A Microfluidic System with Active Mixing for Improved Real-Time Isothermal Amplification
2022-06-30DianlongYangXiaodanJiangYijieZhouXiaobinDongLuyaoLiuLuluZhangXianboQiu
Dianlong Yang, Xiaodan Jiang, Yijie Zhou, Xiaobin Dong,Luyao Liu, Lulu Zhang, Xianbo Qiu
Abstract: To improve the performance of real−time recombinase polymerase amplification (RPA),a microfluidic system with active mixing is developed to optimize the reaction dynamics. Instead of adopting a single typical reaction chamber, a specific reactor including a relatively large chamber in center with two adjacent zig−zag channels at two sides is integrated into the microfluidic chip.Active mixing is achieved by driving the viscous reagent between the chamber and the channel back and forth periodically with an outside compact peristaltic pump. To avoid reagent evapora−tion, one end of the reactor is sealed with paraffin oil. A hand−held companion device is developed to facilitate real−time RPA amplification within 20 min. The whole area of the reactor is heated with a resistance heater to provide uniform reaction temperature. To achieve real−time monitoring,a compact fluorescence detection module is integrated into the hand−held device. A smartphone with custom application software is adopted to control the hand−held device and display the real−time fluorescence curves. The performances of two cases with and without active on−chip mixing are compared between each other by detecting African swine fever viruses. It has been demonstrated that, with active on−chip mixing, the amplification efficiency and detection sensitivity can be signifi−cantly improved.
Keywords: recombinase polymerase amplification (RPA); microfluidic chip; active mixing; optical detection; smartphone
1 Introduction
As an important technique of molecular diagno−sis, isothermal amplification technology (IAT)receives increasing attention with high sensitiv−ity and specificity for the detection and diagno−sis of pathogens [1, 2]. According to the different reaction principle, IAT can be divided into multi−ple types, including recombinase polymerase amplification (RPA) [3], loop−mediated isother−mal amplification (LAMP) [4, 5], cross priming amplification (CPA) [6], helicase−dependent ampli−fication(HDA) [7], strand displacement amplifi−cation (SDA) [8], and etc.
Different integrated microfluidic chips have been developed to automatically complete the whole amplification process from sample prepara−tion to result detection [9, 10]. In recent years,new detection technology based on IAT and microfluidic chips has been insensitively studied to improve the performance of nucleic acid diag−nosis [11, 12 ]. Because of the small size of microfluidic chips, low volume of reagents and high amplification efficiency, the microfluidics−based nucleic acid analysis technology has shown great potentiality on on−site point−of−care detec−tion for infectious diseases [13].
As a highly sensitive and selective technique of IAT, RPA enables to complete amplifying within 20 min, operating at 37-42 ℃. Further−more, RPA can be integrated into microfluidic chips for virus detection without any sophisti−cated equipment, which is convenient in low−resource settings [14, 15]. In 2019, Song et al.proposed a two−stage isothermal amplification method based on RPA and LAMP [16]. However,the method requires repeatedly inverting the tube manually to mix the pre−amplified products and new primers before LAMP amplification, which increases the complexity of the operation.
As one of critical performance characteris−tics for an infectious disease detection system,the limit of detection (LOD) needs to be reduced as much as possible to improve the detection sen−sitivity. There are quite few reported studies about active mixing on microfluidic chips to improve the amplification efficiency of RPA. For RPA amplification, almost all the reported tests only perform active mixing before reaction, and there has no active mixing in reaction [17−19].For example, the reagent on the disk chip is effi−ciently mixed with centrifugal force before ampli−fication [14]. To further optimize the reaction dynamics and simplify the operation, a real−time microfluidic RPA system with active mixing before and in amplification has been developed.Meanwhile, the paper proposes a microfluidic chip integrated with a specific reactor including multiple chambers and two zig−zag channels,which can achieve active mixing by driving the viscous reagent between the chamber and the channel back and forth periodically. For the amplification process, a smartphone with custom software is used to control a hand−held device and receive the detection data in real−time through the Bluetooth. With the microfluidic sys−tem mentioned above, African swine fever virus(ASFV) can be detected successfully in 20 min and the performances of two cases with and with−out active on−chip mixing are compared.
