Application of Micro Electro Mechanical System(MEMS) Technology in Photoacoustic Imaging
2022-06-30LixiaBaoXiaoyingTangZhenqiJiang
Lixia Bao, Xiaoying Tang, Zhenqi Jiang
Abstract: Photoacoustic imaging (PAI) is a new biomedical imaging technology that provides a mixed contrast mechanism and excellent spatial resolution in biological tissues. It is a non−invasive technology that can provide in vivo anatomical and functional information. This technology has great application potential in microscopic imaging and endoscope system. In recent years, the devel−opment of micro electro mechanical system (MEMS) technology has promoted the improvement and miniaturization of the photoacoustic imaging system, as well as its preclinical and clinical appli−cations. This paper introduces the research progress of MEMS technology in photoacoustic micro−scope systems and the miniaturization of photoacoustic endoscope ultrasonic transducers, and points out the shortcomings of existing technology and the direction of future development.
Keywords: photoacoustic imaging; photoacoustic microscope; photoacoustic endoscope; micro elec−tro mechanical system (MEMS); ultrasonic transducer
1 Introduction
Photoacoustic imaging (PAI) is a new rapidly growing biomedical imaging tool that is based on the photoacoustic (PA) effect using the configu−ration of light excitation and the ultrasound(US) capture. It has optical−ultrasound contrast mechanisms and multi−scale imaging ability.Thanks to the characteristics of PA wave genera−tion, PAI enables visualization of relatively deep biological tissues as compared to typical pure optical imaging techniques (i.e., up to 1 mm),while maintaining high spatial resolution[1−5].
PAI technology combines the high contrast characteristics of pure optical imaging and the advantages of high penetration depth of pure ultrasonic imaging, which has become a hot spot and frontier in the field of medical imaging. Com−pared with traditional imaging technologies, such as X−ray computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET), PAI can achieve equivalent resolution with smaller, faster, and less expen−sive systems. Unlike pure ultrasound imaging that only reflects the mechanical properties of tissues with the image contrast provided by acoustic impedance variations, PAI possesses rich optical contrast and is capable of providing bio−logical functions or physiological parameters,such as oxygen saturation of hemoglobin, meta−bolic rate, and the relative concentration of hydrated fat, which can be used for noninvasive diagnosis of various diseases [6]. Compared to pure optical imaging techniques, such as optical coherence tomography (OCT), confocal micros−copy, and multiphoton microscopy, PAI can achieve a significantly larger imaging depth in most human tissues because the attenuation of the echo ultrasound signals in PAI is of two or three orders of magnitude lower than that of the returning optical signals in those pure optical imaging modalities[7]. PAI has been widely used in brain functional imaging[8], early cancer detec−tion[9], vascular visualization[10, 11], and arthri−tis diagnosis[12].
In PAI technology, photoacoustic micro−scope (PAM) and photoacoustic endoscope(PAE) are important components [13, 14], and guide the main development direction [15]. For PAM, the speed of the scanner is the key factor to determine the image acquisition rate. Conven−tional mechanical translation tables and optical galvanometer scanners have either limited scan−ning speed or low signal sensitivity [16], which limits the resolution of the photoacoustic micro−scope. PAE is a special form of PAM, which is characterized by miniaturization of the imaging system and special scanning method to realize the imaging of internal organs [17]. However, the development of photoacoustic endoscopes is chal−lenged by the manufacturing and assembly of micro imaging elements [18].
The micro electro mechanical system(MEMS)−based micromachining technology has several advantages, including size, low weight,low cost with mass production, and exce−llent performance, which can be a good solution to address these existing PAI challenges [19].MEMS technology has also revolutionized sev−eral biomedical tools for fabricating miniaturized diagnostic modalities and screening assays, such as micro−sensors, actuators, micro−channels, micro−optics, etc. [20]. These microdevices based on MEMS have made great contributions to the per−formance improvement of PAI technology in the PAM and PAE systems.
This paper focuses on the research progress of PAM and PAE based on MEMS technology.The development of MEMS technology con−tributes to the miniaturization of instruments and equipment, improves the performance of equipment, and provides help for its preclinical and clinical practice in biomedicine.
