A New Class of Biodegradable Organic Optoelectronic Materials: α-Oligofurans
2022-06-30SiyuJiXuhuiJin
Siyu Ji, Xuhui Jin
Abstract: Organic optoelectronic materials have received considerable attention due to their great potentials in electronic devices,such as organic field−effect transistors (OFETs), organic light−emit−ting diodes (OLED) and organic photovoltaic cells (OPV). Besides, their fascinating properties of flexibility, biocompatibility, molecular diversity, low−cost and solution processability bring new opportunities in bioelectronics in the past decade. While almost all known organic optoelectronic materials are obtained from unrenewable fossil resources and nondegradable, a new family of organic optoelectronic materials is now emerging, which can be obtained from green plants and are biodegradable. Meanwhile, they exhibit excellent optoelectronic properties. This review summarized the synthesis and important molecular properties of this new class of biodegradable organic opto−electronic materials: α−oligofurans. Recent progress of furan−based materials and the existing chal−lenges are also discussed to stimulate further advances in the study of this class of materials.
Keywords: organic optoelectronic materials; oligofurans; biodegradable
1 Introduction
Organic optoelectronic materials are widely used in organic photovoltaic (OPV), organic field−effect transistors (OFETs), organic light−emit−ting diodes (OLEDs), biosensors, bioelectronics and many others [1−5]. Comparing with inor−ganic optoelectronic materials, organic optoelec−tronic materials show great advantages of solu−tion processability, light−weight, high mechanical flexibility, and good biocompatibility. These fas−cinating properties originate from their various conjugated molecular structures. During the past decades, many efforts have been devoted to design and synthesize novel organic optoelec−tronic materials [6−7]. Although huge numbers of materials with new structures have been pre−pared, the types of basic aromatic building blocks which were used to construct the conjugated backbone of organic optoelectronic materials remain limited. The common building blocks include aromatic benzene rings and some unsatu−rated five−membered heterocycles such as thio−phene or pyrrole (Fig. 1).
Fig. 1 Molecular structures of common unsaturated five−mem−bered heterocycles
Furan is one of the representative unsatu−rated heterocycles. It is widely presented in natu−ral organic compounds [8]. As one of the sim−plest heteroaromatic compounds [9−11], it was discovered in 1870. Unlike thiophene− or acene−based materials, furan can be obtained entirely from renewable resources. Its main precursor, fur−fural, has been industrially produced from biolog−ical waste (Fig. 2(a)) [12]. More importantly,furan−only materials and many furan−based derivatives are biodegradable. Therefore, noncon−jugated furan−containing polymeric materials have been widely used for biomedical applica−tions. Nevertheless, because furan is more elec−tron rich and less aromatic than thiophene, it exhibits great reactivity which results in syn−thetic challenge in the preparation of long conju−gated oligofurans. Over a long period of time,furan has received less attention as a building block for constructing optoelectronic materials[13]. Even short α −oligofurans have never been structurally characterized until 2010 when Bendi−kov and colleagues successfully achieved long unsubstituted α−oligofurans up to nine rings (Fig.2(b)). Here we summarize great advantages of α−oligofurans and provide a general overview of the optoelectronic properties of furan−based conju−gated materials.