2 Material and Methods
2.1 Optimized RPA Chip with Active Mixing
It is reasonable that efficient mixing is essential before or during the amplification reaction, which determines the performance of the amplification reaction directly. However, compared to conven−tional polymerase chain reaction (PCR) reagent,the viscosity of RPA reagent is higher, which is unfavorable for amplification on static RPA chip due to the low diffusion efficiency. Therefore, an optimized RPA chip (30 mm×45 mm×1.2 mm)with active mixing is developed to improve the performance.
As shown in Fig. 1(a), the microfluidic chip consists of three layers (top layer with two seal−ing parts, middle layer, bottom layer), in which the top and bottom layers are cut from polycar−bonate (PC) with a thickness of 0.1 mm by a laser machine (HTC−0906−W80, Jinan Hantong Digital Control Device Enterprise Co. Ltd.,Jinan), and the middle layer is made of poly−methyl methacrylate (PMMA) with a thickness of 1 mm. All layers are laminated together with the double−sided tape (Type #9731, 3 M). The picture of the microfluidic chip is shown in Fig.1(b).
As shown in Fig. 1(c) , the middle layer holds the main structure of the microfluidic chip,which consists of four chambers ( two mixing chambers, a detection chamber, an air chamber),four ports, two adjacent zig−zag channels and buffering channel to a total reaction volume of 40 μL. Here, the zig−zag channels are used to achieve active mixing by driving the viscous reagent between the mixing chambers and the buffering channel back and forth periodically.
Fig. 1(d) shows the detailed geometry dimensions of channels and chambers of the RPA chip. By taking advantage of the geometry change between the chambers and the buffering channel as well as the zig−zag mixing channel,efficient mixing can be achieved conveniently.Meanwhile, fabricated with an elastic rubber film, the air chamber is used to balance air pres−sure. To ensure that the paraffin oil is injected in a single direction to the chip, venting and sam−ple loading ports are sealed by tape at sealing part firstly, and then they are released and the loading port for paraffin oil injection is sealed after paraffin oil is filled in the chip. When RPA reagent is being injected to chip from the sample loading port, venting port is used to release air,and afterwards, venting and sample loading ports are sealed by tape at sealing part again to avoid reagent evaporation.
For temperature control, a resistive heater covering the entire reaction area under the chip is adopted for uniform heating. As shown in Fig.1(e), the real reaction temperatures at different chambers of the RPA chip are quite close. To perform real−time RPA amplification with accu−rate temperature control, a proportional−integral−derivative (PID) controller is developed. At mix−ing process, once the actual temperature reaches the setpoint temperature, reagent will be mixed for 40 s through the active mixing module auto−matically, furthermore, which will be mixed again for 40 s after 10 min in RPA amplification.
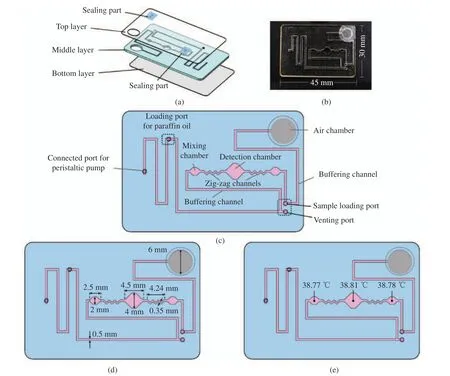
Fig. 1 RPA chip with active mixing: (a) exploded diagram; (b) picture of RPA chip; (c) layout of fluid control; (d) RPA chip with detailed geometry dimensions of channels and chambers; (e) RPA chip with temperature distribution
2.2 System Overview of the Hand-Held Device
As shown in Fig. 2, a microfluidic system with active mixing for improving real−time isothermal amplification is developed. The smartphone with the custom software is used to control the experi−mental process through Bluetooth. Once receiv−ing the instructions from the smartphone, the microfluidic device (105 mm × 105 mm × 56 mm)will start to run and the fluorescence curve will be displayed on a liquid crystal display (LCD) in amplification. Meanwhile, fluorescence data and curve are shown on the smartphone screen.

