敲减SET8表达通过上调p53/DRAM1信号通路促进大鼠血管平滑肌细胞自噬和凋亡*
2022-03-28刘兰张东雪朱荣芳李晖梁向楠张胜雷白亚玲
刘兰, 张东雪, 朱荣芳, 李晖, 梁向楠, 张胜雷, 白亚玲
敲减表达通过上调p53/DRAM1信号通路促进大鼠血管平滑肌细胞自噬和凋亡*
刘兰, 张东雪, 朱荣芳, 李晖, 梁向楠, 张胜雷, 白亚玲△
(河北医科大学第四医院肾内科,河北 石家庄 050011)
探讨赖氨酸甲基转移酶SET8(SET domain-containing protein 8)低表达通过p53/DRAM1(DNA damage-regulated autophagy modulator 1)通路调控大鼠血管平滑肌细胞(vascular smooth muscle cells, VSMCs)自噬和凋亡的作用及机制。体外原代培养大鼠VSMCs,敲减表达,将细胞分为3组:正常组、空载体对照组和SET8-shRNA组。流式细胞术检测细胞凋亡,MTT法检测细胞活力,Western blot检测SET8、p53、DRAM1、LC3、Bax和Bcl-2的蛋白水平。过表达,将大鼠VSMCs分为3组:正常组、空载体对照组和DRAM1组。检测各组细胞活力,凋亡及DRAM1、LC3、Bax和Bcl-2的蛋白水平。敲减表达的同时敲减或表达,观察LC3、Bax和Bcl-2的蛋白表达情况。(1)敲减表达后,大鼠VSMCs凋亡显著增多,活力显著降低(<0.05)。Western blot结果显示,敲减表达后,SET8和Bcl-2表达显著降低(<0.05),p53、DRAM1、LC3-II和Bax表达显著升高(<0.05)。(2)过表达后,大鼠VSMCs凋亡显著增多(<0.05),活力显著降低(<0.05)。Western blot结果显示,过表达后,DRAM1、LC3-II和Bax表达显著升高(<0.05),Bcl-2表达显著降低(<0.05)。(3)敲减或表达后,LC3-II和Bax表达降低,Bcl-2表达升高(<0.05)。敲减表达可促进大鼠血管平滑肌细胞发生自噬和凋亡,可能机制是通过上调p53和DRAM1表达,促进自噬蛋白LC3-II及凋亡蛋白Bax表达实现的。
血管平滑肌细胞;SET8蛋白;DRAM1蛋白;自噬;细胞凋亡
