaved caspase-3 in LV-miR-29c+TNF-α group were significantly decreased (<0.05), while those in LV-miR-29c sponge+TNF-α group were significantly increased (<0.05). (4) The results of Hoechst and TUNEL staining showed that the number of apoptotic cells in LV-vector group was significantly increased after TNF-α application (<0.05). Compared with LV-vector+TNF-α group, the number of apoptotic cells in LV-miR-29c+TNF-α group was significantly decreased (<0.05), while that in LV-miR-29c sponge+TNF-α group was significantly increased (<0.05).miR-29c attenuates the injury of mouse hippocampal neuronal HT22 cells induced by TNF-α, and its mechanism may be related to the inhibition of TNFR1 signaling pathway.
MicroRNA-29c; Tumor necrosis factor-α; TNFR1 signaling pathway; Apoptosis; Hippocampal neurons
1000-4718(2022)09-1537-10
2022-04-07
2022-07-29
13765253805; E-mail: junweizeng@sohu.com
R338.2; R363.2
A
10.3969/j.issn.1000-4718.2022.09.001
[基金项目]国家自然科学基金资助项目(No. 31860291);贵州省教育厅创新群体重大研究项目(黔教合KY字[2018]025)
(责任编辑:宋延君,李淑媛)