Fig. 2 Hand−held companion device with smartphone
In order to ensure RPA reaction, real tem−perature in the reaction chamber of the RPA chip needs to reach 39 ℃, which can be achieved by setting the temperature of the heating mod−ule at 47 ℃. A PID controller has been used for accurate temperature control.
The hand−held companion device consists of a smartphone, a Bluetooth module, a controller module, a power module, a heating module, an optical detection module and a mixing module.With a resistance heater, the heating module provides a constant temperature to heat the RPA chip uniformly. For the power module,besides external power supply, an internal rechargeable battery with 2 960 mWh is also included to allow 5 tests. Especially, with con−cise system design and low−cost components, the total cost of the hand−held device is $85.
2.3 Mixing and Optical Modules of the Hand-Held Device
As shown in Fig. 3, it can be seen the internal structure diagrams of optical detection module and the mixing module, which are the major components for the hand−held device.
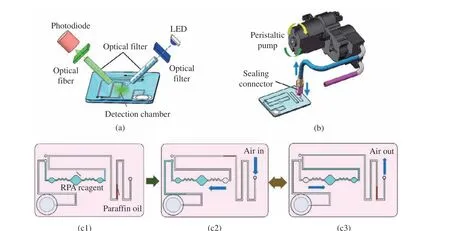
Fig. 3 Schematic illustration of the hand−held device: (a) optical module; (b) mixing module; (c) mixing procedure of RPA reagent
As shown in Fig. 3(a), the optical detection module consists of a LED, a photodiode, two optical fibers and two optical filters. To improve the detection sensitivity, two specific optical fibers with 2 mm diameter are used to collect flu−orescence signal from the detection chamber with high efficiency. During fluorescence collection, a LED is used to illuminate the detection chamber area in microfluidic chip with the optical fiber through a common optical filter, and fluores−cence signal is collected by a photodiode through another set of optical fiber and optical filter.
To optimize the reaction dynamics and improve the performance of RPA chip, the strat−egy with active mixing has been studied and compared with the mode of free diffusion. There−fore, as shown in Fig. 3(b), to perform active mixing, an active mixing module with a peri−staltic pump and a sealing connector is included to assist RPA mixing in amplification. Here, a sealing connector is used to connect the chip and peristaltic pump. Air is pumped in and out through the control of the peristaltic pump. As shown in Fig. 3(c1), before mixing, one end of the chip is sealed with paraffin oil to avoid reagent evaporation. In mixing, the air in the chip is pumped in and out repeatedly by the peristaltic pump, and the change of internal air pressure drives the reagent back and forth peri−odically. By taking advantage of the geometry change between the chambers and the buffering channel as well as the zig−zag mixing channel,efficient mixing can be achieved conveniently.The mixing cycles can be seen in Fig. 3(c2) and Fig. 3(c3). With the air chamber and paraffin oil, the detection chamber is always filled with reagent to avoid the influence of air bubbles in detection.
The detailed mechanism of the zig−zag−based mixing method can be found in the published lit−erature [20]. Briefly, when the reagent flows in zig−zag mixing channels back and forth, the lami−nar recirculation occurs, which induces a tran−sversal component of the velocity to improve the mixing process effectively. The frequency of the shift of the flow direction is 6.25 Hz, which can be programed by setting the operation frequency of the peristaltic pump.
3 Results and Discussion
3.1 Evaluation of Mixing Efficiency on RPA Chip
In order to evaluate the effect of mixing effi−ciency on RPA chip, there are two cases with dif−ferent reagents (RPA and water) that are com−pared respectively, including active mixing and free diffusion.
For experiment process, firstly, 40 μL of RPA reagent and water were injected into two RPA chips respectively, and then, for each of them, 10 μL of blue dye is injected into the chip as well, and finally the sample loading and the venting ports are sealed with tape. Experimental results are shown in Fig. 4.