血管钙化是增加心血管疾病发病率和死亡率的重要危险因素[1-2]。血管钙化的重要机制有细胞的自噬、凋亡、表型转化等[3-4]。SET8 (SET domain-containing protein 8)是现今发现的唯一能够特异性催化H4赖氨酸20位的赖氨酸甲基转移酶,能够甲基化p53和Twist等非组蛋白,参与调控细胞增殖凋亡等生理学功能[5]。自噬基因(DNA damage-regulated autophagy modulator 1)是p53下游调节自噬和凋亡的基因,2006年由Crighton等[6]提出,可调节原发性肿瘤细胞的自噬和凋亡。作为DRAM依赖的一种自噬凋亡途径,自噬能够作为一种细胞死亡程序,诱导肝细胞凋亡及肝损伤[7]。现今SET8和DRAM1在大鼠血管平滑肌细胞(vascular smooth muscle cells, VSMCs)自噬和凋亡中的作用研究均较少,并且机制尚不明确。鉴于此,本项工作以体外培养的大鼠血管平滑肌细胞为研究对象,探讨SET8是否通过p53/DRAM1通路调控大鼠血管平滑肌细胞的自噬和凋亡。
材料和方法
1 主要实验试剂
选取胎牛血清(Gibco);SET8质粒和DRAM1质粒(广州复能基因有限公司);Lipofectamine™ 3000(Invitrogen);SET8抗体(Abcam);DRAM1和LC3抗体(华安生物技术有限公司);Bcl-2和Bax抗体(Abcam);GAPDH抗体(Bioworld)。
2 实验方法
2.1实验模型的制备与分组选取河北医科大学动物实验中心的健康雄性清洁级SD大鼠(证书号:1305090)6只,8~10周龄,体重80~100 g,做原代培养[8]。取胸主动脉中膜层,剪成小块,均匀铺于平底进行培养。取第3~4代细胞,给与刺激。将大鼠VSMCs分为3组:(1)正常normal)组:细胞未转染;(2)对照(vector)组:转染浓度为2 μg/L的对照NS-shRNA质粒;(3)SET8-shRNA组:转染浓度为2 μg/L的SET8-shRNA质粒。检测细胞自噬及凋亡情况。为验证DRAM1对VSMCs自噬及凋亡的影响,将大鼠VSMCs分为3组:(1)normal组:细胞未转染;(2)vector组:转染对照质粒,浓度为2 μg/L;(3)DRAM1组:转染DRAM1过表达质粒,浓度为2 μg/L。检测细胞自噬及凋亡情况。
2.2MTT法检测细胞活力取第3代大鼠血管平滑肌细胞接种于96孔板中,细胞融合达60%~70%给予刺激,每组设3个复孔。检测前4 h将5 g/L的MTT 按每孔20 μL加入各检测孔中,培养4 h,之后弃去培养液,将二甲基亚砜按每孔150 μl加入各检测孔,室温振荡10 min。于酶标仪490 nm波长处测定吸光度()值,记录结果。
