Nanoscale Metal-Organic Frameworks:Stimulus-Response and Applications
2022-06-30ChenZhaoRuiJingShanshanWangXiaoyingTang
Chen Zhao, Rui Jing, Shanshan Wang, Xiaoying Tang
Abstract: Metal−organic frameworks (MOFs), a crystalline porous material with a periodic net−work structure formed by the self−assembly of transition metal ions and organic ligands, have been widely applied in various fields due to their rich composition and structural diversity. Among vari−ous types of MOFs, stimuli−responsive MOFs have gained increasing attention in recent years,because of their broad application in the field of physics, biology, and chemistry. In this review, we analyzed and classified the mechanism of stimulus−response MOFs (pH response, glucose response,GSH response, light response, temperature response) and their applications in drug delivery,adsorption and luminescence functions, magnetization and catalysis functions, probe and sensor.
Keywords: metal−organic frameworks (MOFs); stimuli−responsive; drug delivery
1 Introduction
Metal−organic framework (MOF) is a crystalline porous material with a periodic network struc−ture formed by the self−assembly of transition metal ions and organic ligands. It was first syn−thesized in the mid−1990s, but its pore size and stability were limited. At the beginning of this century, some studies have gradually realized the functions of maintaining the integrity of the skeleton after removing the guest molecules in the pore channels, and the successful transition from micropores to mesopores [1, 2].
At present, the preparation methods of MOFs are relatively mature and can be selected according to different synthetic conditions and requirements, including in−situ solvothermal method, seed crystal method, microwave method,and layered method. MOFs have been widely used in many fields such as gas storage, adsorp−tion and separation, luminescent materials, catal−ysis, sensors, and drug delivery due to its large specific surface area, adjustable pore channels,and functionalization. Although MOF has many advantages and application scenarios, it still has such problems as 1) crystals cannot grow contin−uously with high quality, 2) the growth of shapes and orientations cannot be controlled, 3) homo−geneous MOFs films are still defective, and so on.