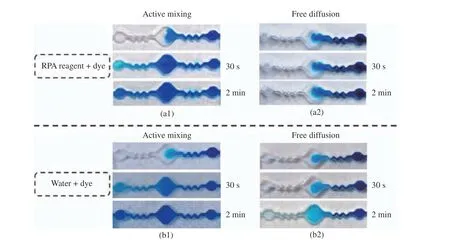
Fig. 4 Comparison between active mixing and free diffusion: (a1) active mixing with RPA reagent and dye; (a2) free diffusion with RPA reagent and dye; (b1) active mixing with water and dye; (b2) free diffusion with water and dye
Fig. 4(a1) and Fig. 4(b1) are RPA chips with active mixing, and Fig.4(a2) and Fig.4(b2)are RPA chips relying on free diffusion. For active mixing, it usually includes diffusion and convection. It can be seen that compared with free diffusion mode, the mixing efficiency of the RPA chips with active mixing has been signifi−cantly improved.
As shown in Fig. 4(a1), the RPA reagent can be mixed efficiently with dye within 30 s with the developed active mixing method. How−ever, as shown in Fig. 4(a2), with free diffusion,the RPA reagent cannot be mixed efficiently with dye within limited time due to its high vis−cosity. Moreover, the similar conclusion can be made based on experiment results from Fig.4(b1) and Fig. 4(b2), and for both of them,instead of using RPA reagent, water is used to evaluate the mixing efficiency.
Meanwhile, simplified simulation analysis to the mixing efficiency of both active mixing and free diffusion has been performed with the stan−dard toolbox of COMSOL 5.6. In simulation, the dynamic viscosity of RPA reagent is set to 7.154 mPa·s which is previously measured with a viscosity meter, and its density is set to 1 031 kg/m3which is also previously measured with a density meter. The diffusion coefficient is set to 10-9m2/s.At the beginning, the original concentration of RPA reagent in section A and B of the com−bined mixing channels and chambers is set to 1 μmol/mL and 0 μmol/mL, respectively. The mixing frequency is set to 6.25 Hz. As shown in Fig. 5, with active mixing (Fig. 5(a)), the mix−ing efficiency of RPA chip can be significantly improved comparing to free diffusion (Fig. 5(b)).
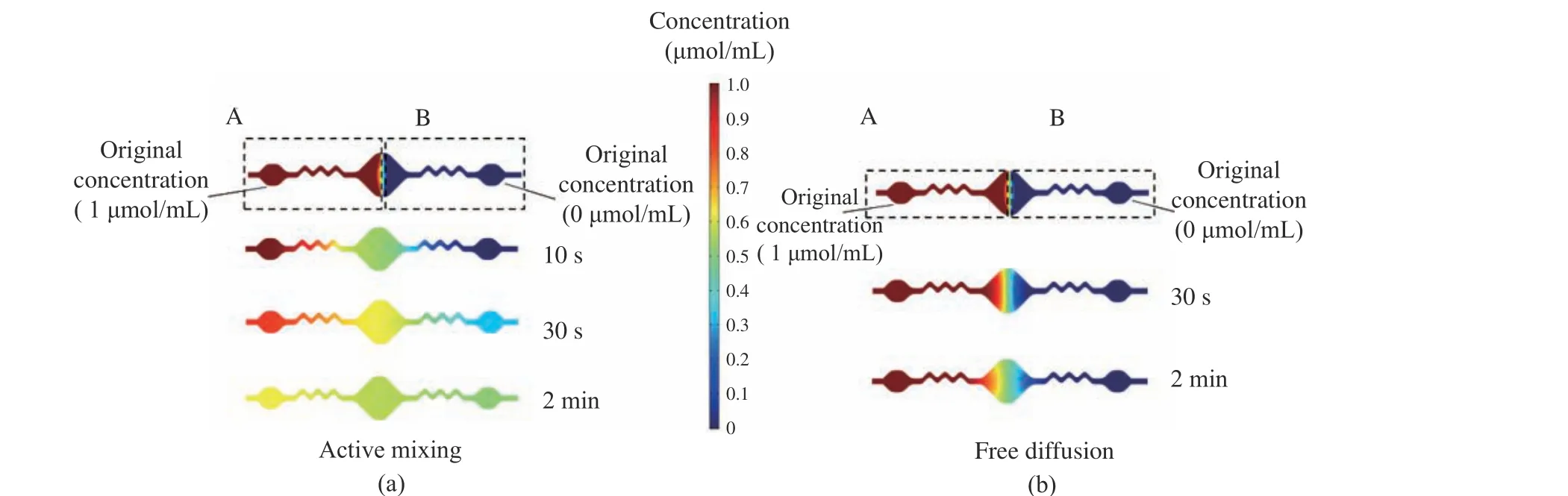
Fig. 5 Simulation analysis for both active mixing and free diffusion: (a) active mixing; (b) free diffusion
Based on both experimental results and sim−ulation analysis, RPA chip with the developed mixing module can improve the mixing effi−ciency significantly, which is helpful to achieve high amplification efficiency and more sensitive detection.