2 Photoacoustic Microscope (PAM)Based on MEMS Technology
PAM is a high−resolution PAI technique, where a nanosecond pulsed laser beam at a high laser rep−etition rate irradiates the sample. In PAM, it is the tissue optical properties that determine the contrast. Acoustic waves generated due to ther−mal expansion are detected by a high−frequency transducer [21−23]. According to whether the lat−eral resolution is through optical focusing or acoustic focusing, PAM can be classified as opti−cal−resolution PAM (OR−PAM) and acoustic res−olution PAM (AR−PAM). In OR−PAM, imaging depth is limited by the optical diffusion limit(about 1 mm in biological tissue), which limits the application of OR−PAM technology in pre−clinical and clinical imaging. In AR−PAM, the lateral resolution is reduced, but it overcomes the optical diffusion limit ( about 1 mm) and improves the penetration depth in biological tis−sue. AR−PAM can be used to explore a variety of biomedical information, such as anatomical imag−ing [24], functional imaging [25], and molecular imaging [26].
Normally in conventional AR−PAM, two−dimensional mechanical raster scanning is emplo−yed to reconstruct three−dimensional images. The use of stepper motor−based mechanical stages results in slow scanning speed and is highly unlikely to build miniaturized devices [27, 28].Due to these slow acquisition speeds, the wides−pread uses of conventional AR−PAM systems are not practical in preclinical and clinical studies.Thus, it is important to develop an AR−PAM system that has the capability of wide−field scan−ning and high−speed imaging to make an AR−PAM system suitable for widespread clinical use.With the development of MEMS technology,compared to the grating scanning system, the MEMS scanner used in PAI will help to signifi−cantly improve the image acquisition time, so as to realize most real−time image visualization. The ability to use MEMS to obtain OR−PAM and AR−PAM images will help to visualize high−reso−lution surface blood vessels and low−resolution deep blood vessels, so as to make full use of the potential of high−resolution photoacoustic tech−nology at high speed [29], making it possible for AR−PAM system to achieve a wide range of pre−clinical and clinical applications that have not been possible so far.
2.1 OR-PAM System Based on MEMS
OR−PAM is one of the fastest developing micro−scopic imaging technologies [30], which uses inherent biological contrast and has lateral reso−lution with large penetration depth. In tradi−tional OR−PAM, a two−dimensional (2D) elec−tromechanical scanner is required to raster scan the overlapping focus of Optics and acoustics to form a three−dimensional (3D) image [31, 32].However, due to the limitation of scanning speed and volume, OR−PAM is difficult to improve time resolution and miniaturization.
Using MEMS scanner, a high−speed OR−PAM system was developed earlier. W. Z. Qi et al. [33] reported a miniaturized optical resolu−tion PAM system based on a MEMS scanning mirror. A two−dimensional MEMS scanning mir−ror was used to achieve raster scanning of the excitation optical focus. The wideband photoa−coustic signals were detected by a flat ultra−sound transducer with a center frequency of 10 MHz and an active area of 2 mm in diameter.The size and weight of this device were 60 mm ×30 mm × 20 mm and 40 g, respectively. Sharp blades, carbon fibers, and a silver strip target were used to evaluate this system. In vivo experi−ments of imaging vasculatures in the mouse ear,brain, and human lip were completed to demon−strate its potential for biological and clinical applications.
The introduction of a water−immersible MEMS scanner has improved the OR−PAM imaging speed significantly compared to raster mechanical stage scanning and also helps to develop handheld−based devices [34−36]. J. Yao et al. [35] developed a special uniaxial water immersion MEMS mirror based on OR−PAM and studied in vivo vascular imaging, as shown in Fig. 1. In this system, the planar transducer with a center frequency of 50 MHz is equipped with an acoustic lens to improve the sensitivity. The one−dimensional immersion MEMS scanner is imme−rsed in water to reflect laser pulses and sound waves. The system can significantly improve the imaging speed, maintain the confocal alignment of optical and acoustic beams in a wide field of view (Fig. 1 (a)), retain the high spatial resolu−tion and imaging sensitivity of the traditional OR−PAM system, and achieve a B−scan imaging speed of 400 Hz in a 3 mm scanning range.MEMS−OR−PAM of blood flow dynamics of the vasculature in a mouse ear was shown in Fig.1(b). Capillaries were clearly resolved, and the flow dynamics over a 2 mm × 5 mm area were imaged with a 0.8 Hz volumetric frame rate and 400 Hz B−scan rate. J. Yao et al. conducted a preclinical study on the mouse brain using a high−speed MEMS scanner [37]. Two pulsed lasers ( i.e. picosecond and nanosecond pulses)with a single wavelength of 532 nm are used for a high−resolution display of blood oxygen.