This review aims to sort out and summarize the relevant research on MOF materials in recent years. These studies were discussed and analyzed to provide directions for the future development of MOF−based materials. In this review, we first organized the related studies on the stimulation response of MOFs, and then classified and sum−marized its applications in various fields. In this review, the current research status and existing problems of MOF−based materials are discussed,which would arouse the attention of scientists in different fields and cooperation, to achieve the significance of developing the comprehensive properties of MOFs.
2 Stimulus-Responsive MOFs
Because of the introduction of stimulus−respon−sive groups in the MOF structure, the physical and chemical properties of stimulus−responsive MOFs will change to a certain extent under cer−tain conditions (after being stimulated either in vivo or in vitro), leading to changes in molecular conformation, hydrolytic cleavage or protonation.The loaded material is released. The common response groups in MOFs are the imidazole group(pH response), disulfide bond (GSH response),porphyrin (light response), temperature, etc. In this section, we will focus on describing and sum−marizing the release mechanism based on MOF−loaded materials according to stimuli such as pH,GSH, light and temperature (Tab. 1).
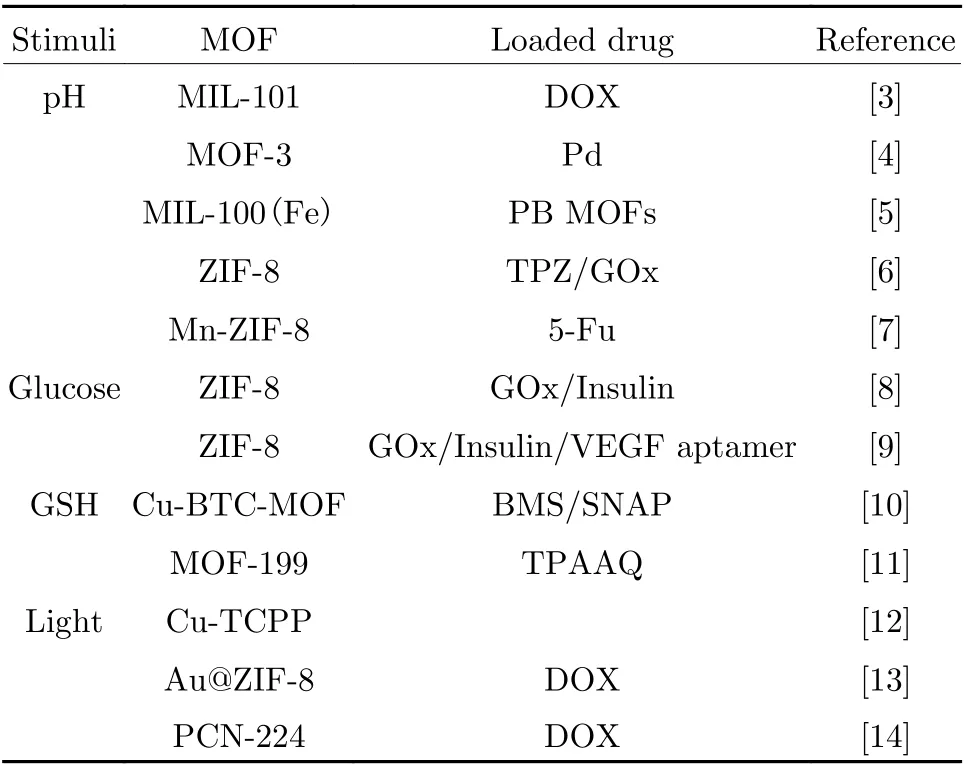
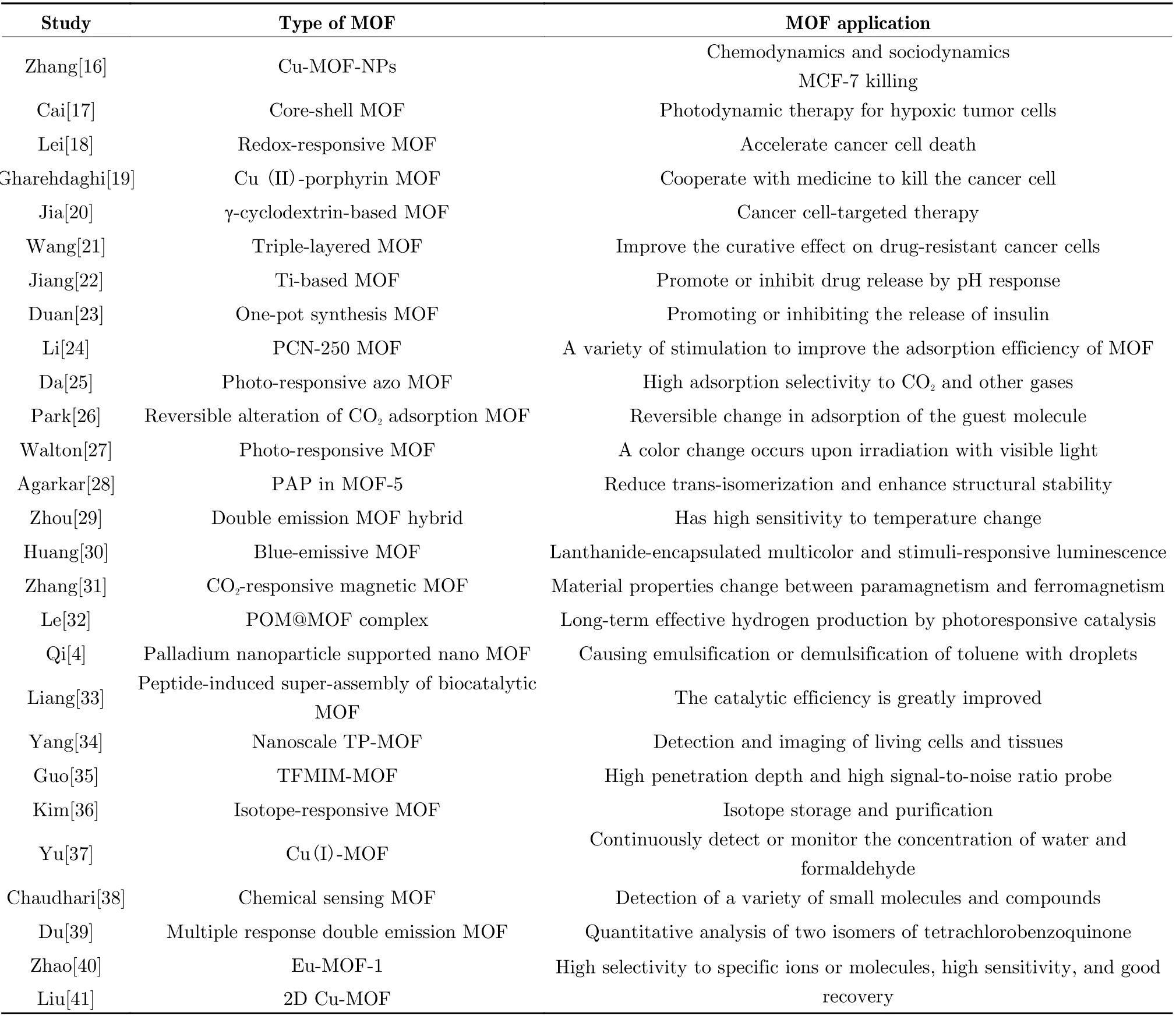
Tab. 1 Stimuli-responsive MOFs and their components
2.1 pH-Responsive MOFs
Since the pH value of the tumor microenviron−ment is relatively acidic compared to normal tis−sues’ (pH 4.5-7.8), many researchers design and synthesize MOFs with an acidic response by selecting organic ligand materials with response to pH stimuli. Wang et al. [3] synthesized a MOF−based drug delivery system. The authors load DOX into the pH−response MOF through the one−pot method. The drug delivery system exhibited tumor acid−environment−enhanced cel−lular uptake performance, because of the pH−responsive benzoic imine bond. The resulting pH−stimuli MOF shows tumor−targeting perfor−mance and efficient tumor cell inhibition with the stimulus−responsive release of DOX (Fig. 1(a)).