3.2 Temperature Performance of the RPA Chip
It has been found that the temperature gradi−ents of the entire RPA chip are within ±0.5℃.Meanwhile, with accurate PID control, the tem−perature control errors at the steady state are within ±0.2 ℃. Therefore, the amplification effi−ciency of the entire RPA chip can be ensured with accurate and uniform heating.
3.3 Amplification of ASFV on RPA Chip
In order to further evaluate the performance of RPA chip with active mixing, purified ASFV DNA samples were used to perform RPA amplifi−cation. With the initial concentration of 3×104copy/μL, ASFV DNA samples were diluted to different concentration gradients (3×103copy/μL, 1×103copy/μL) by molecular−biol−ogy grade water. Multiple experiments with dif−ferent samples with different concentrations have been performed to demonstrate that with active mixing, more sensitive RPA amplification can be achieved consistently.
As shown in Fig. 6, all the ASFV samples with different concentrations were successfully amplified and detected within 20 min, and differ−ent real−time fluorescence curves with mixing and diffusion were displayed in solid and dashed lines respectively.
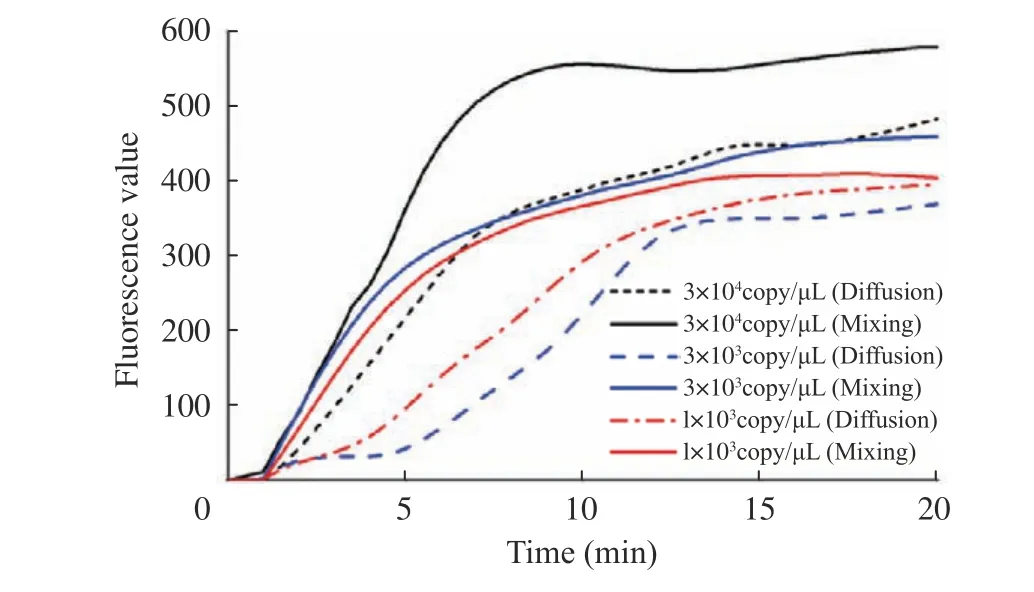
Fig. 6 ASFV DNA detection on RPA chips
For more reasonable analysis and compari−son, all fluorescence curves were preprocessed by data smoothing and filtering. For each concentra−tion, the performance of RPA amplification with active mixing is obviously superior to diffusion,in terms of the beginning time of rising up, the slope of the curve, and the final fluorescence amplitude. Moreover, with active mixing, more sensitive RPA amplification can be achieved by improving the reaction dynamics of isothermal amplification especially with viscous reagent.
