Kir2.1在LPS或IL-4诱导的巨噬细胞M1/M2型极化中的作用*
2022-03-28王璐杨瑞张莹莹李新芝马克涛
王璐, 杨瑞, 张莹莹, 李新芝, 马克涛△
· 论著 ·
Kir2.1在LPS或IL-4诱导的巨噬细胞M1/M2型极化中的作用*
王璐1,2,3, 杨瑞1,2,3, 张莹莹1,2,3, 李新芝1,2,4△, 马克涛1,2,3△
(1石河子大学医学院新疆地方与民族高发病教育部重点实验室,新疆 石河子 832000;2石河子大学医学院第一附属医院国家卫健委中亚高发病防治重点实验室,新疆 石河子 832000;3石河子大学医学院生理学教研室,新疆 石河子 832000;4石河子大学医学院病理生理学教研室,新疆 石河子 832000)
通过激活或阻断内向整流钾通道2.1(inwardly-rectifying potassium channel 2.1, Kir2.1),检测巨噬细胞M1/M2型极化标志物及相关细胞因子的改变,探讨Kir2.1在脂多糖(lipopolysaccharide, LPS)或白细胞介素4(interleukin-4, IL-4)诱导的巨噬细胞M1/M2型极化过程中的具体作用。采用RAW264.7小鼠巨噬细胞系,将未转染慢病毒的RAW264.7细胞分为对照(control)组、LPS组、IL-4组、LPS+zacopride(Kir2.1选择性激动剂)组及IL-4+ML133(Kir2.1选择性阻断剂)组,将转染慢病毒的RAW264.7细胞分为vector组、LPS+vector组、IL-4+vector组、KD-shKir2.1组、LPS+KD-shKir2.1组及IL-4+KD-shKir2.1组。应用ELISA技术检测巨噬细胞培养液上清中IL-1β、IL-6和肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)的浓度;应用实时荧光定量PCR技术检测巨噬细胞中Kir2.1、诱导型一氧化氮合酶(inducible nitric oxide synthase, iNOS)、CD86、CD206、IL-6及TNF-α的mRNA表达;应用细胞免疫荧光技术和Western blot技术检测巨噬细胞中Kir2.1、iNOS、CD86及CD206的定位和表达。Kir2.1在RAW264.7细胞的胞膜及胞质中均有表达,并且LPS干预RAW264.7细胞后Kir2.1的蛋白表达显著下降(<0.01),IL-4干预巨噬细胞后Kir2.1的蛋白表达显著升高(<0.01)。激活Kir2.1能够显著减少炎症细胞因子的释放(<0.05或<0.01),降低M1型极化标志物iNOS和CD86的mRNA和蛋白表达(<0.05或<0.01),而阻断或敲减Kir2.1能够显著增加炎症细胞因子的释放(<0.05或<0.01),升高M1型极化标志物iNOS和CD86的mRNA和蛋白表达(<0.05或<0.01),降低M2型极化标志物CD206的mRNA和蛋白表达(<0.05或<0.01)。激活Kir2.1能够抑制LPS诱导的巨噬细胞向M1型极化;而阻断或敲减Kir2.1能够促进LPS诱导的巨噬细胞向M1型极化,抑制IL-4诱导的巨噬细胞向M2型极化。
巨噬细胞极化;内向整流钾通道;炎症;动脉粥样硬化