2.3流式细胞术检测细胞凋亡收集大鼠血管平滑肌细胞,根据试剂说明书进行Annexin V-PE/7-AAD双重染色,使用流式细胞仪进行检测,右下象限Annexin V阳性、7-AAD阴性,为早期凋亡细胞;右上象限Annexin V和7-AAD均为阳性,为晚期凋亡细胞。实验重复3次。
2.4应用 Western blot检测各指标蛋白的表达提取各组大鼠VSMCs的蛋白质,配制成12%的SDS-聚丙烯酰胺凝胶,测蛋白浓度并取20 μg蛋白质加样,电泳条件95 V、1.5 h。使用PVDF膜转膜,转膜条件95 V、1 h。洗膜3次,牛血清白蛋白(5%)封闭1 h。加Ⅰ抗稀释液(SET8, 1∶500; DRAM1, 1∶1 000; LC3, 1∶1 000; Bax, 1∶2 000; Bcl-2, 1∶1 000; p53, 1∶2 000; GAPDH, 1∶5 000),4 ℃孵育过夜;次日洗膜,加入II抗稀释液(1∶5 000),37 ℃孵育1 h。使用蛋白成像系统照膜,实验重复3次。
2.5共转染实验为验证SET8对p53和DRAM1的调节作用,在大鼠VSMCs中转染SET8-shRNA,并在转染SET8-shRNA的同时共转染p53-shRNA和DRAM1-shRNA,vector作为对照组,观察VSMCs凋亡和自噬蛋白的表达情况。收集转染48 h后的各组细胞,提取蛋白,检测Bax、Bcl-2和LC3蛋白表达。
3 统计学处理
采用SPSS 20.0软件进行统计学处理。呈正态分布的计量资料以均数±标准差(mean±SD)表示,两组间比较采用检验,多组间的比较采用单因素方差分析,组间两两比较采用Student-Newman-Keuls(SNK)检验,<0.05为差异有统计学意义。
结果
1 敲减SET8对大鼠VSMCs凋亡的影响
给予敲减表达后,采用Annexin V-PE/7-AAD染色,应用流式细胞仪检测细胞凋亡的变化情况。结果显示,与normal组和vector组比较,SET8-shRNA组细胞凋亡显著增多(<0.05),见图1。
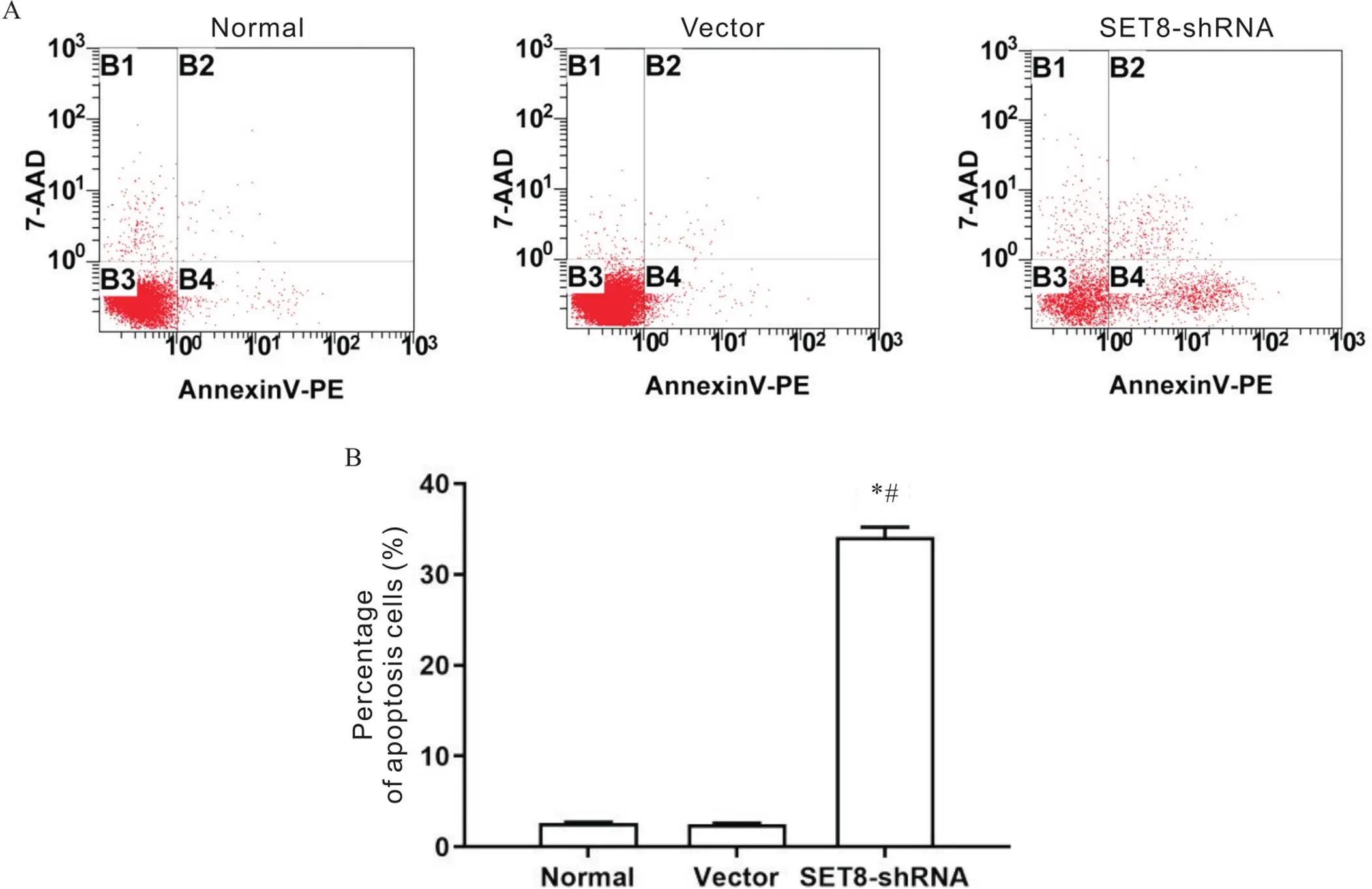
Figure 1.Effect of SET8 knockdown on apoptosis of rat VSMCs. A: flow cytometry results; B: percentage of apoptotic cells. Mean±SD. n=3. *P<0.05 vs normal group; #P<0.05 vs vector group.