Jiang et al. [4] designed a post−synthetic approach to graft the poly[2−(diethylamino)ethyl methacrylate)] (PDEAEMA) chains onto UiO−66−type NPs to generate PDEAEMA−g−UiO−66 NMOF (termed as MOF−3) (Fig. 1(b)).The obtained Pd@MOF−3 is pH−responsive and can trigger the emulsification (at the neutral condi−tion) and demulsification (at the acidic condi−tion) of toluene droplets. Because of its pH−responsive property, it can be in situ separated and recycled by demulsifying via simply tuning the pH value at the end of the reaction. This smart Pickering emulsion catalytic system is robust and can be recycled at least five times with its catalytic activity. Wang and coworkers developed PB@MIL−100(Fe) dual metal−organic−frameworks (d−MOFs) nanoparticles (Fig. 1(c))[5]. In the acidic microenvironment of the tumor cells, a high loading content of artemisinin is released from the d−MOFs. The theranostic nano agent becomes a promising next generation of nanomedicine for efficient and safe cancer ther−apy because of the distinctive multimodal imag−ing capability, excellent synergistic therapy effect through the combined chemo−photothermal ther−apy together with the low toxicity of both d−MOFs and artemisinin. Zhang et al. [6] fabri−cated a MOF−based biomimetic nanoreactor encapsulating glucose oxidase (GOx) and pro−drug for colon tumor therapy. The fabricated TGZ@eM nanoreactor can help deliver GOx to tumor cells and then exhaust endogenous glu−cose and O2to starve tumors efficiently (Fig.1(d)). Notably, the TPZ could release from the nanoreactor in the acid endosome environment for enhancing colon tumor therapy. Pan et al. [7]designed Mn−ZIF−8/5−Fu nanoparticles to com−bine MR imaging, ultrahigh anti−glioma drug loading, and pH−responsive drug release(Fig. 1(e)). Mn−ZIF−8, a low toxicity bimetallic zeolitic imidazolate framework with good dis−persibility and high specific surface area was syn−thesized for potential high drug loading. Besides,they used Mn−ZIF−8 in vivo magnetic resonance imaging (MRI) for the first time. After injecting Mn−ZIF−8 intravenously, it could be observed that T1−weighted MR signals at tumor sites con−tinuously increased over time, and Mn2+in tumors accumulated at a relatively high level.The therapeutic efficacy of Mn−ZIF−8/5−Fu in U87−MG tumor−bearing mice was significantly improved by targeted delivery. Mn−ZIF−8/5−Fu performs well in vivo biocompatibility at a given dose. Hence, efficient 5−Fu loaded Mn−ZIF−8 can be a promising framework for diagnosing and treating glioma simultaneously because of its good performance in vivo biocompatibility, pH responsiveness, and T1−weighted contrast MRI of tumors.