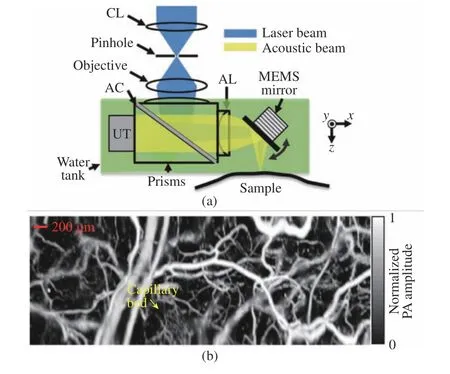
Fig. 1 An OR−PAM based on water immersion MEMS mirror and its application: (a) schematic of MEMS−OR−PAM;(b) MEMS−OR−PAM of blood flow dynamics of the vasculature in a mouse ear (Authorized by the authors according to the CC BY license)
The 1−axis water immersible MEMS scan−ning mirror can greatly increase the B−scan imag−ing speed of OR−PAM. However, it still has limi−tations such as bulky system size due to the additional motorized stage for volumetric imag−ing. For clinical translation, such as endoscopy,laparoscopy, or handheld systems, it is essential to have both high imaging speed and small sys−tem size. To overcome these limitations, two kinds of 2−axis water immersible MEMS scan−ning mirrors were developed [38, 39]. Similar to the 1−axis water immersible MEMS scanning mir−ror, they are also made of flexible polymer instead of brittle silicon. One was fabricated by a laser cutting of biaxially−oriented polyethylene terephthalate (BOPET) film, and the other was made by soft lithography of polydimethylsilox−ane (PDMS) . The 2−axis water immersion MEMS scanning mirrors simultaneously con−trolled the optical and acoustic beams along two axes on a scanner. The Aluminized Silicon Mir−ror improved the reflectivity of the optical and acoustic beams. The strong electromagnetic drive along the two axes was used to overcome the water resistance. Without using any motorized stage, this OR−PAM system can achieve a high B−scan rate of 50 Hz and a volumetric imaging rate of 0.25 Hz. For this system, lateral and axial resolutions were 3.6 μm and 27.7 μm, respec−tively.
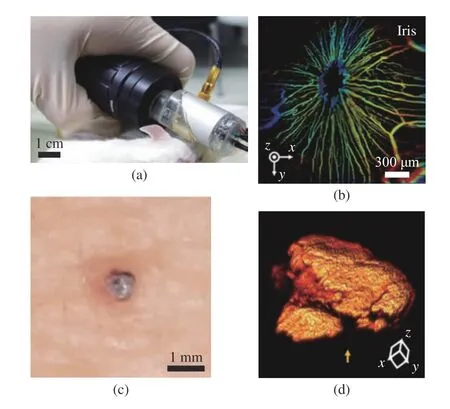
L. Lin et al. demonstrated a handheld PAM system based on 2−axis water immersible MEMS scanning mirror, as shown in Fig. 2 [40]. This handheld OR−PAM system has a dimension of 80 mm×115 mm×150 mm and is more flexible than conventional benchtop systems. The lateral resolution of the handheld system was 5 μm, and the 3D volumetric imaging rate over a region of 2.5 mm × 2.0 mm × 0.5 mm was 2 Hz. To ver−ify the usage of the handheld PAM in clinical applications, they acquired PA images of a human cuticle and a mole on a volunteer’s leg, as shown in Fig. 3 . Handheld OR−PAM provides higher spatial resolution in the superficial region,and it is possible to evaluate several tumor edges by measuring the vascular system and blood oxy−gen saturation. K. Park et al. reported a much smaller handheld OR−PAM probe, as shown in Fig. 3(a) [41]. They modified the water immer−sible MEMS scanning mirror to a round shape to reduce the system size. In this system, all acous−tical, optical, and mechanical components are integrated into a single probe with a diameter of 17 mm and a weight of 162 g. A PA image with 700 pixel × 700 pixel is obtained, with measured B−scan and volumetric imaging speeds of 35 Hz and 0.05 Hz, and the laser is operated at 532 nm with a 50−kHz−repetition rate. The measured lat−eral and axial resolutions are 12 μm±1.6 μm and 30 μm±3.1 μm, respectively. Due to its small size and fast imaging speed, this handheld probe is suitable for imaging small animals and humans.Fig. 3(b) showed the depth map image of the front view of mouse iris microvessels, and Fig. 3(d)showed the 3D image of volunteer finger nevus.