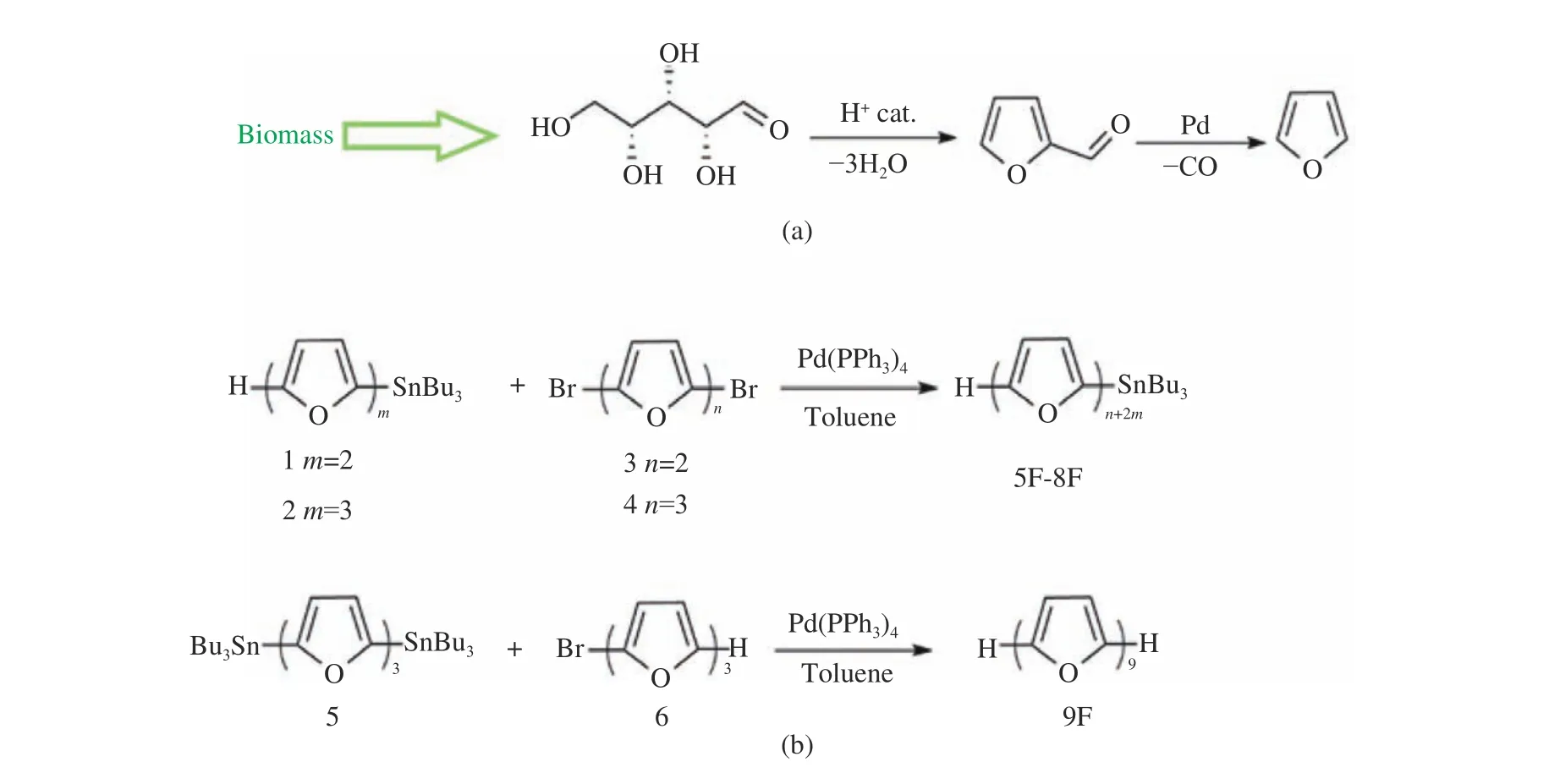
Fig. 2 Synthesis of furan and oligofuran: (a) synthesis of furan from D−xylose[12]; (b) synthesis of long oligofuran
2 Oligofuran
2.1 Solubility
Both oxygen and sulfur belong to the sixth main group in the periodic table, and oxygen has greater electronegativity than sulfur. The elec−tronegativity of oxygen and sulfur are around 3.5 and 2.5, respectively, so furan is less aromatic than thiophene [14]. Comparing with oligothio−phenes, oligofurans typically show better solubil−ity.
In 2009, the Bendikov group firstly reported the synthesis of α −oligofurans 5F−9F [15]. They found that the solubility of oligofurans was sig−nificantly better than that of the corresponding oligothiophenes, for example, the solubility of 6F in chloroform was 0.7 mg/mL, while that of 6T was only <0.05 mg/mL. Higher solubility enables better processability of furan materials. The heats of fusion of 4F and 4T are 6.5 kcal·mol-1and 10.5 kcal·mol-1, respectively, accounting for the weak intermolecular interactions in the solid state. Better solubility results in better process−ability of furan materials.