动脉粥样硬化(atherosclerosis, AS)是一种以粥样斑块为特征的心血管疾病[1-3],巨噬细胞作为斑块中炎症因子的主要来源,能够影响AS进程及结局[4-6]。巨噬细胞在各种因素诱导下,能够转变为不同的极化类型[7-8],即M1型巨噬细胞(经典激活型巨噬细胞)和M2型巨噬细胞(替代激活型巨噬细胞),二者在各种因素调节下处于动态平衡状态[9-10]。同时,巨噬细胞极化受多种钾离子通道调节,如内向整流钾离子通道(inwardly-rectifying potassium channels, Kir)等[11-12]。Kir2.1作为其中重要的成员之一,参与动作电位复极化等过程[13-15]。有文献报道,Kir2.1参与细胞分化,具有控制骨髓源性巨噬细胞增殖、活化和凋亡的作用[16-17]。因此,我们推测:Kir2.1可能通过调节脂多糖(lipopolysaccharide, LPS)或白细胞介素4(interleukin-4, IL-4)诱导的M1/M2型巨噬细胞极化,影响相关炎症细胞因子的释放,进而影响AS进展。为验证此假设,本实验采用LPS或IL-4建立巨噬细胞极化模型,一方面给予Kir2.1选择性阻断剂ML133或选择性激动剂zacopride干预,另一方面采用慢病毒转染巨噬细胞以降低Kir2.1的表达,应用ELISA、实时荧光定量PCR、Western blot及细胞免疫荧光技术,探讨Kir2.1在LPS或IL-4诱导的M1/M2型巨噬细胞极化中的具体功能,阐明巨噬细胞的Kir2.1在病理生理过程中的作用。
材料和方法
1 主要试剂及仪器
1.1试剂LPS(Sigma);IL-4(PeproTech);ML133和zacopride(APExBIO);抗Kir2.1抗体、抗诱导型一氧化氮合酶(inducible nitric oxide synthase, iNOS)抗体、抗CD86抗体和抗CD206抗体(Abcam);辣根过氧化物酶标记的Ⅱ抗(北京中杉金桥生物技术有限公司)。
1.2仪器生物安全柜[阿尔泰实验室设备(北京)有限公司];CO2恒温培养箱(Thermo);LSM510激光共聚焦显微镜(Carl Zeiss)。
2 方法
2.1细胞培养RAW264.7小鼠巨噬细胞系来源于中国科学院上海细胞库。将RAW264.7细胞用含有10%胎牛血清(fetal bovine serum, FBS)的DMEM培养液在37 ℃、5% CO2培养箱中孵育。将细胞分为control组、LPS组(100 μg/L LPS孵育24 h[18])、IL-4组(20 μg/L IL-4孵育24 h[19])、LPS+zacopride组(100 μmol/L zacopride预处理60 min后加入100 μg/L LPS共同孵育24 h)和IL-4+ML133组(20 μmol/L ML133预处理60 min后加入20 μg/L IL-4共同孵育24 h)。
2.2慢病毒转染用携带有绿色荧光蛋白(green fluorescent protein, GFP)标记的空白对照慢病毒以及携带有GFP标记小鼠shRNA的慢病毒(上海汉恒生物技术公司)感染RAW264.7细胞。实验分组为vector组(感染HBLV-GFP-PURO)、LPS+vector组(在vector组基础上加入100 μg/L LPS孵育24 h)、IL-4+vector组(在vector组基础上加入20 μg/L IL-4孵育24 h)、KD-shKir2.1组(感染HBLV-m-shRNA2-GFP-PURO)、LPS+KD-shKir2.1组(在KD-shKir2.1组基础上加入100 μg/L LPS孵育24 h)和IL-4+KD-shKir2.1组(在KD-shKir2.1组基础上加入20 μg/L IL-4孵育24 h)。