Fig. 2 OR−PAM of the human skin by the handheld probe: (a)OR−PAM image of capillaries in a cuticle; (b) photo−graph of the OR−PAM handheld probe imaging a red mole on a volunteer’s leg; (c) photograph of the mole;(d) OR−PAM image of the mole. The color bar shows the normalized PA signal amplitude (Authorized by the authors according to the CC BY license)
MEMS technology improves the imaging rate and miniaturization of scanning mirrors,improves the resolution of PAM, and acceleratesthe development of handheld PAM. At the same time, its potential for biological and clinical applications has been proved through in vivo experiments.
2.2 AR-PAM System Based on MEMS
Acoustic−resolution photoacoustic microscopy(AR−PAM) is an emerging biomedical imaging modality that combines superior optical sensitiv−ity and fine ultrasonic resolution in an optical quasi−diffusive regime (about 1 - 3 mm in tis−sues). AR−PAM has been explored for anatomi−cal, functional, and molecular information in bio−logical tissues. Heretofore, AR−PAM systems have suffered from a limited field−of−view (FOV)and slow imaging speed, which have precluded them from routine preclinical and clinical appli−cations.
The scanner is the key component to limit the scanning speed of PAM. Improving the work−ing speed of the scanner can improve the imag−ing speed of PAM. J. W. Baik et al. [42] devel−oped an ultra−wide field AR−PAM system based on a waterproof MEMS scanner, which over−comes the limitations of traditional AR−PAM.The developed system utilizes a custom−designed MEMS scanner for high−speed imaging and is integrated with two linear stepper motors for wide−field scanning. The key specifications of our system include fine spatial resolutions (84 μm for lateral and 38 μm for axial), fast imaging speed(83 Hz for B−scan over 2.5 mm), wide FOV(36 mm × 80 mm with an acquisition time of 224 s), signal noise ratio (SNR) (32 dB in mice and 23 dB in humans in vivo) and imaging depth(2.3 mm in mice and 1.6 mm in humans).Exploiting these strengths, the researchers per−formed whole−body in−vivo AR−PAM imaging of small animals at multiplanes from ventral, sagit−tal, and dorsal views, and successfully obtained multi−layered distinct structures of the animals non−invasively including small capillaries, major blood vessels as well as internal organs. More−over, anatomical structures of subcutaneous microvascular networks in human fingers, palms,and forearms were identified. Due to its superior imaging capability for microvascular networks with wide FOV, the developed MEMS−AR−PAM system could potentially contribute significantly to the preclinical and clinical evaluation of con−stitutional diseases, peripheral vascular diseases,and dermatological diseases.
The use of uniaxial MEMS and mechanical stage helps to obtain images faster than scan−ning only through the mechanical stage, but it is not conducive to the construction of handheld devices or microdevices. M. Moothenchery et al.[43] used a 2−axis MEMS scanner for high−speed AR−PAM imaging. The structure of the AR−PAM system is shown in Fig. 4(Ⅰ). The perfor−mance of this system includes lateral resolution,axial resolution, maximum imaging depth, in vivo vascular imaging, and phantom imaging. At the same time, the influence of transducer fre−quency on resolution characteristics and imaging depth is also studied. MEMS scanner was used for high−speed deep tissue imaging. A lateral res−olution of 84 μm and an axial resolution of 27 μm with about 2.7 mm imaging depth were achieved using a 50 MHz transducer−based AR−PAM sys−tem. The use of a higher frequency transducer at 75 MHz has further improved the resolution characteristics of the system with a reduction in imaging depth and a lateral resolution of 53 μm and an axial resolution of 18 μm with about 1.8 mm imaging depth were achieved. Using the two−axis MEMS scanner, a 2.0 mm × 2.5 mm area was imaged in 3 s, as shown in Fig. 4(Ⅱ,Ⅲ). The capability of achieving acoustic resolu−tion images using the MEMS scanner makes it beneficial for the development of high−speed miniaturized systems for deeper tissue imaging,which is related to many clinical skin imaging applications and can perform almost real−time imaging.