2.2 Planarity
In conjugated systems, planarity is a prerequi−site for obtaining good p−orbital overlap and elec−tron delocalization. However, non−fused ring con−jugated systems have very flexible conjugated backbones due to their ability to rotate around C−C bonds [16−17]. Oligofurans have rigid conju−gated backbones and are more planar. According to literature reports, twisting each interring bond of 6F to 35° requires an energy of 12.5 kcal·mol-1,while the similar twisting in 6T requires only 2.3 kcal·mol-1(Fig. 3(a)) [10,18]. In 2013, Sharma et al. calculated by DFT theory that the bond length between oligofuran rings is shorter than that of oligothiophene, indicating that the car−bon−carbon bonds between oligofuran rings have more obvious double bond characteristic (Fig.3(b)). This indicates that oligofurans have more pronounced quinoid resonance structures (Fig.3(c)), stronger rigidity, and better planarity than their corresponding oligothiophene analogs[19−20].
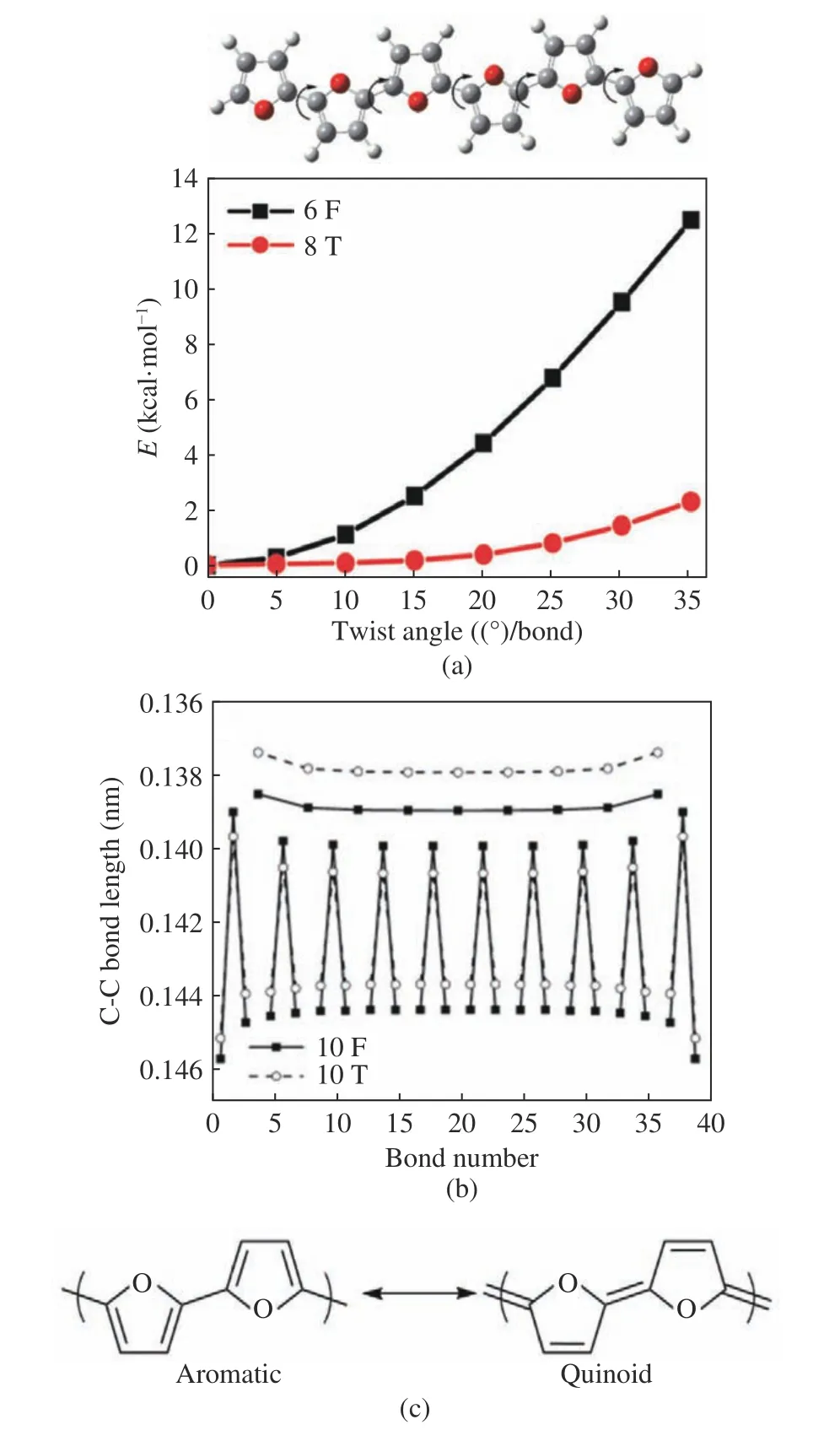
Fig. 3 Calculation explained for the planarity of furan: (a) cal−culated relative energy versus twist angle for spiral twisting of 6F and 6T[10]; (b) bond−length alternation patterns in 10F and 10T[19]; (c) aromatic and quinoid resonance structures of oligofuran
The smaller diameter of the oxygen atom compared to the sulfur atom may also con−tribute to a significant increasing in rigidity, as it reduces the steric hindrance between the het−eroatoms and the β−hydrogen atoms in the adja−cent ring. The same head−to−head modified hexa−furan, the molecular dihedral angle is almost 0°(Fig. 4(a)), while the same hexathiophene, the dihedral angle is 26° [21], the small cyclic α−oligo−furan also shows a planar or nearly planar struc−ture [22−24]. In 2014, Jin et al. reported the longest oligofuran, 16F−6C6(Fig. 4(c)) , and it remained highly coplanar despite head−to−head modifications [25]. In general, oligofurans show clear shoulders (Fig. 4(b)) in their UV−Vis absorption spectra, with significantly smaller stokes shifts compared to oligothiophenes[10,14,26]. All of these indicate that the furans material is more rigid and has better planarity.