2.3ELISA检测培养液上清中IL-1β、IL-6和肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)的浓度取传代3~5次的RAW264.7细胞,铺板干预后收集各组细胞培养液上清于EP管中并做好标记,根据制造商说明书,应用ELISA试剂盒检测RAW264.7细胞培养液上清中IL-1β、IL-6和TNF-α的含量,每个样品测定3次。
2.4实时荧光定量PCR检测巨噬细胞中Kir2.1、IL-6、TNF-α及M1/M2型极化标志物的mRNA表达用标准Trizol试剂从RAW264.7细胞中提取总RNA。应用20 μL反转录体系反转录合成cDNA,反应程序为:25 ℃ 5 min,42 ℃ 60 min,70 ℃ 15 min,4 ℃。接着进行实时荧光定量PCR,引物序列见表1。
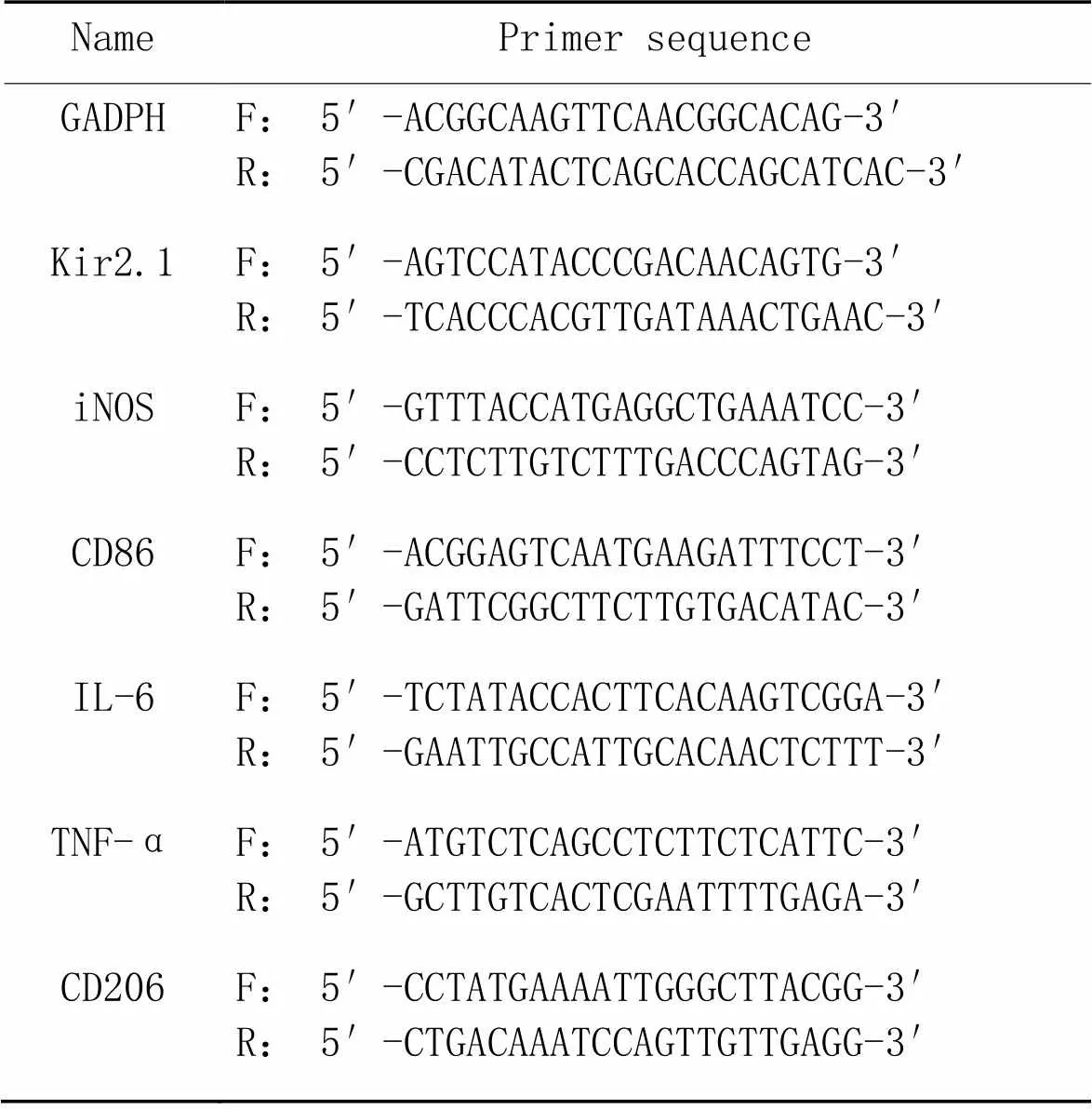
表1 引物序列
F: forward; R: reverse.
2.5Western blot检测巨噬细胞中Kir2.1及M1/M2型极化标志物的蛋白表达取出已干预好的细胞,PBS洗涤后于冰上裂解30 min,4 ℃、12 000 r/min离心15 min。采用BCA法测定蛋白浓度后,取蛋白样品加入适量上样缓冲液,于SDS聚丙烯酰胺凝胶进行电泳分离,然后将凝胶上的蛋白转移至PVDF膜上,5%脱脂奶粉室温封闭2 h,分别加入Ⅰ抗4 ℃过夜,洗膜后再加入相应Ⅱ抗室温孵育2 h,再次洗膜后与发光试剂反应,置于X线胶片暗盒中曝光、显影。应用ImageJ 2x分析软件检测并分析条带的灰度值。
2.6细胞免疫荧光检测巨噬细胞中Kir2.1及M1/M2型极化标志物的定位和表达取出已干预好的细胞,PBS洗涤3次,固定,破膜,BSA于37 ℃封闭30 min,滴加Ⅰ抗于湿盒内37 ℃孵育2 h,PBS漂洗3次,滴加Ⅱ抗,湿盒内37 ℃孵育1 h,PBS漂洗3次,滴加DAPI避光染色,PBS漂洗3次,封片。激光扫描共焦显微镜观察。
3 统计学分析
应用SPSS 22.0分析软件进行统计学分析。所有实验数据均采用均数±标准差(mean±SD)表示。两组间均数比较采用独立样本检验,多组间均数比较采用单因素方差分析(one-way ANOVA)。以<0.05为差异有统计学意义。
结果
1 LPS和IL-4能够调节巨噬细胞中Kir2.1的蛋白表达和mRNA表达
Kir2.1在巨噬细胞的胞膜及胞质中均有表达,LPS干预RAW264.7细胞后显著降低了Kir2.1蛋白和mRNA的表达水平,而IL-4干预RAW264.7细胞后Kir2.1的蛋白和mRNA表达水平显著提高(<0.01),见图1。

Figure 1.LPS and IL-4 regulated Kir2.1 expression in macrophages. A: the protein expression of Kir2.1 in RAW264.7 cells after LPS treatment; B: the protein expression of Kir2.1 in RAW264.7 cells after IL-4 treatment; C: the mRNA expression of Kir2.1 in RAW264.7 cells after LPS treatment; D: the mRNA expression of Kir2.1 in RAW264.7 cells after IL-4 treatment; E: the expression and localization of Kir2.1 in RAW264.7 cells (scale bar=50 μm). Mean±SD. n=6. **P<0.01 vs control group.