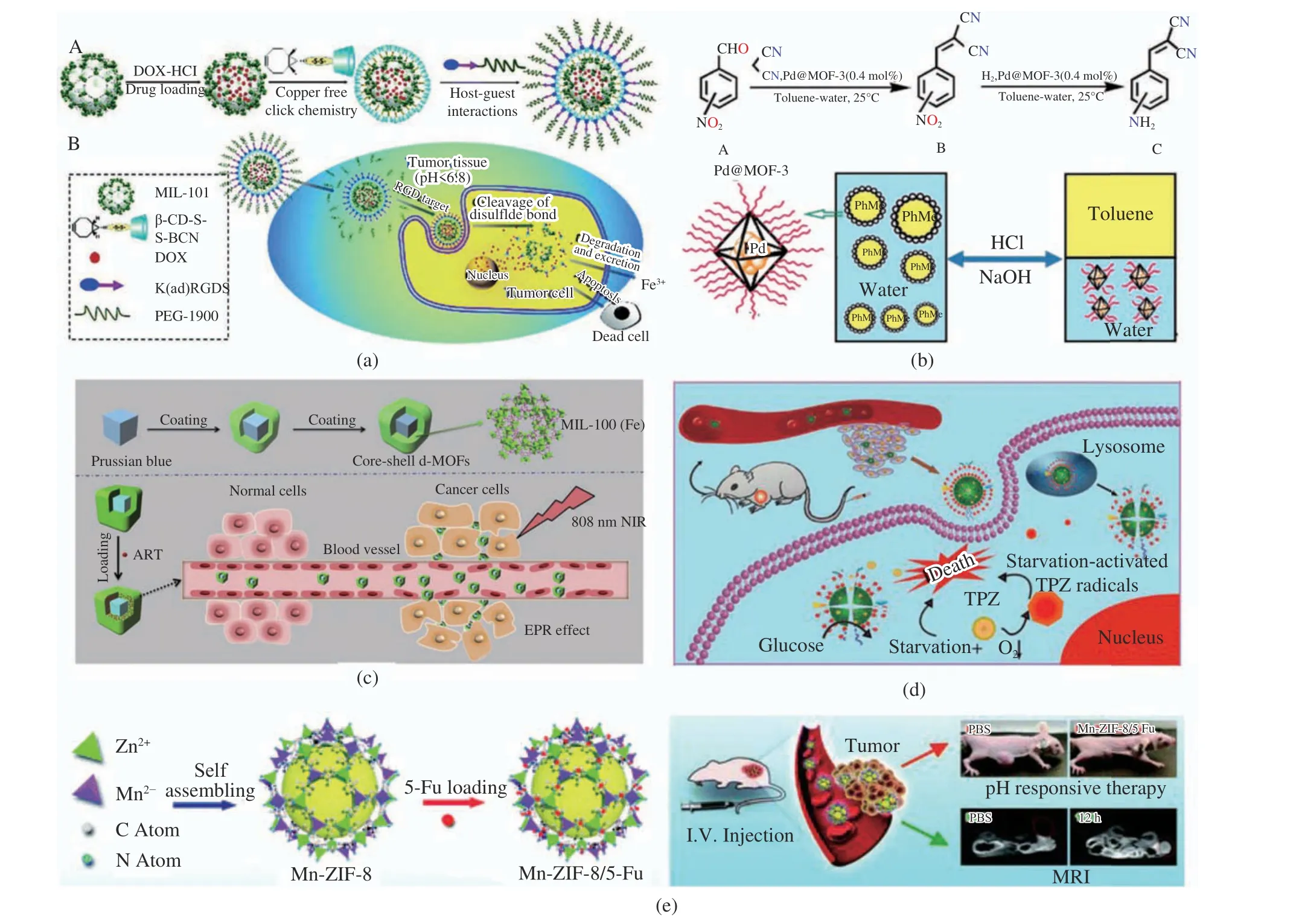
Fig. 1 Schematic illustration of pH−responsive MOFs: (a) the fabrication of DOx loaded pH−stimuli MOF[3]. Copyright 2015, Royal Society of Chemistry; (b) the fabrication of Pd@MOF−3[4]. Copyright 2017, American Chemical Society; (c) synthesis of core−shell d−MOFs[5]. Copyright 2016, Elsevier Ltd; (d) mechanism of pH−responsive behavior of TGZ@eM and tumor therapy[6].Copyright 2018, American Chemical Society; (e) fabrication of Mn−ZIF−8/5−Fu nanoparticles and their bioimaging property[7].Copyright 2019, Royal Society of Chemistry
2.2 Glucose-Responsive MOFs
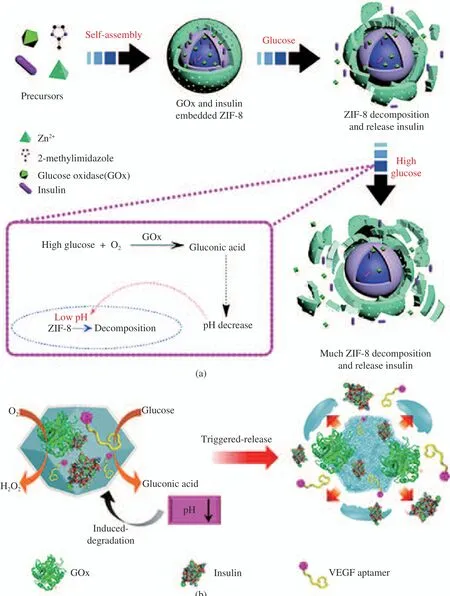
Fig. 2 Schematic diagram of glucose−responsive MOFs: (a) the construction of insulin-GOx/ZIF−8[8]. Copyright 2018, Royal Society of Chemistry; (b) the design and preparation of MOFs−insulin−Gox−AVEGF[9]. Copyright 2018, American Chemical Society
Duan et al. [8] developed a novel glucose−respon−sive MOF−based insulin delivery nanosystem(insulin-GOx/ZIF−8) (Fig. 2(a)). It can self−reg−ulate insulin release according to the glucose con−centration. It is self−assembled by mixing aque−ous solutions of Zn2+, 2−methylimidazole, GOx,and insulin. When glucose reaches a high level, a certain amount of glucose enters the composites’pores and contacts with GOx, which makes glu−cose oxidized to gluconic acid and H2O2. The composites are decomposed due to the resulting pH lowering effect in the particles. Thus, the release of insulin loaded in ZIF−8 is promoted so that the blood glucose level is reduced. Chen et al. [9] fabricated the ZIF−8 MOFs and loaded them with insulin, GOx, and antivascular endo−thelial growth factor aptamer (AVEGF) (Fig.2(b)). ZIF−8 MOFs are used as glucose−respon−sive carriers. GOx could cause the oxidation of glucose to produce H2O2and gluconic acid, then resulting in the degradation of ZIF−8 and the release of insulin, GOx, and AVEGF. The func−tional MOFs−insulin−GOx−AVEGF could be used as smart nanoparticles for the treatment of mac−ular diseases.