In 2021, the Helten group reported boron−doped oligofuran compounds BB1F-BB4F (Fig.5). Whether in the oligofuran bridge or the boron center, the furan rings were almost coplanar,with an average twist angle of about 5.5° [27].While the boron−doped oligothiophenes reported by Jäkle had a twist angle of 14.6° [28].
2.3 Photoluminescence
According to literature reports, the fluorescence quantum yields of 3F−6F (Fig. 6(a)) are 0.78,0.8, 0.74, and 0.69, while the fluorescence quan−tum yields of the corresponding polythiophenes are 0.07, 0.18, 0.36, and 0.41 [14]. There are two reasons for the high fluorescence quantum yield of furan materials. First, there is no spin−orbit coupling caused by the heavy atom effect, which reduces the probability of electrons traversing between S0→T1 and S1→T1 systems [29]; Sec−ond, the rigidity of furan molecular skeleton reduces the vibration of excited state molecules and reduces the energy loss of vibrational relax−ation [10].
In 2013, the Bendikov group investigated two structural isomers (Fig. 6(b)) containing bisfuran (TFFT) and bisthiophene (FTTF) [30].This work shows that the introduction of a short oligofuran ring is sufficient to produce strong flu−orescence (Fig. 6(c)), whereas a single furan ring cannot enhance the fluorescence of the conju−gated backbone. Among the boron−doped oligofu−rans reported by Helten, the quantum yield of diborane ranges from 89% to 97%, and the quan−tum yield of the difuran−bridged polymer PB2F is as high as 87%. Their emission colors can be effectively tuned in the visible range from blue to orange via the length of the oligofuran linker(Fig. 6(d)) [27].