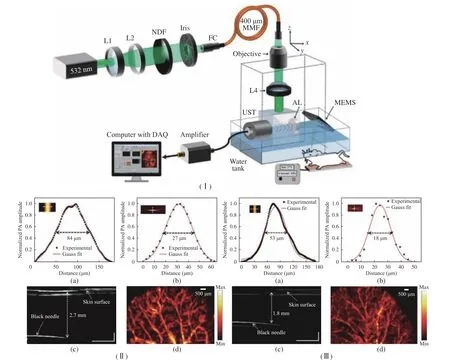
Fig. 4 Structure, performance and application of AR−PAW system: (Ⅰ) schematic of the AR−PAM system; (Ⅱ) characteristics of the 50 MHz AR−PAM system; (Ⅲ) characteristics of the 75 MHz AR−PAM system; (a) lateral resolution; (b) axial resolution; (c)maximum imaging depth; (d) MIP image of the mouse ear (Copyrights @ 2019, John Wiley and Sons)
2.3 OR-AR-PAM System Based on MEMS
Typically, OR−PAM is used to image superficial vasculatures and AR−PAM is used to image deeper vasculatures under the skin. Since the same optical and acoustic components can be used for both OR−PAM and AR−PAM, it would be of great advantage to combine them and co−register superficial optical resolution images and deep acoustic resolution images. The scalable res−olution and penetration depth achievable using such a combined OR−AR system makes complete utilization of high−resolution PAI technology at a reduced cost.
M. Moothanchery et al. [29] developed a high−speed and wide−area scanning integrated OR−AR−PAM system combining MEMS scanner and raster mechanical movement, which can achieve high−resolution shallow depth images and low−resolution images at increased imaging depth.The structure of this system is shown in Fig. 5(Ⅰ). Using a 50 MHz transducer, 5 μm lat−eral resolution with 0.9 mm imaging depth in vivo was achieved using OR−PAM and 84 μm lat−eral resolution with 2 mm imaging depth in vivo was achieved using AR−PAM. The axial resolu−tion of the system was 27 μm. The combined effect of the system was demonstrated by per−forming in vivo imaging of the mouse ear and abdomen region, which was shown in Fig. 5(Ⅱ,Ⅲ). The combined OR−AR−PAM imaging achiev−able using the MEMS scanner will be beneficial for the clinical translation of miniaturized hand−held scanning devices, mainly for a skin imaging application.