For zig−zag−based mixing method, active mixing can be conveniently achieved by driving the reagent back and forth inside the mixing channels with a compact peristaltic pump. Com−paring to zig−zag−based mixing, other mixing method, for example, micro−stirrer−based, vortex−based, or ultrasonic−based method, usually requires more complicated set up with bigger−size components or more elaborate control. Therefore,zig−zag mixing method is adopted to achieve effi−cient mixing with reduced cost [21−23].
This manuscript gives an insight that how active mixing in reaction can improve the perfor−mance of RPA amplification, for example, the detection sensitivity. In other words, the current work tries to present the potential solution to improve RPA performance by adopting active mixing during amplification beside the initial mixing before amplification. In the future, more systematical study can be performed to find out the limit of detection (LOD) of RPA detection to different types of clinical samples.
4 Conclusions and Outlook
A microfluidic system for efficient real−time RPA amplification was developed. The viscosity of RPA reagent is significantly higher than that of conventional PCR reagent, which could cause suboptimal reaction dynamics due to the low dif−fusion efficiency and nonuniform distribution of different reagent. Witnessing the negative effect caused by viscous RPA reagent, the optimiza−tion strategy with active on−chip mixing is adopted. A specific reactor including a relatively large chamber in center with two adjacent thin zig−zag channels at two sides, which was uni−formly heated at a constant temperature, was integrated into the RPA chip. To assist active on−chip mixing, an outside compact peristaltic pump was used to drive the viscous reagent auto−matically between the chamber and the channel back and forth periodically in RPA amplification.By taking advantage of the geometry change between the chamber and the channel as well as the zig−zag mixing channel, efficient mixing can be achieved conveniently. Meanwhile, to avoid reagent evaporation, one end of the RPA chip is filled with paraffin oil.
A hand−held companion device, which con−sisted of a heating, detection, and fluidic control modules, was developed to perform real−time RPA amplification on microfluidic chips. A resis−tance heater was adopted for uniform heating.For fluorescence detection, a photodiode was used for fluorescence signal collection, and a LED was used for fluorescence excitation. With con−cise system design, the total cost of the compo−nents of the hand−held device is as low as $85. To provide more friendly operation, a smartphone with custom application software is adopted to control the hand−held device and display the real−time fluorescence curves with Bluetooth. With the smartphone platform, more complicated data analysis can be performed, and it also allows the detection result to be conveniently transmitted to another place, for example, medical center for further analysis.
Based on experimental results, it has been demonstrated that, with active on−chip mixing,the RPA amplification efficiency and detection sensitivity can be significantly improved compar−ing to the case without active on−chip mixing. It is well−known that, for pathogen detection based on nucleic acid amplification, for example, detec−tion of viruses from different infectious diseases,the LOD of the developed system is one of criti−cal performance characteristics. In other words,when the LOD of the developed system is not as low as required, parts of positive samples, such as low positive samples, cannot be detected and dis−criminated successfully, which will cause serious problem especially for a quickly transmitted infectious disease, for example, COVID−19.Therefore, to improve the detection sensitivity of isothermal amplification based on RPA is quite helpful to further optimize its performance and extend its application based on its existing char−acteristics, for example, simple heating at a con−stant temperature, short amplification time, e.g.,20 min, and concise and low−cost companion device.
杂志排行
Journal of Beijing Institute of Technology的其它文章
- Application of Micro Electro Mechanical System(MEMS) Technology in Photoacoustic Imaging
- A New Class of Biodegradable Organic Optoelectronic Materials: α-Oligofurans
- Design of Implant Prosthesis for Bone Injury Repair Considering Stress Shielding Effect
- Mixing Dynamics and Synthesis Performance of Staggered Herringbone Micromixer for Limit Size Lipid Nanoparticles
- Effect of Auxiliary Gas Flow Parameters on Microstructure and Properties of Ta Coatings Prepared by Three-Cathode Atmosphere Plasma Spraying
- Nanoscale Metal-Organic Frameworks:Stimulus-Response and Applications