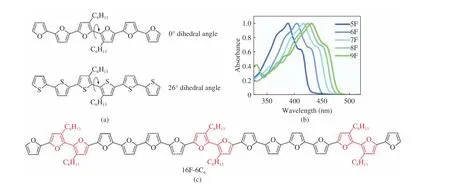
Fig. 4 Experimental data explained for the planarity of furan: (a) twisting between head−to−head defects in oligofurans and oligothio−phenes [21]; (b) absorption (left) and emission (right) of oligofurans [10]; (c) molecular structure of 16F−6C6 [25]
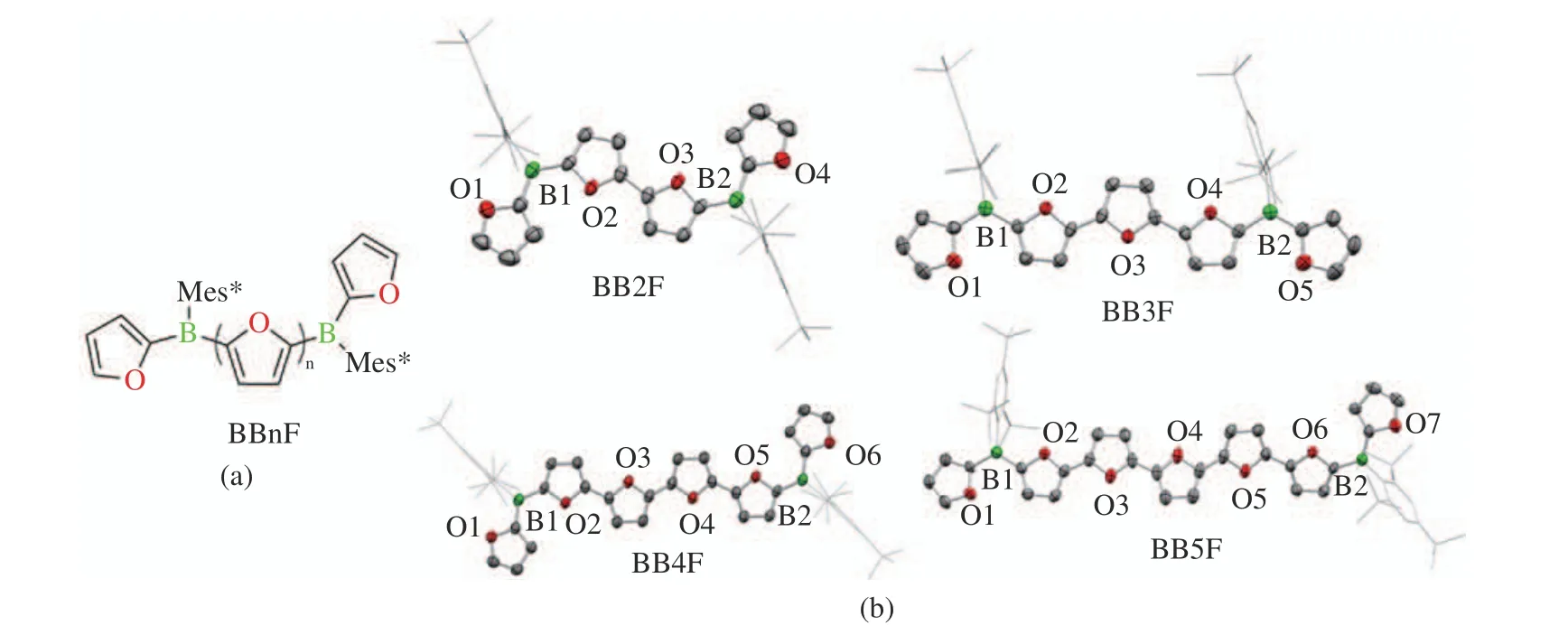
Fig. 5 Structures of BBnF: (a) molecular structures of BBnF; (b) molecular structures of BB2F−BB5F by single−crystal X−ray diffrac−tometry [27]
3 Furan-Based Materials
The research on furan−based materials is earlier than the research on oligofuran materials, where furan mainly present at π bridges and benzofu−ran. In fact, the introduction of furan resulted in great improvements in solubility, planarity, lumi−nescence, and device efficiency.