2 激活Kir2.1抑制LPS诱导的巨噬细胞向M1型极化
如图2所示,经LPS干预后,RAW264.7细胞IL-6、TNF-α、iNOS和CD86的mRNA表达水平,培养液上清中IL-1β、IL-6和TNF-α的浓度,以及iNOS和CD86的蛋白表达水平均显著升高(<0.05或<0.01);与LPS组相比,LPS+zacopride组IL-6、TNF-α、iNOS和CD86的mRNA表达水平,培养液上清中IL-1β、IL-6和TNF-α的浓度,以及iNOS和CD86的蛋白表达水平均显著降低(<0.05或<0.01)。可见,激活Kir2.1能够抑制LPS诱导的巨噬细胞向M1型极化。

Figure 2.Activation of Kir2.1 inhibited LPS-induced polarization of macrophages to M1 phenotype. A to D: the mRNA expression of iNOS, CD86, IL-6 and TNF-α in RAW264.7 cells; E to G: the concentrations of IL-1β, IL-6 and TNF-α in culture supernatants of RAW264.7 cells; H: the protein expression of of iNOS and CD86 in RAW264.7 cells; I: the expression and localization of iNOS in RAW264.7 cells; J: the expression and localization of CD86 in RAW264.7 cells. Scale bar=50 μm Mean±SD. n=6. *P<0.05, **P<0.01 vs control group; #P<0.05, ##P<0.01 vs LPS group.
3 敲减Kir2.1促进LPS诱导的巨噬细胞向M1型极化
如图3A所示,未转染慢病毒的control组无绿色荧光,而vector组和KD-shKir2.1组有绿色荧光,表明慢病毒已成功转染RAW264.7细胞。为进一步验证慢病毒敲减Kir2.1的效率,我们检测了RAW264.7细胞中Kir2.1的mRNA和蛋白表达。与vector组相比,KD-shKir2.1组RAW264.7细胞中Kir2.1的mRNA和蛋白表达水平均显著降低(<0.01),见图3B、C。

Figure 3.Verification of the transfection efficiency of lentivirus (A) and its knockdown efficiency for Kir2.1 (B and C). Scale bar=100 μm. Mean±SD. n=6. **P<0.01 vs vector group.
如图4所示,敲减Kir2.1能够提高RAW264.7细胞培养液上清中IL-1β、IL-6和TNF-α水平,增加IL-6、TNF-α、iNOS和CD86的mRNA表达,以及iNOS和CD86的蛋白表达(<0.05或<0.01),表明敲减Kir2.1能促进LPS诱导的巨噬细胞向M1型极化。

Figure 4.Knockdown of Kir2.1 promoted LPS-induced polarization of macrophages to M1 phenotype. A to D: the mRNA expression of iNOS, CD86, IL-6 and TNF-α in RAW264.7 cells; E to G: the concentrations of IL-1β, IL-6 and TNF-α in culture supernatants of RAW264.7 cells; H: the protein expression of iNOS and CD86 in RAW264.7 cells. Mean±SD. n=6. *P<0.05, **P<0.01 vs vector group; #P<0.05, ##P<0.01 vs LPS+vector group.