2.3 GSH-Responsive MOFs
Du et al.[10] applied benzene−1,3,5−tricarboxy−late (BTC) as the organic ligand and Cu as metal nodes to fabricated Cu−BTC−MOF. BMS−986205 (BMS) and NO donor s−nitrosothiol groups (SNAP) are loaded in MOF, then coated with F127 to improve its biocompatibility. Cu2+in Cu−BTC−MOF can be converted to Cu+by redox reactions in the GSH−enriched tumor microenvironment, degrading the Cu−BTC−MOF to release the encapsulated drugs. This approach for assembling responsive MOF−based drug carri−ers would open new potentials for tumor immunotherapy. Wang et al . [11] synthesized Cu(II) carboxylate−based MOFs and then loaded with photosensitizers ( chlorin e6, Ce6) (Fig.3(b)). After cellular uptake, Cu (II), metal nodes of MOFs could efficiently scavenge endoge−nous GSH and induce the decomposition of Cu(II) carboxylate−based MOF to release the encapsulated Ce6, then recovers their1O2genera−tion.
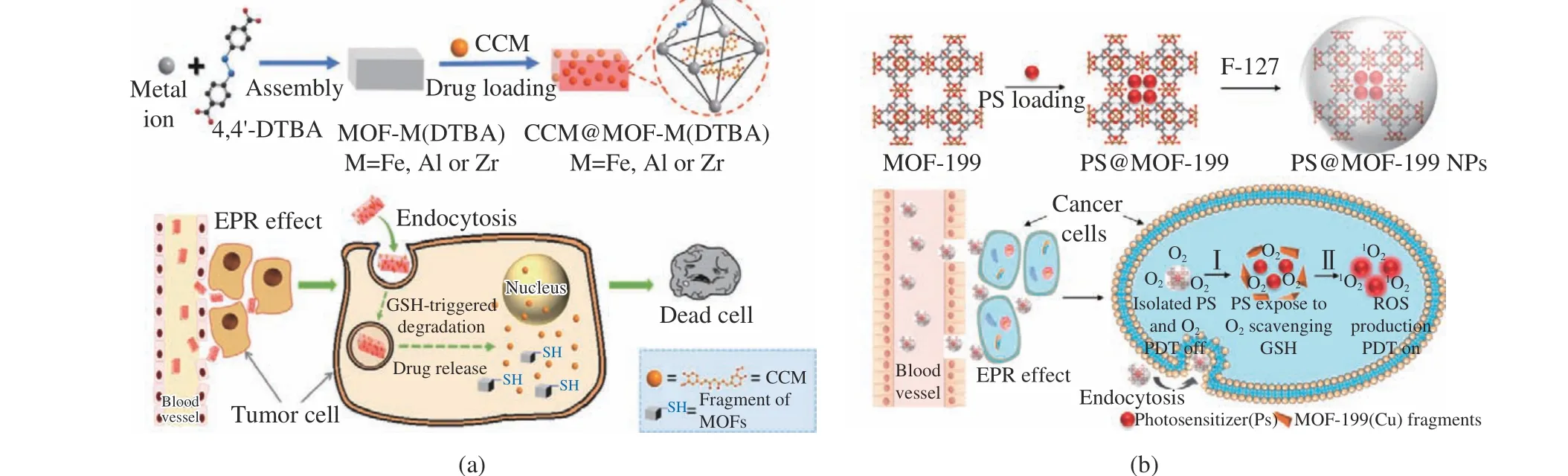
Fig. 3 Schematic illustration of GSH−responsive MOFs: (a) synthesis of MOF−M(DTBA) and the stimulus−response mechanism[10].Copyright 2018, American Chemical Society; (b) the preparation of Ce6 loaded Cu(II) carboxylate−based MOFs and the pro−cess of tumor treatment[11]. Copyright 2019, American Chemical Society
2.4 Light-Responsive MOFs
Li et al. [12] fabricated Cu−TCPP MOF nano−sheets through the solvothermal method. The strong NIR absorption under 808 nm laser irradi−ation, resulting from the d−d energy band transi−tion of Cu2+, leads to the effective temperature rising. Cu−TCPP MOF nanosheets could be used for the photothermal treatment of cancer. Huang et al. [13] designed and synthesized Au@ZIF−8 as the carrier of DOX. Au@ZIF−8 shows a good photothermal effect under 808 nm laser irradia−tion. Through the combination of photothermal therapy and photodynamic therapy, the prolifera−tion of cancer cells is effectively suppressed.Zhang et al. [14] revealed that DNA−functional−ized MOFs (PCN−244) could trace tumor cells targeted by accurate drug delivery and effective photodynamic therapy. This functionalization of PCN−224 provides a pivotal opportunity to develop MOFs−based multimodal treatment.