In 2010, the Fréchet group reported the first furan−containing low−bandgap polymers,PDPP2FT and PDPP3F (Fig. 7(a)) [31]. They introduced furan units into DPP polymers to replace thiophene units, as a result,the solubil−ity of the polymers was increased. Although the use of longer and larger alkyl substituents can also improve solubility, it also increases π−π stacking distances, bad for charge carrier trans−port. Therefore, furan units plays an important role in reducing the length and volume of side chains [32]. In 2014, Liang et al. synthesized the polymers PDTPTDP and PDTPFDP(Fig. 7(b)),and the solubility of furan−containing polymers was significantly improved [33]. In 2019, Qian et al. tuned the properties of near−infrared dyes by changing the heteroatoms of the donor (Fig.7(d)). The results show that the furan ring−con−taining dyes not only have longer absorption wavelengths, but also have better planarity [34].In 2017, Tang’s group reported a case of furan−containing AIEgen [35] and compared it with thiophene analogs. The results show that furan is superior to thiophene in fluorescence, chromatic−ity and charge transport. The OLED device with TPE−F (Fig. 7(c)) as the light−emitting layer exhibits excellent device performance, with a high brightness of 24 298 cd·m-2and an electrolu−minescence efficiency of 3.67%.
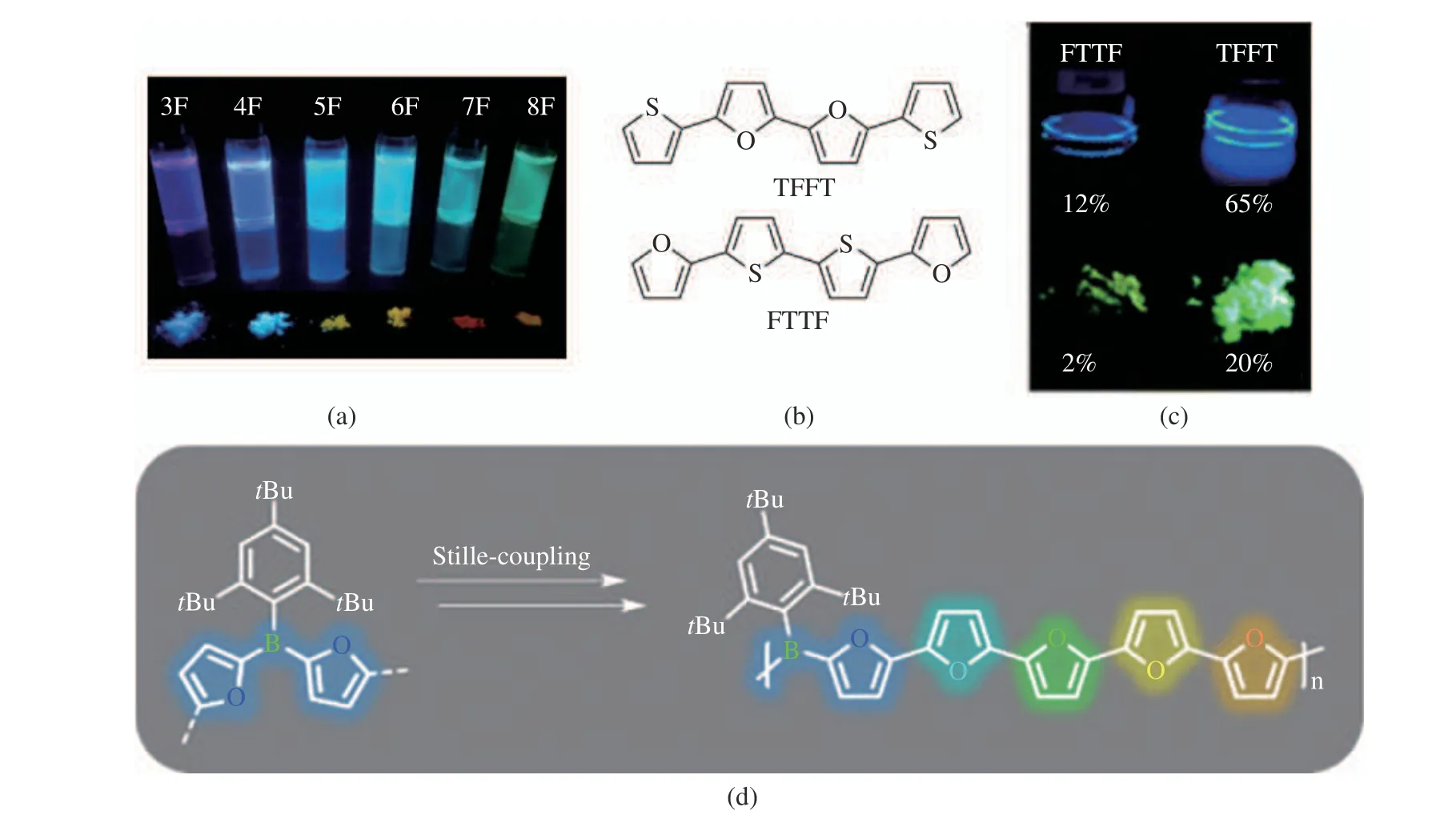
Fig. 6 Luminescence of compounds containing furan: (a) photograph of irradiated samples of 3F-8F in 1,4−dioxane solutions and as powders[12]; (b) molecular structure of FTTF and TFFT; (c) luminescence of FTTF and TFFT[30]; (d) emission colors of boron−doped oligofurans [27]