4 阻断或敲减Kir2.1抑制IL-4诱导的巨噬细胞向M2型极化
CD206在RAW264.7细胞的胞膜和胞质中均有表达;RAW264.7细胞经IL-4干预后,CD206的mRNA和蛋白表达显著上调(<0.01);阻断或敲减Kir2.1后,CD206的mRNA和蛋白表达显著下调(<0.05或<0.01),见图5。
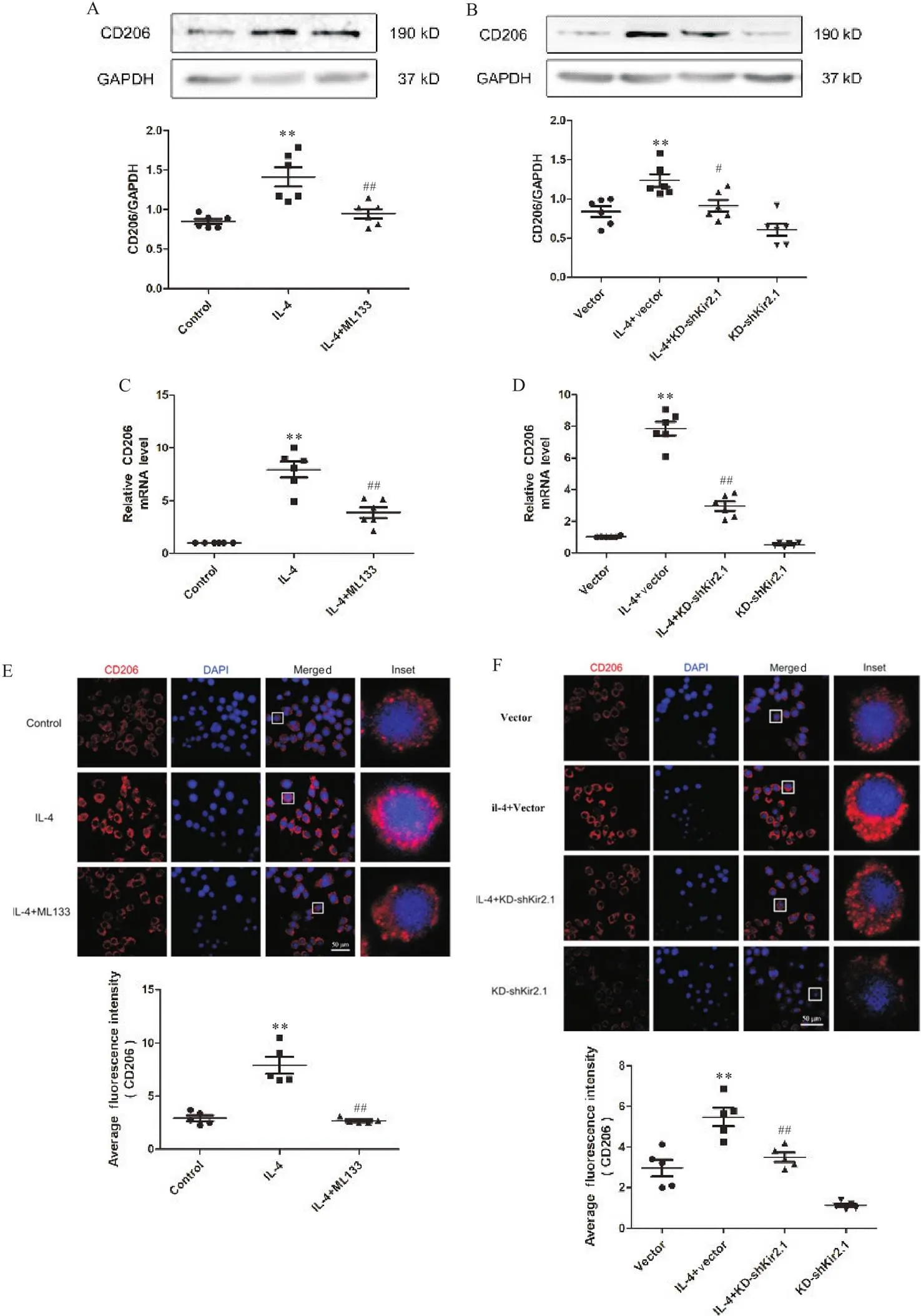
Figure 5.Blockage or knockdown of Kir2.1 inhibited IL-4-induced polarization of macrophages to M2 phenotype. A and B: the protein expression of CD206 in RAW264.7 cells after ML133 intervention or knockdown of Kir2.1; C and D: the mRNA expression of CD206 in RAW264.7 cells after ML133 intervention or knockdown of Kir2.1; E and F: the expression and localization of CD206 in RAW264.7 cells after ML133 intervention or knockdown of Kir2.1. Mean±SD. n=6. **P<0.01 vs control or vector group; #P<0.05, ##P<0.01 vs IL-4 or IL-4+vector group.