2 敲减SET8对大鼠VSMCs活力的影响
MTT结果显示,与normal组和vector组比较,SET8-shRNA组细胞活力于12 h、24 h、36 h和48 h均显著降低(<0.05);normal组与vector组比较,差异无统计学意义(>0.05),见图2。
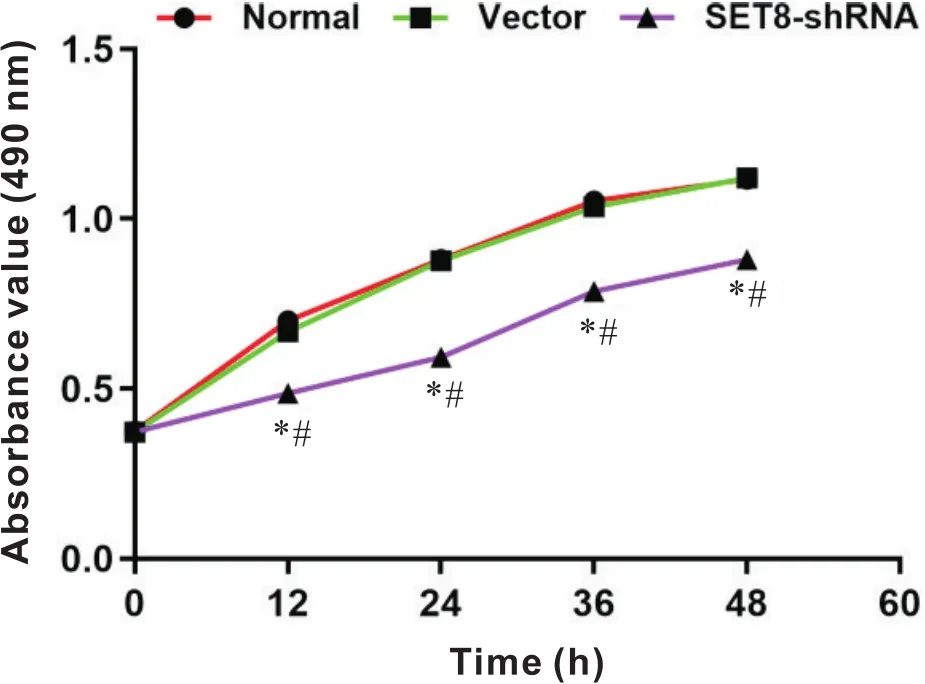
Figure 2.Effect of SET8 knockdown on rat VSMCs viability. Mean±SD. n=3. *P<0.05 vs normal group; #P<0.05 vs vector group.
3 敲减SET8表达后对各组大鼠VSMCs p53、DRAM1、LC3、Bcl-2和Bax表达的影响
Western blot结果显示,与normal组和vector组比较,SET8-shRNA组SET8和Bcl-2蛋白相对表达量显著降低,p53、DRAM1、LC3-II和Bax蛋白相对表达量显著升高(<0.05),见图3。

Figure 3.Effect of SET8 knockdown on the protein expression of p53, DRAM1, LC3, Bcl-2 and Bax in rat VSMCs in each group. A: Western blot results for each protein; B: relative protein expression. Mean±SD. n=3. *P<0.05 vs normal group; #P<0.05 vs vector group.
4 过表达DRAM1对大鼠VSMCs凋亡的影响
流式细胞术结果显示,与normal组和vector组比较,DRAM1组细胞凋亡显著增多(<0.05),见图4。

Figure 4.Effect of overexpression of DRAM1 on apoptosis of rat VSMCs. A: flow cytometry results; B: percentage of apoptotic cells. Mean±SD. n=3. *P<0.05 vs normal group; #P<0.05 vs vector group.
5 DRAM1对大鼠VSMCs活力的影响
MTT结果显示,与normal组和vector组比较,DRAM1组细胞活力于12 h、24 h、36 h和48 h均显著降低(0.05);normal组与vector组比较,差异无统计学意义(0.05),见图5。

Figure 5.Effect of overexpression of DRAM1 on the viability of rat VSMCs. Mean±SD. n=3. *P<0.05 vs normal group; #P<0.05 vs vector group.
6 DRAM1过表达对各组大鼠VSMCs LC3、Bcl-2和Bax表达的影响
Western blot结果显示,与normal组和vector组比较,DRAM1组Bcl-2蛋白相对表达量显著降低,DRAM1、LC3-II和Bax蛋白相对表达量显著升高(<0.05),见图6。

Figure 6.Effects of overexpression of DRAM1 on the expression of LC3, Bcl-2 and Bax proteins in rat VSMCs. A: Western blot results for each protein; B:r elative protein expression. Mean±SD. n=3. *P<0.05 vs normal group; #P<0.05 vs vector group.