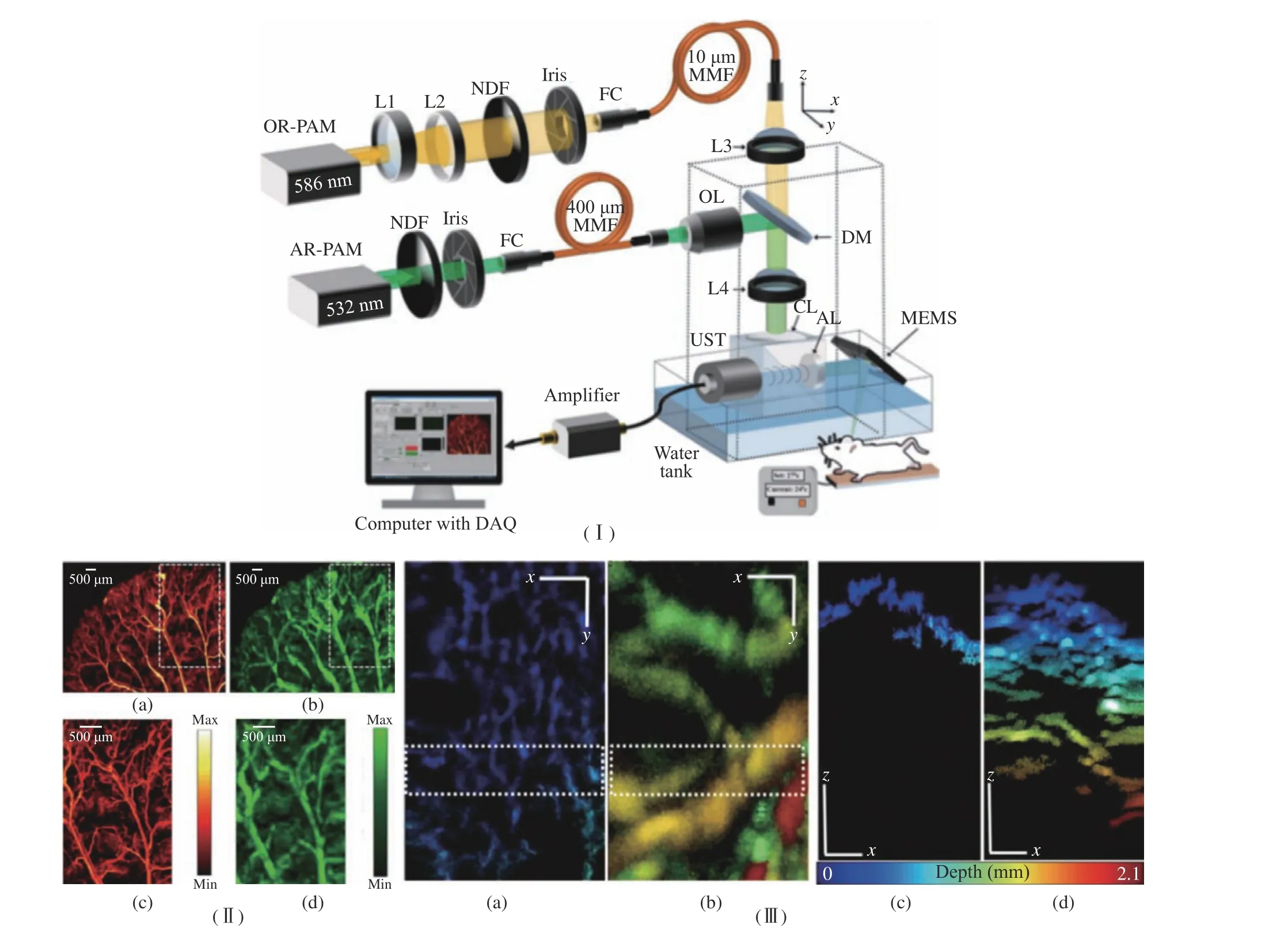
Fig. 5 Structure and application of OR−AM−PM system: (Ⅰ) schematic diagram of the OR−AR−PAM system; (Ⅱ) MIP images of a mouse ear: (a) OR−PAM, (b) AR−PAM, (c) ROI in OR−PAM, and (d) ROI in AR−PAM; (Ⅲ) MIP images of the dorsal side of the mouse abdomen: (a) OR−PAM, (b) AR−PAM, (c) depth profile MIP of the ROI shown in OR−PAM image,(d) depth profile MIP of the ROI shown in OR−PAM image (Authorized by the authors according to the CC BY license)
C. Zhang et al. [44] presented an integrated optical−resolution (OR) and acoustic−resolution(AR) multiscale PAM based on free−space light transmission and fast MEMS scanning. The lat−eral resolution for OR is 4.9 μm, and the lateral resolution for AR is 114.5 μm. The maximum imaging depth for OR is 0.7 mm, and the maxi−mum imaging depth for AR is 4.1 mm. The imaging speed can reach 5 × 104aline/s. The high signal−to−noise ratios and wavelength throughput are achieved by delivering light via free−space, and the high speed is achieved by a MEMS scanning mirror.