讨论
AS是一种常见的慢性炎性疾病[20-21]。巨噬细胞不同的极化类型被认为是AS斑块发生发展的关键动力[22-25]。巨噬细胞上有多种钾离子通道表达[26]。目前已有研究,证实电压门控性钾通道1.3在巨噬细胞M1/M2型极化过程中发挥关键作用[27],但Kir2.1是否参与调节巨噬细胞M1/M2型极化尚不明确。本研究采用LPS和IL-4分别诱导构建巨噬细胞M1和M2极化模型,探讨Kir2.1是否通过调节巨噬细胞M1/M2型极化,影响炎症细胞因子的释放,进而参与AS进展。
Kir2.1作为巨噬细胞中主要的离子通道之一,参与了巨噬细胞的分化和成熟。多项研究表明,Kir2.1在不同组织中调节巨噬细胞活化、增殖等多项生理功能[28-29],但Kir2.1在巨噬细胞M1/M2型极化过程中发挥的具体作用尚未明确报导。因此,本实验通过LPS或IL-4诱导巨噬细胞M1/M2型极化,观察到Kir2.1主要表达在巨噬细胞的胞膜和胞质中,并且LPS能够下调巨噬细胞中Kir2.1的蛋白表达,而IL-4能够上调巨噬细胞中Kir2.1的蛋白表达。国内外研究表明,zacopride作为一种Kir2.1选择性激动剂,能够剂量依赖性地增强内向整流钾电流,在心血管疾病中具有关键性的保护作用[30-31]。Wang等[32]也检测出ML133对Kir2.x家族的其他成员几乎没有选择性,是迄今报道的具选择性的Kir家族小分子阻断剂。因此本研究采用zacopride激活Kir2.1,结果显示激活Kir2.1能够抑制巨噬细胞向M1型极化,另一方面采用ML133阻断Kir2.1,显示阻断Kir2.1能够抑制巨噬细胞向M2型极化。另有研究通过应用转染技术检测到Kir2.1具有控制多种细胞类型电活动的作用。本研究也通过慢病毒转染巨噬细胞RAW264.7,观察到敲减Kir2.1能够促进巨噬细胞向M1型极化,抑制巨噬细胞向M2型极化。
综上所述,本研究表明巨噬细胞中存在Kir2.1的表达,激活Kir2.1可以抑制巨噬细胞向M1型极化,阻断或敲减Kir2.1可抑制巨噬细胞向M2型极化并促进巨噬细胞向M1型极化。这些结果表明,Kir2.1能够调节巨噬细胞极化过程,由此推测上调Kir2.1对AS具有一定的缓解作用,但它具体通过哪些信号通路调节巨噬细胞极化尚不明确。目前考虑Kir2.1参与NF-κB信号通路和JAK/STAT信号通路并在其中发挥调节作用,因此后续研究将会从Kir2.1在这2条信号通路中的具体作用机制这一方面做进一步探讨。
[1] Wu YL, Wang F, Fan LH, et al. Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF-κB and p38 MAPK signaling pathways[J]. Biomed Pharmacother, 2018, 97:1673-1679.
[2]樊碧娆, 姚伟娟. 氧化型低密度脂蛋白受体在动脉粥样硬化发病机制中的作用[J]. 中国病理生理杂志, 2020, 36(10):1897-1901.
Fan BY, Yao WJ. Role of oxidized low density lipoprotein receptors in the pathogenesis of atherosclerosis[J]. Chin J Pathophysiol, 2020, 36(10):1897-1901.
[3] Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates[J]. Clin Sci (Lond), 2018, 132(12):1243-1252.
[4] Silveira LS, Antunes BMM, Minari ALA, et al. Macrophage polarization: implications on metabolic diseases and the role of exercise[J]. Crit Rev Eukaryot Gene Expr, 2016, 26(2):115-132.
[5] Tian F, Yu BL, Hu JR. mTOR mediates the cross-talk of macrophage polarization and autophagy in atherosclerosis[J]. Int J Cardiol, 2014, 177(1):144-145.
[6] Li YQ, Shen SX, Ding SK, et al. Toll-like receptor 2 downregulates the cholesterol efflux by activating the nuclear factor-κB pathway in macrophages and may be a potential therapeutic target for the prevention of atherosclerosis[J]. Exp Ther Med, 2018, 15(1):198-204.
[7] Tang D, Chen SY, Hou D, et al. Regulation of macrophage polarization and promotion of endothelialization by NO generating and PEG-YIGSR modified vascular graft[J]. Mater Sci Eng C Mater Biol Appl, 2018, 84:1-11.
[8]吴莲凤, 陆红, 洪炜龙, 等. M1/M2型巨噬细胞极化参与肾组织炎症损伤和修复进程[J]. 中国病理生理杂志, 2017, 33(12):2245-2251.
Wu LF, Lu H, Hong WL, et al. M1/M2 macrophage polarization is involved in the inflammatory injury and repair process of renal tissue[J]. Chin J Pathophysiol, 2017, 33(12):2245-2251.