7 SET8-shRNA与p53-shRNA和DRAM1-shRNA共转染
与vector组比较,将SET8-shRNA转染至VSMCs后,Bax及LC3-II蛋白表达增高,Bcl-2蛋白表达显著降低(0.05);与SET8-shRNA组比较,SET8-shRNA与p53-shRNA共同转染VSMCs后,Bax及LC3-II蛋白表达降低,Bcl-2蛋白表达显著升高(0.05);与SET8-shRNA组比较,SET8-shRNA与DRAM1-shRNA共同转染VSMCs后,Bax及LC3-II蛋白表达降低,Bcl-2蛋白表达升高(0.05),见图7。

Figure 7.Effects of co-transfection of SET8-shRNA with p53-shRNA or DRAM1-shRNA on the expression of Bax, Bcl-2 and LC3 proteins. A: Western blot results for each protein; B: relative protein expression. 1: vector group; 2: SET8-shRNA group; 3: SET8-shRNA+p53-shRNA group; 4,SET8-shRNA+DRAM1-shRNA group. Mean±SD. n=3. *P<0.05 vs vector group; #P<0.05 vs SET8-shRNA group.
讨论
血管钙化是心血管疾病死亡率增高的独立危险因素[9]。而细胞的凋亡、自噬和表型转化等是引起血管钙化的重要机制[10-11]。参与细胞凋亡和自噬的通路有多种。本项工作以体外培养的大鼠血管平滑肌细胞为研究对象,探讨SET8调控大鼠血管平滑肌细胞自噬和凋亡的发生机制。研究结果显示SET8低表达可诱导大鼠血管平滑肌细胞发生自噬与凋亡,其可能机制是通过促进p53及DRAM1表达实现的。
赖氨酸甲基转移酶SET8可以特异性单甲基化抑癌基因的382赖氨酸位点(K382me1),能够抑制的靶基因转录,进而影响细胞的增殖、凋亡等生物学功能[12-13]。而抑癌基因参与细胞生存和分裂的控制,对细胞凋亡、自噬和细胞周期有重大的影响[14-15]。Zhang等[12]显示敲减表达可通过促进p53表达进而促进胃癌细胞发生凋亡。也有研究显示在血管平滑肌细胞凋亡中SET8也起重要作用[16]。但SET8在大鼠血管平滑肌细胞凋亡和自噬中的具体作用机制尚不明确,因此本研究以体外培养大鼠血管平滑肌细胞为模型,通过敲减表达,观察大鼠血管平滑肌细胞凋亡情况及自噬蛋白DRAM1和LC3-II等蛋白变化。本研究结果显示敲减表达后,p53、DRAM1和LC3-II表达上调,促凋亡蛋白Bax表达升高,抑凋亡蛋白Bcl-2表达降低,大鼠血管平滑肌细胞增殖能力降低,凋亡增多。提示敲减后,可通过上调p53、DRAM1、LC3-II和Bax表达,抑制Bcl-2的表达,进而促进大鼠血管平滑肌细胞发生凋亡。
有研究显示过表达能够上调自噬蛋白LC3-II表达,进而诱导癌细胞发生自噬[17]。也有研究表明DRAM1能够上调促凋亡蛋白Bax表达,进而促进宫颈癌细胞凋亡[18]。而Bax和Bcl-2是一对正负凋亡调节因子,Bax起促进凋亡作用,Bcl-2起抗凋亡作用[19]。因此,我们推测DRAM1可能通过调控Bax、Bcl-2和LC3,进而诱导大鼠血管平滑肌细胞发生自噬和凋亡。本研究以体外培养大鼠血管平滑肌细胞为模型,给予过表达,观察大鼠血管平滑肌细胞凋亡及自噬情况。结果显示给予过表达后,自噬相关蛋白LC3-II表达升高,促凋亡蛋白Bax表达升高,抑凋亡蛋白Bcl-2表达降低,大鼠血管平滑肌细胞凋亡增多,增殖显著减少。结果提示,DRAM1可通过上调自噬蛋白LC3-II和凋亡蛋白Bax表达,抑制Bcl-2的表达,进而促进大鼠血管平滑肌细胞发生自噬和凋亡。2006年Crighton等[6]提出,是下游调节自噬的重要基因。编码238个氨基酸,是调控自噬和凋亡的溶酶体膜蛋白[20-21]。为验证SET8是否通过p53和DRAM1通路调控大鼠血管平滑肌细胞自噬和凋亡,给予抑制表达的同时抑制和表达,结果显示与只抑制表达组相比,抑制同时抑制或表达后,Bax和LC3-II显著降低,Bcl-2显著升高。
综上所述,SET8低表达可以促进大鼠血管平滑肌细胞发生自噬和凋亡,可能机制是通过调节p53/DRAM1表达,促进自噬蛋白LC3-II和凋亡蛋白Bax表达,抑制抗凋亡蛋白Bcl-2的表达,进而引起大鼠血管平滑肌细胞发生了自噬和凋亡。
[1] Yamada S, Giachelli CM. Vascular calcification in CKD-MBD: roles for phosphate, FGF23, and Klotho[J]. Bone, 2017, 100:87-93.