2.5 Temperature-Responsive MOFs
Temperature−sensitive MOF materials could sense small changes of temperature and produce corresponding physicochemical changes, so they are often used in the field of drug delivery. Silva et al. [15] designed a ZIF−8 based (ZIF−8, EuxTby)@AuNP nanomaterial. When the plasmonic absorption band is excited, it causes a tempera−ture increase and induces a thermal response.The wavelength and power density of the excita−tion source could be used to control the tempera−ture rise of the nanoparticles, and the exposure time of the irradiation source could control the ambient temperature rise of the ZIF−8@NP mate−rials. This thermal−responsive material can be activated by visible light radiation to release small molecules in a controlled manner. This work provides a new perspective for the design and preparation of self−assembled multifunc−tional thermal−responsive materials.
3 Application of MOF-Based Stimuli-Responsive
Adding different stimuli−responsive groups or materials to MOF structure and different physi−cal or chemical changes of these materials under stimulation, such as molecular conformation change, protonation, or hydrolytic cleavage, will eventually lead to the release of guest molecules,which will make MOF−based stimulus−response materials be used in different fields. In this sec−tion, the application directions of MOF−based stimulus−response materials will be described and summarized, such as drug delivery, adsorption and luminescence functions, magnetization and catalysis functions, probe and sensor applications,etc. (Tab. 2).
Tab. 2 Stimuli-responsive MOFs and their application
3.1 Drug Delivery
In this section, the applications of some typical MOF−based stimulation response materials in the treatment of cancer and other diseases are intro−duced and the treatment principle and experi−mental process of some materials are highlighted,which is significant for the more efficient selec−tion of MOF−based stimuli−response materials in the clinic application.
3.1.1 Cancer Treatment
A novel anoxic−reactive copper metal−organic framework nanoparticles ( Cu−MOF NPs) was first reported last year for chemodynamic and sonodynamic therapy (CDT/SDT)[16]. This nano−material will achieve targeted and efficient MCF−7 killing through Cu2+reduction reaction and SDT/CDT synergy in the hypoxic tumor microenvironment (Fig. 4(a)). Another smart nanocomposite based on core−shell metal−organic framework (MOF) can also carry out effective NIR/H2O2responsive photodynamic therapy for hypoxic tumor cells [17]. Lei et al.[18] developed an inherently redox−responsive MOF carrier and this nanoparticle, m(DTBA) (M = Fe, Al or Zr),showed faster release action in vitro experiment and could accelerate the process of cancer cell death (Fig.4(b)). The study of another copper por−phyrin metal−organic framework/graphene oxide nanocomposite also proves that the killing abil−ity of cancer cells carried by this pH−response−based nanocarrier is much higher than that of free form drugs [19]. The γ−cyclodextrin−based metal−organic framework (γ−CD−MOF) complex could obtain cancer cell targeting by fixing AS1411 aptamer on it, which has the application prospect of effective doxorubicin hydrochloride release and tumor growth inhibition both in vivo and vitro and can also be used for targeted anticancer drug delivery or cancer treatment[20]. Wang et al.[21]developed a triple−layered metal−organic frame−work hybrid based on a tandem−response strat−egy. This nanomaterial will improve the curative effect of drug−resistant cancer cells by producing highly active hydroxyl radicals and inhibiting the expression of drug−resistant P−glycoprotein in the tumor microenvironment (Fig.4(c)).
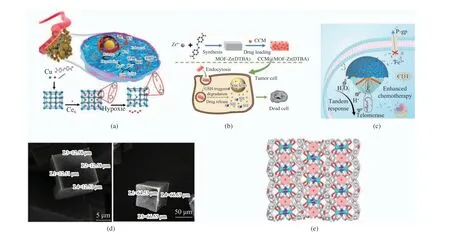
Fig. 4 Schematic diagram of MOFs for cancer treatment: (a) preparation of hypoxia−responsive copper MOF for improved cancer treatment[16]. Copyright 2020, Elsevier Ltd; (b) preparation of DTBA and principle of drug delivery. Copyright 2018, Ameri−can Chemical Society; (c) triple−layered MOF tandem response strategy. Copyright 2021, Wiley−V C H Verlag GmbbH & Co;(d) SEM images of MOF−5 and MOF−5 inserted with PAP. Copyright 2019, Elsevier Ltd; (e) crystal stacking pattern of MOF−1 along the b axis. Copyright 2017, American Chemical Society
3.1.2 Drug Delivery for Other Diseases
As the pH of the extracellular matrix in inflam−matory tissue is more acidic than that in the healthy cell tissue, the efficacy needs to be improved by these pH−responsive drug carriers. A biocompatible titanium−based metal−organic fra−mework MIL−125 (MIL represents the material of Lavoisier Institute) was developed, which could promote or inhibit drug release based on pH response for MIL−125 that has different stability in different environments [22]. Diabetes can cause hypothyroidism, insulin resistance, and other symptoms, leading to a series of metabolic disor−ders. Duan et al. [23] have developed a glucose−responsive metal−organic framework based insulin delivery nano−system, which relies on the stimu−lation of nanomaterials by glucose to promote or inhibit the release of insulin, and it has been suc−cessfully applied to the drug carrier platform of diabetes. Metal−organic framework nanomateri−als show giant application potential in the biomedical field. However, its application in bio−logical and clinical fields is limited due to its poor physiological stability in specific media. Liu et al. [42] researched the dissociation degree of MOF−based materials under different physiologi−cal conditions through in vivo and in vitro exper−iments and developed an original polymerization strategy based on MOF nanoparticles to increase its stability and drug release efficiency.