[9] Han XB, Boisvert WA. Interleukin-10 protects against atherosclerosis by modulating multiple atherogenic macrophage function[J]. Thromb Haemost, 2015, 113(5):505-512.
[10] Khallou-Laschet J, Varthaman A, Fornasa G, et al. Macrophage plasticity in experimental atherosclerosis[J]. PLoS One, 2010, 5(1):e8852.
[11] Grizel A, Popinako A, Kasimova MA, et al. Domain structure and conformational changes in rat KV2.1 ion channel[J]. J Neuroimmune Pharmacol, 2014, 9(5):727-739.
[12] Fang W, Xia L, Sun XX, et al. Lipopolysaccharides increase Kir2.1 expression in lung endothelial cells[J]. Int J Clin Exp Pathol, 2018, 11(6):2959-2967.
[13] Chen KH, Zuo DC, Liu Z, et al. Kir2.1 channels set two levels of resting membrane potential with inward rectification[J]. Pflugers Arch, 2018, 470(4):599-611.
[14] Zuo DC, Chen KH, Zhou M, et al. Kir2.1 and K2P1 channels reconstitute two levels of resting membrane potential in cardiomyocytes[J]. J Physiol, 2017, 595(15):5129-5142.
[15] Wendt S, Wogram E, Korvers L, et al. Experimental cortical spreading depression induces NMDA receptor dependent potassium currents in microglia[J]. J Neurosci, 2016, 36(23):6165-6174.
[16] Nguyen HM, Grössinger EM, Horiuchi M, et al. Differential Kv1.3, KCa3.1, and Kir2.1 expression in "classically" and "alternatively" activated microglia[J]. Glia, 2017, 65(1):106-121.
[17] Lam D, Lively S, Schlichter LC. Responses of rat and mouse primary microglia to pro- and anti-inflammatory stimuli: molecular profiles, K+channels and migration[J]. J Neuroinflammation, 2017, 14:166.
[18] 李建设, 何文龙, 孟珂, 等. 姜黄素对LPS诱导的大鼠星形胶质细胞NF-κB炎症因子信号通路的影响[J]. 中国老年学杂志, 2021, 41(5):1081-1085.
Li JS, He WL, Meng K, et al. Effects of curcumin on LPS-induced NF-κB inflammatory factor signaling pathway in rat astrocytes[J]. Chin J Gerontol, 2021, 41(5):1081-1085.
[19]李鹏辉, 梁世倩, 胡昳旸, 等. 小鼠脊髓损伤急性期不同来源巨噬细胞活化和功能比较分析[J]. 神经解剖学杂志, 2021, 37(3):265-273.
Li PH, Liang SQ, Hu YY, et al. Comparative analysis of activation and function of macrophages from different sources during acute spinal cord injury in mice[J]. Chin J Neuroanat, 2021, 37(3):265-273.
[20] Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis[J]. Nat Rev Cardiol, 2015, 12(1):10-17.
[21] Kurkowska-Jastrzebska I, Karlinski MA, Błazejewska-Hyzorek B, et al. Carotid intima media thickness and blood biomarkers of atherosclerosis in patients after stroke or myocardial infarction[J]. Croat Med J, 2016, 57(6):548-557.
[22] Leroyer AS, Isobe H, Lesèche G, et al. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques[J]. J Am Coll Cardiol, 2007, 49(7):772-777.
[23] Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions[J]. Nat Rev Cardiol, 2010, 7(2):77-86.
[24] Chinetti-Gbaguidi G, Baron M, Bouhlel MA, et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways[J]. Circ Res, 2011, 108(8):985-995.
[25] Zhang BC, Ma YF, Xiang CH. SIRT2 decreases atherosclerotic plaque formation in low-density lipoprotein receptor-deficient mice by modulating macrophage polarization[J]. Biomed Pharmacother, 2018, 97:1238-1242.
[26] Cong B, Chai YM, Wang B, et al. Molecular characterization and functional analysis of four teleostean K+channels in macrophages of sea perch ()[J]. Fish Shellfish Immunol, 2017, 60:426-435.
[27] 刘雪琴, 王彦富, 张慧玲, 等. 动脉粥样硬化中钾通道Kv1.3阻断剂对巨噬细胞极化的影响[J]. 临床心血管病杂志, 2016, 32(9):901-904.
Liu XQ, Wang YF, Zhang HL, et al. Effect of potassium channel Kv1.3 blocker on polarization of macrophages in atherosclerosis[J]. J Clin Cardiol, 2016, 32(9):901-904.