[2] Disthabanchong S, Srisuwarn P. Mechanisms of vascular calcification in kidney disease[J]. Adv Chronic Kidney Dis, 2019, 26(6):417-426.
[3] Liu R, Leslie KL, Martin KA. Epigenetic regulation of smooth muscle cell plasticity [J]. Biochim Biophys Acta, 2015, 1849(4):448-453.
[4]周薇, 徐金升, 白亚玲, 等. miR-30b在高磷诱导的大鼠血管平滑肌细胞钙化中的作用[J]. 中国病理生理杂志, 2021, 37(1):54-59.
Zhou W, Xu JS, Bai YL, et al. Role of miR-30b in calcification of rat vascular smooth muscle cells induced by high phosphorus[J]. Chin J Pathophysiol, 2021, 37(1):54-59.
[5] Yang C, Wang K, Zhou Y, et al. Histone lysine methyltransferase SET8 is a novel therapeutic target for cancer treatment[J]. Drug Discov Today, 2021, 26(10):2423-2430.
[6] Crighton D, Wilkinson S, O'Prey J, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis[J]. Cell, 2006, 126(1):121-134.
[7] Xu J, Zang Y, Liu D, et al. DRAM is involved in hypoxia/ischemia-induced autophagic apoptosis in hepatocytes[J]. Aging Dis, 2019, 10(1):82-93.
[8] Cui L, Bai Y, Zhang J, et al. Effects of extracellular acid stimulation on rat vascular smooth muscle cell in gas6/Axl or PI3K/Akt signaling pathway[J]. Clin Exp Hypertens, 2016, 38(5):451-456.
[9] Kakani E, Elyamny M, Ayach T, et al. Pathogenesis and management of vascular calcification in CKD and dialysis patients[J]. Semin Dial, 2019, 32(6):553-561.
[10] Gu Q, Li F, Ge S, et al. CDC20 knockdown and acidic microenvironment collaboratively promote tumorigenesis through inhibiting autophagy and apoptosis[J]. Mol Ther Oncolytics, 2020, 17:94-106.
[11] Grootaert MOJ, Moulis M, Roth L, et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis[J]. Cardiovasc Res, 2018, 114(4):622-634.
[12] Zhang X, Peng Y, Yuan Y, et al. Histone methyltransferase SET8 is regulated by miR-192/215 and induces oncogene-induced senescence via p53-dependent DNA damage in human gastric carcinoma cells[J]. Cell Death Dis, 2020, 11(10):937.
[13] Watts AJ, Storey KB. Hibernation impacts lysine methylation dynamics in the 13-lined ground squirrel, ictidomys tridecemlineatus[J]. J Exp Zool A Ecol Integr Physiol, 2019, 331(4):234-244.
[14] Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network[J]. Free Radic Biol Med, 2019, 133:162-168.
[15]王美月, 贺燕婷, 于洁, 等. p53在层流剪切应力下对EPCs分化及功能的影响[J]. 中国病理生理杂志, 2021, 37(2):225-231.
Wang MY, He YT, Yu J, et al. Role of p53 in differentiation and function of EPCs under laminar shear stress[J]. Chin J Pathophysiol, 2021, 37(2):225-231.