3 Photoacoustic Endoscope (PAE)Based on MEMS Technology
Photoacoustic imaging technology has great application potential in the field of endoscopy.The combination of photoacoustic imaging tech−nology and traditional endoscopes gave birth to photoacoustic endoscopy (PAE) [45 ]. Photoa−coustic endoscopes have better contrast than ultrasonic endoscopes and optical coherence tomography endoscopes. Traditional PAE usu−ally needs to introduce rotating motor for tan−gential scanning [46, 47], combining with axial scanning to obtain volume imaging data. This scanning method has high requirements for micro electric shock, and the imaging speed is slow and expensive. Moreover, during mechanical scan−ning, the micro−motor will introduce crosstalk noise in the process of rotation, and meanwhile,the mechanical jitter caused by the moving pro−cess will affect the quality of the photoacoustic image. The development of photoacoustic endo−scopes is challenged by the manufacturing and assembly of micro imaging elements. In the past decade, MEMS technology has greatly promoted the development of a photoacoustic endoscope.H. Guo et al. [33] integrated MEMS mirrors to develop photoacoustic endoscopic probes with high system compactness and data acquisition speed. Compared to previous endoscopes based on hybrid mechanical scanning, 2D internal scan−ning with a maximum scanning frequency of 500 Hz can be achieved by using micro MEMS endoscopes. In addition, MEMS−based scanning does not produce electromechanical noise on pho−toacoustic signals and is more accurate than mechanical motor scanning.
Micromachined ultrasonic transducers(MUTs), as the key components of the photoa−coustic endoscope, include piezoelectric ultra−sonic transducers (pMUTs) [48] and capacitive ultrasonic transducers (cMUTs) [49, 50 ]. To improve the detection sensitivity and bandwidth of PAE, different methods are proposed in the field of MEMS research to design cMUT and pMUT. Next, the ultrasonic transducer based on MEMS technology for a photoacoustic endoscope is mainly introduced.
3.1 Photoacoustic Endoscope (PAE) pMUT Based on MEMS
In the PAE system, pMUT possesses low elec−tromechanical coupling and does not need the high polarization voltage and small capacitance gap required by cMUT, so it has a wider applica−tion.
Chen et al. [48] reported a novel pMUT based on aluminum nitride (AlN) and its appli−cation in photoacoustic imaging. The device con−sists of SiO2, a bottom electrode, AlN films, an upper electrode, and a polyimide protective layer.AlN was grown on a 300 nm−thick SiO2film and a 200 nm−thick bottom electrode at room temper−ature. An area ratio of 0.45 was used between the upper electrode and the vibration area of the pMUT to provide an optimal sensitivity of the transducer. Its resonant frequency was measured to be 2.885 MHz, and the coupling coefficient was in the range of 2.38% - 3.71%. The fabri−cated pMUT was integrated with a photoacous−tic imaging system and photoacoustic image of human hair tissue based on agar. The resolution of the system is 240 μm. Based on AlN pMUT,the pMUT array will be integrated into the future MEMS−based photoacoustic endoscope for in vivo imaging of tissue samples. At the same time, the bandwidth of pMUT must be improved to use micro PAE for deeper and higher resolu−tion imaging [51].
3.2 Photoacoustic Endoscope cMUT Based on MEMS
As a new generation of MEMS ultrasonic trans−ducer, cMUT uses the capacitance change related to the energy conversion between the silicon sub−strate and thin−film layer to detect US signals.
Compared with traditional bulk piezoelec−tric transducers, cMUT has many advantages,including wide bandwidth, high electromechani−cal coupling coefficient, and easy fabrication of arrays with a wide range of frequencies and sizes[52]. In 2010, Nikoozadeh et al. reported linear and circular cMUT arrays for intracardiac imag−ing applications [53]. They developed a 24−ele−ment 1D cMUT phased−array and a 64−element annular cMUT array both with a center fre−quency of 10 MHz, and assembled them into a 3 mm diameter catheter and a 4 mm diameter catheter, respectively. The one−dimensional cMUT array had a dimension of 1.7 mm × 1.3 mm,while the ring cMUT array had an inner and an outer diameters of 1.6 mm and 2.5 mm, respec−tively. Because the capacitance of the cMUT ele−ment is small (0.3 pf) and the parasitic capaci−tance of the micro coaxial cable is large (about 200 pf), it is necessary to customize the designed integrated circuit (IC) as a low−noise amplifier to improve the signal−to−noise ratio and imaging quality.