3.2 Adsorption Function
MOFs are used as an ideal carbon capture adsor−bent for its physical and chemical diversity and extremely high specific surface area, nevertheless,adsorbent regeneration has a giant energy penalty. Recently, Li et al. [24] proposed a dual stimuli−responsive MOF by both ultraviolet light and magnetic induction, PCN−250 and this is a new exploration to improve MOF adsorption effi−ciency by utilizing a variety of stimulations. The same year, a metal−organic framework combin−ing azo and amide ligands was developed, which showed high adsorption selectivity to CO2and some other specific gases under light response research, also with excellent performance in the separation of xylene isomers in the liquid phase[25]. As early as 2012, a metal−organic frame−work that can reversibly adsorb CO2was found[26]. This MOF inserted azobenzene functional groups into the organic linker, resulting in changes in the adsorption of guest molecules after photochemical or heat treatment.
3.3 Luminescence Function
A report in 2013 revealed the preparation and characterization of the first MOF containing DTE (a photochromic object) [27]. This mate−rial will be observed to change the absorption peak when irradiated with ultraviolet light, while it is irradiated with visible light and this color change will be reversed. In a research of Journal of Molecular Structure in 2019, the characteriza−tion of inserting the photoresponsive molecule 2−phenazopyridine (PAP) into MOF−5 as a guest was also reported (Fig. 4(d)) [28]. Through the research of its optical switch and invisible spec−trum, it is found that PAP has a lower possibil−ity of trans isomerization and stronger structural stability than azobenzene as a guest. In addition to light−response luminescence (photolumines−cence) MOF, some light−emitting MOFs based on thermal−response were also developed in recent years. Zhou et al. [29] proposed a double−emis−sion MOF hybrid in the Journal of Materials Chemistry C published in 2015. This inherent wide emission MOF with high sensitivity to tem−perature change shows a completely different thermal dependence from the traditional lan−thanide emission and intrinsic wide emission.Huang et al. [30] also synthesized a new mixed ligand two−dimensional MOF, mof−1 (Fig.4 (e)).By adding a certain proportion of Eu3+/Tb3+Co−doped material to the pores of MOF and a cer−tain thermal−induced emission conversion, the main body of the frame changes from blue light emission to white light emission.
3.4 Magnetization Function
The magnetization function of MOFs introduced here is similar to the CO2adsorption function in Section 3.2 above. Because of its high absorption and specific selectivity for CO2, MOF materials are expected to be developed into a carrier com−bining magnetic ordering and porosity. Generally,the magnetization function of MOFs is based on its stimuli−response to CO2. When the material absorbs and desorbs CO2, the material properties change between paramagnetism and ferromag−netism. Its electronic conductivity and the change of allowable activity will determine its ability to carry out electron transfer and struc−ture conversion when absorbing and releasing CO2. Based on these principles, Zhang et al. [31]reported a CO2responsive magnetic MOF, which consists of trifluorobenzoic acid bridged impeller type ruthenium (II) clusters as electron donors(D) and diethyloxytetracyanodimethylamine as electron acceptors (A) to form a MOF layered with [D+−A−−D] formula.
3.5 Catalysis Function
In the field of solar energy−driven pyrolysis, the recyclable heterogeneous catalyst is a significant scientific research topic that puzzles researchers for several decades. A functional homogeneous polyoxometalate MOF was reported on Applied Catalysis B: Environmental in 2021 (POM@MOF composite), which is constructed by a sim−ple and broad−spectrum impregnation strategy,can produce hydrogen through photo−responsive catalysis in a long term effect, and has the advantages of recyclability, stability, and high efficiency [32]. Similar to the application of drug carriers of MOF−based stimuli−response nanoma−terials, MOFs can also be used in catalysis based on pH−response. The nano MOFs supported by palladium nanoparticles are a kind of Pickering emulsifier, which is also synthesized by solution impregnation [4]. It is worth noting that this nanomaterial is pH−responsive, leading to the emulsification of toluene droplets under neutral conditions and demulsification under acidic con−ditions. In addition to the above−mentioned (pH,glucose, GSH, light, temperature, etc.) stimula−tion response methods, controllable and pre−dictable biologically induced intelligent MOF−based materials are also gradually applied and developed. Liang et al. [33] developed a new strategy of peptide−induced MOF super assem−bly with two or three single enzymes guest in.The coiled peptide chain on the surface of MOF ceases its surface structure decomposition and programmed enzyme cascade reaction, which greatly improves the catalytic efficiency of these particles compared with those unassembled MOF.