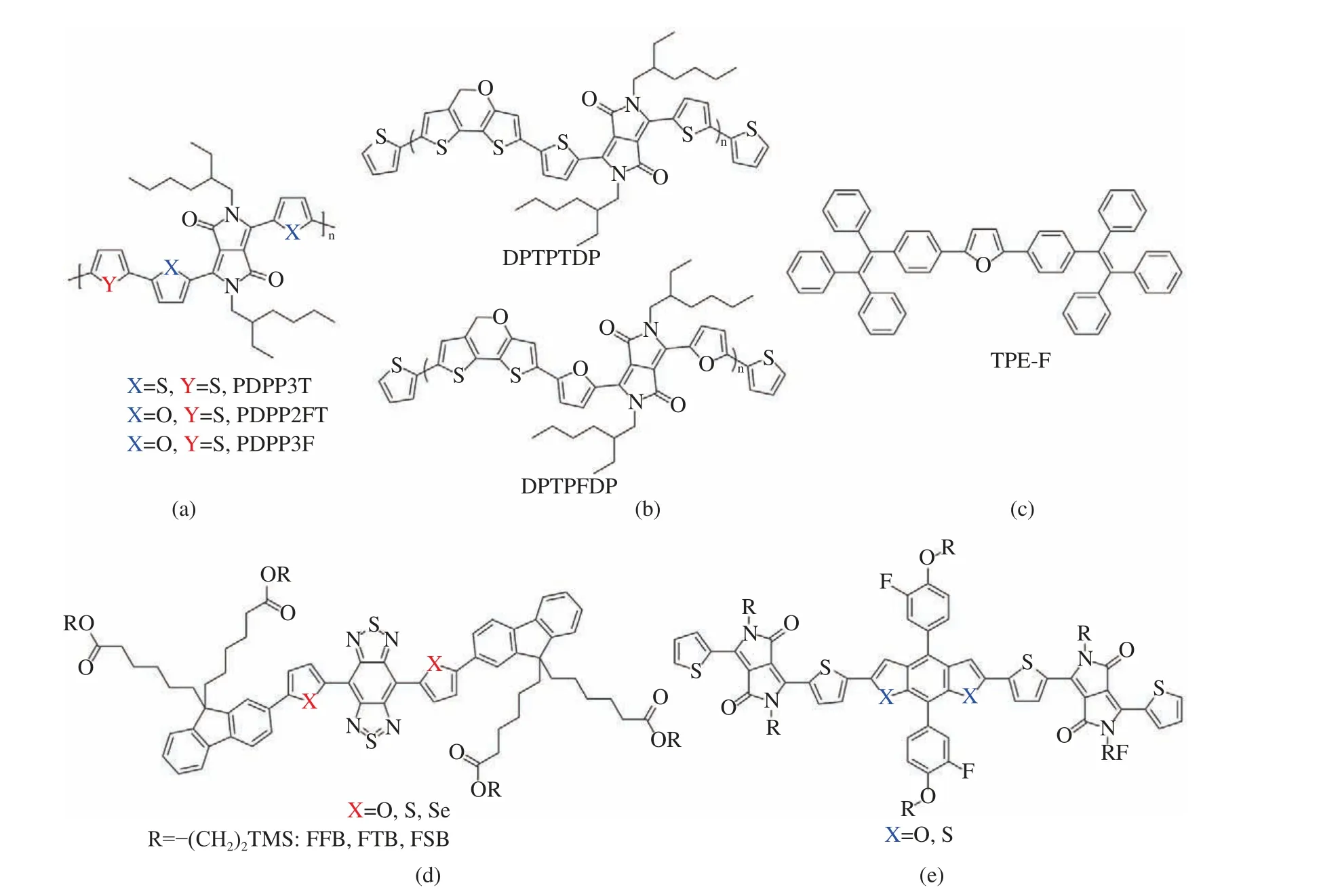
Fig. 7 Molecular structures of furan−based materials: (a) PDPP2FT and PDPP3F; (b) PDTPTDP and PDTPFDP; (c) TPE−F;(d)FFB, FTB and FSB; (e) small molecule using BDF and BDT
Benzodifuran (BDF) has a good planar structure [16,17,36], which helps to improve the intermolecular π−π stacking, thereby enhancing the charge carrier mobility. In 2015, Qian et al.synthesized two small molecule using BDF and BDT as electron−rich units respectively (Fig.7(e)). Containing BDF units exhibits proper aggregation and denser π−π packing. Solar cells based on BDF exhibit more than double the pho−tocurrent of devices based on BDT and yield a power conversion efficiency of 5.5% [16,37].
Meanwhile, Bendikov et al. used electro−chemical, spectroscopic and theoretical calcula−tions to demonstrate the high charge delocaliza−tion in oligofuran, coupled with the strong fluo−rescence and high rigidity of previously reported oligofuran−based materials [38−40].
4 Summary and Prospects
Furan has emerged as an attractive class of building blocks for constructing organic optoelec−tronic materials although it has been less consid−ered over a long period of time. In comparison with long oligo− and polythiophenes which are the most widely used and best−studied conju−gated material to date, oligofurans exhibit higher fluorescence, better solubility, greater rigidity,and tighter solid−state packing than the corre−sponding oligothiophenes. In