In 2012, Nikoozadeh et al. further demon−strated the combined ultrasound and photoacous−tic imaging based on the developed 3 mm catheter based on cMUT array [54]. The 3 mm cMUT catheter was assembled into a fiber−opti−cal catheter with an outer diameter of 8 mm. In their imaging experiment, the cMUT elements were biased at 50 V and excited with 55 Vp−p alternating current (AC) pulses. Photoacoustic images of the mouse kidney tumor model were obtained to show the blood vessels in the tumor.A. S. Savoia et al. [55] reported the development of an integrated two−dimensional cMUT array for volumetric ultrasound imaging. Acoustically opti−mized 3−D packaging technology had been applied to the design, manufacture, and charac−terization of the multichip module. The multi−chip module obtained a low−noise receiver and programmable transmit beamformer through the 3−D interconnection of 256 elements cMUT spi−ral array and 256 channel analog front−end appli−cation−specific integrated circuit (ASIC) with the integrated pulse generator.
cMUT array is used in photoacoustic endo−scope systems because of its wide frequency band and high sensitivity. However, cMUT also has limitations in manufacturing and operation.First, the small capacitance gap of cMUT (usu−ally in the range of 100-200 nm) is a manufac−turing challenge, which requires complex and accurate manufacturing process control. Second,because the active capacitance of cMUT is very small, its performance is greatly affected by para−sitism. Third, the high bias voltage is needed to achieve high sensitivity, which may cause safety problems in biomedical in vivo imaging. There−fore, high−frequency cMUT with high−resolution photoacoustic endoscope application is very chal−lenging.
4 Summary
Photoacoustic imaging technology has developed rapidly in the biomedical field and has been widely studied in preclinical and clinical applica−tions. Photoacoustic microscope and photoacous−tic endoscope system are the main development directions of photoacoustic imaging technology,but a common challenge in the development of these devices is the design and integration of micro−optical and mechanical components of the devices. Based on the precision machinery, micro−machining technology, and optical functions of MEMS, the miniaturization and integration of various scanning mirrors, ultrasonic transducers,movable and tunable mirror lenses, and other optical structures are possible. MEMS technol−ogy improves the resolution of the photoacoustic microscope and accelerates the development of a handheld photoacoustic microscope. At the same time, in vivo experiment also proves its potential in biological and clinical applications. MEMS−based scanning mirror is applied to the probe of the photoacoustic endoscope, which can improve the scanning rate and accuracy without introduc−ing electromechanical noise to the photoacoustic signal. Micromachined ultrasonic transducer is the key component of a photoacoustic endoscope.The development of MEMS technology promotes its miniaturization and integration and improves the detection sensitivity and bandwidth of the endoscope.
Although the development of MEMS tech−nology has made great progress in photoacoustic imaging technology, its clinical application still needs to be further optimized. For example,MEMS scanning mirror has not reached the bit size, which limits its application in endoscopic devices. At the same time, photoacoustic imag−ing system needs complex and accurate manufac−turing process requirements to improve the per−formance and stability of the system. It is expected that advanced MEMS technology can realize photoacoustic imaging technology, pro−vide cellular and molecular characteristics through deep tissue penetration, promote the development of high performance and obtain bet−ter clinical practical results.
Acknowledgments
The authors want to thank the Analysis & Test−ing Center at Beijing Institute of Technology.
杂志排行
Journal of Beijing Institute of Technology的其它文章
- A New Class of Biodegradable Organic Optoelectronic Materials: α-Oligofurans
- Design of Implant Prosthesis for Bone Injury Repair Considering Stress Shielding Effect
- A Microfluidic System with Active Mixing for Improved Real-Time Isothermal Amplification
- Mixing Dynamics and Synthesis Performance of Staggered Herringbone Micromixer for Limit Size Lipid Nanoparticles
- Effect of Auxiliary Gas Flow Parameters on Microstructure and Properties of Ta Coatings Prepared by Three-Cathode Atmosphere Plasma Spraying
- Nanoscale Metal-Organic Frameworks:Stimulus-Response and Applications