[28] 雷新军, 马爱群, 席雨涛, 等. 阻断Kv1.3和Kir2.1抑制人巨噬细胞源性泡沫细胞分化[J]. 中南大学学报(医学版), 2006, 31(4):493-498.
Lei XJ, Ma AQ, Xi YT, et al. Inhibition of Kv1.3 and Kir2.1 inhibited the differentiation of human macrophage-derived foam cells[J]. J Cent South Univ (Med Sci), 2006, 31(4):493-498.
[29] Wu SY, Chen YW, Tsai SF, et al. Estrogen ameliorates microglial activation by inhibiting the Kir2.1 inward-rectifier K+channel[J]. Sci Rep, 2016, 6:22864.
[30] Liu QH, Li XL, Xu YW, et al. A novel discovery ofK1channel agonist: zacopride selectively enhancesK1current and suppresses triggered arrhythmias in the rat[J]. J Cardiovasc Pharmacol, 2012, 59(1):37-48.
[31] Wu BW, Cao JM. On the risk concerns of zacopride, a moderateK1channel agonist with cardiac protective action[J]. J Cardiovasc Pharmacol, 2014, 64(4):357-359.
[32] Wang HR, Wu M, Yu HB, et al. Selective inhibition of the Kir2 family of inward rectifier potassium channels by a small molecule probe: the discovery, SAR, and pharmacological characterization of ML133[J]. ACS Chem Biol, 2011, 6(8):845-856.
Role of Kir2.1 in M1/M2 polarization of macrophages induced by LPS or IL-4
WANG Lu1,2,3, YANG Rui1,2,3, ZHANG Ying-ying1,2,3, LI Xin-zhi1,2,4△, MA Ke-tao1,2,3△
(1,,,832000,;2,,,832000,;3,,832000,;4,,832000,)
To explore the specific role of inwardly-rectifying potassium channel 2.1 (Kir2.1) in the M1/M2 polarization of macrophage induced by lipopolysaccharide (LPS) or interleukin-4 (IL-4).Mouse RAW264.7 macrophages were used in this study. The RAW264.7 cells were divided into control group, LPS group, IL-4 group, LPS+zacopride (a selective agonist) group and IL-4+ML133 (a selective blocker of Kir2.1) group. The RAW264.7 cells infected with lentivirus were divided into vector group, LPS+vector group, IL-4+vector group, KD-shKir2.1 group, LPS+KD-shKir2.1 group and IL-4+KD-shKir2.1 group. The concentrations of IL-1β, IL-6 and tumor necrosis factor-α (TNF-α) in the supernatant of macrophage medium were detected by ELISA. The mRNA levels of Kir2.1, inducible nitric oxide synthase (iNOS), CD86, CD206, IL-6 and TNF-α in the RAW264.7 cells were detected by real-time fluorescence quantitative PCR. The location and protein expression of Kir2.1, iNOS, CD86 and CD206 in the RAW264.7 cells were detected by immunofluorescence and Western blot.The Kir2.1 was mainly expressed in both membrane and cytoplasm of the RAW264.7 cells. The protein expression of Kir2.1 was significantly decreased after LPS intervention and significantly increased after IL-4 intervention in the RAW264.7 cells (<0.01). Activation of Kir2.1 significantly reduced the release of inflammatory cytokines (<0.05 or<0.01), decreased the mRNA and protein expression of iNOS and CD86 (<0.05 or<0.01). Inhibition of Kir2.1 significantly increased the release of inflammatory cytokines (<0.05 or<0.01) and the mRNA and protein levels of iNOS and CD86 (<0.05 or<0.01), but decreased the mRNA and protein expression of CD206 (<0.05 or<0.01).Activation of Kir2.1 inhibits the M1 polarization of LPS-induced macrophages. Inhibition of Kir2.1 promotes the M1 polarization of LPS-induced macrophages and decreases the M2 polarization of IL-4-induced macrophages.
Macrophage polarization; Inwardly-rectifying potassium channels; Inflammation; Atherosclerosis
R363; R541.4
A
10.3969/j.issn.1000-4718.2022.03.001
1000-4718(2022)03-0385-09
2021-09-23
2022-01-20
[基金项目]国家自然科学基金资助项目(No. 81860286);兵团国际合作项目(No. 2020BC004);中国医学科学院中央级公益性科研院所基本科研业务费专项资金资助(No. 2020-PT330-003)
马克涛 Tel: 15001645180; E-mail: maketao@ hotmail.com; 李新芝 Tel:13309939180; E-mail: lixinzhi@shzu.edu.cn
(责任编辑:卢萍,罗森)