[16] 张东雪, 高少辉, 张胜雷. SET8介导AKT信号通路在调控高磷诱导的血管平滑肌细胞钙化中的作用[J]. 中华肾脏病杂志, 2020, 36(3):214-220.
Zhang D, Gao S, Zhang S. SET8 regulates high-phosphorus-induced vascular smooth muscle cell calcification via AKT signaling pathway[J]. Chin J Nephrol, 2020, 36(3):214-220.
[17] Lu T, Zhu Z, Wu J, et al. DRAM1 regulates autophagy and cell proliferation via inhibition of the phosphoinositide 3-kinase-Akt-mTOR-ribosomal protein S6 pathway[J]. Cell Commun Signal, 2019, 17(1):28.
[18] Zhang X, Wang Y, Zhao A, et al. Long non-coding RNA LINC00511 accelerates proliferation and invasion in cervical cancer through targeting miR-324-5p/DRAM1 axis[J]. Onco Targets Ther, 2020, 13:10245-10256.
[19] Zhang Y, Yang X, Ge X, et al. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice[J]. Biomed Pharmacother, 2019, 109:726-733.
[20] Hu W, Chen S, Thorne RF, et al. TP53, TP53 target genes (DRAM, TIGAR), and autophagy[J]. Adv Exp Med Biol, 2019, 1206:127-149.
[21] Lee HY, Oh SH. Autophagy-mediated cytoplasmic accumulation of p53 leads to apoptosis through DRAM-BAX in cadmium-exposed human proximal tubular cells[J]. Biochem Biophys Res Commun, 2021, 534:128-133.
(责任编辑:卢萍,李淑媛)
Knockdown ofpromotes autophagy and apoptosis of rat vascular smooth muscle cells by up-regulating p53/DRAM1 signaling pathway
LIU Lan, ZHANG Dong-xue, ZHU Rong-fang, LI Hui, LIANG Xiang-nan, ZHANG Sheng-lei, BAI Ya-ling△
(,,050011,)
To investigate whether lysine methyltransferase SET domain-containing protein 8 () knockdown regulates the autophagy and apoptosis of rat vascular smooth muscle cells (VSMCs) through p53/DNA damage-regulated autophagy modulator 1 (DRAM1) signaling pathway.Rat VSMCs were divided into 3 groups: normal group, vector group and SET8-shRNA group. Apoptosis was detected by flow cytometry, viability was detected by MTT, and the protein levels of SET8, p53, DRAM1, LC3, Bax and Bcl-2 were detected by Western blot. VSMCs of rats were divided into 3 groups: normal group, vector group and DRAM1 overexpression group. Cell viability, apoptosis and protein levels of DRAM1, LC3, Bax and Bcl-2 were detected.The protein expressions of LC3, Bax and Bcl-2 were observed in VSMCs of rats following suppression oforwhile interfering with.The apoptosis of VSMCs was increased significantly and viability was decreased significantly afterknockdown(<0.05). Western blot showed that the expressions of SET8 and Bcl-2 were decreased, and the expressions of p53, DRAM1, LC3-II and Bax were increased significantly afterknockdown(<0.05). Apoptosis of VSMCs was increased significantly and viability was decreased significantly after overexpression of(<0.05). Western blot showed that the expressions of DRAM1, LC3-II and Bax were increased and the expression of Bcl-2 was decreased after overexpression of DRAM1 (<0.05). The expressions of LC3 and Bax were decreased and the expression of Bcl-2 was increased after inhibiting the expression ofor(<0.05).Autophagy and apoptosis in vascular smooth muscle cells of rats were promoted by knockdown ofThe possible mechanism is to promote the expression of autophagy protein LC3-II and apoptotic protein Bax by upregulating the expressions of p53 and DRAM1.
Vascular smooth muscle cells; SET8; DRAM1; Autophagy; Apoptosis
R363.2; R543
A
10.3969/j.issn.1000-4718.2022.03.006
1000-4718(2022)03-0427-07
2021-08-20
2022-01-04
[基金项目]河北省医学科学研究重点课题(No. 20180513; No. 20190702)
Tel: 15081150811; E-mail: snbyl@163.com