addition, device investigation also demonstrated that oligofurans possess the field effect mobility similar to that of the corresponding oligothiophenes. Considering furan can be manufactured from renewable resources, and are expected to be biodegradable,furan−containing materials could be promising candidates for bioapplications, such as biosen−sors or bioelectronics.
This review holds out the potential of α−oligofurans as a new kind of biodegradable organic optoelectronic materials. However, the current knowledge is far from complete. Furan−based monomeric precursors which is suitable for metal−catalyzed condensation polymerizations are still lacking due to the instability of furan ring[41]. Meanwhile, there remains a need to gener−ate syntheses of novel furan−containing materials based on the requirement of high−MW polymers with minimized defects and outstanding optoelec−tronic properties.
杂志排行
Journal of Beijing Institute of Technology的其它文章
- Application of Micro Electro Mechanical System(MEMS) Technology in Photoacoustic Imaging
- Design of Implant Prosthesis for Bone Injury Repair Considering Stress Shielding Effect
- A Microfluidic System with Active Mixing for Improved Real-Time Isothermal Amplification
- Mixing Dynamics and Synthesis Performance of Staggered Herringbone Micromixer for Limit Size Lipid Nanoparticles
- Effect of Auxiliary Gas Flow Parameters on Microstructure and Properties of Ta Coatings Prepared by Three-Cathode Atmosphere Plasma Spraying
- Nanoscale Metal-Organic Frameworks:Stimulus-Response and Applications