3.6 Probe and Sensor Applications
This section mainly introduces the application of some typical stimuli−response MOFs in probes and sensors, and briefly introduces the principle and development process of some of these probes and sensors. This would facilitate the develop−ment of new probes and sensors based on origi−nal stimuli−response or modern stimuli−response.
3.6.1 Probe Function
MOF materials with light−response and pH−response stimulation are introduced respectively.These materials can also be used in the field of biomedical sensors or probes. However, the previ−ous MOF probes have the defects of low signal−to−noise ratio, poor light stability, weak tissue penetration, and low transformation efficiency.To overcome these problems, Yang et al. [34]designed a two−photon MOF sensing platform(TP−MOF) based on NMOF combined with a two−photon organic part of target−response. The two−photon fluorophore in this probe can be excited in the near−infrared ( ~820 nm) band,which can be applied to the detection and imag−ing of living cells or tissues, and has favorable light stability and tissue penetration. Guo et al.[35] prepared a19F magnetic resonance imaging MRI nanoprobe with MOF core and TFMIM(providing fluorine), which depends on pH−response and has the advantages of outstanding penetration depth and high signal−to−noise ratio.Some modern probes, such as molecular recogni−tion probe MIL−53 (AL) based on isotopic−response, were also developed in 2020 [36]. This novel probe is different from those mentioned above such as light−response and pH−response stimulation probes. It uniquely uses secondary respiratory conversion to identify isotopic molecules, which solves the problem that storage and purification cannot be realized because of almost the same physical and chemical proper−ties of isotopes.
3.6.2 Sensor Function
A chemical sensor is a kind of instrument that is sensitive to various chemicals and converts its concentration into electrical signals for detection.The new generation of chemical sensors can be designed with MOF materials with adjustable functions. Yu et al. [37] developed a porous Cu(I)−MOF naked−eye colorimetric sensor for con−tinuously detecting or monitoring the concentra−tion of water or formaldehyde. The guest exchange ( water molecules and formaldehyde molecules) of this sensor is reversible and the mono−crystallinity of it is maintained in the pro−cess. As a modern type of chemical sensor, the fluorescence chemical sensor is many times more reliable than the ordinary visual system. A chem−ical sensing MOF based on photonics is synthe−sized by rapid supramolecular self−assembly [38].Through the photochemical interaction between host and guest nano−molecules, it is applied to the detection of a variety of volatile small molecules or compounds. Du et al. [39] prepared a multi−response dual−emission MOF, which can increase the signal channel to solve the problem of mutual interference between different analytes,and was applied to the quantitative analysis of two isomers of tetrachlorobenzoquinone. Lumi−nescent MOF sensors also have great potential in the biological and environmental fields, such as Eu−MOF and Cu−MOF, which were just devel−oped this year [40, 41]. Both of these two types of fluorescent three−dimensional or two−dimen−sional sensors exhibit high selectivity to specific ions or molecules, high sensitivity, and good recy−clability. Both these three−dimensional or two−dimensional fluorescence sensors show high selec−tivity, high sensitivity, and good recoverability for specific ions or molecules.
4 Conclusion
In this review, the stimuli−responsive MOFs were categorized and their applications were summa−rized. Different structures of MOFs have differ−ent release mechanisms under the action of pH,GSH, light, and temperature. The diversity and easy functionalization of MOFs make them prime candidates for the application of drug delivery,absorption, luminescence, magnetization, cataly−sis, and probe. Moreover, the designed porous structure of MOFs can be applied as monomers that respond to a specific environment, for exam−ple, pH, glucose, GSH, light, temperature, etc.This review revealed the relationship between the structure and stimuli−responsive properties of MOFs and focuses on their applications, which have reference value for the development of MOF−based stimuli−responsive materials and the exploration of new applications.
杂志排行
Journal of Beijing Institute of Technology的其它文章
- Application of Micro Electro Mechanical System(MEMS) Technology in Photoacoustic Imaging
- A New Class of Biodegradable Organic Optoelectronic Materials: α-Oligofurans
- Design of Implant Prosthesis for Bone Injury Repair Considering Stress Shielding Effect
- A Microfluidic System with Active Mixing for Improved Real-Time Isothermal Amplification
- Mixing Dynamics and Synthesis Performance of Staggered Herringbone Micromixer for Limit Size Lipid Nanoparticles
- Effect of Auxiliary Gas Flow Parameters on Microstructure and Properties of Ta Coatings Prepared by Three-Cathode Atmosphere Plasma Spraying
