ATP regulates the phosphorylation and degradation of myofibrillar proteins in ground ovine muscle
2021-12-14RENChiHOUChengliZHANGDequanLlXinXlAOXiongBAlYuqiang
REN Chi,HOU Cheng-li,ZHANG De-quan,Ll Xin,XlAO Xiong,BAl Yu-qiang
Institute of Food Science and Technology,Chinese Academy of Agricultural Sciences/Key Laboratory of Agro-Products Processing,Ministry of Agriculture and Rural Affairs,Beijing 100193,P.R.China
Abstract Phosphorylation post-translational modification plays an important role in postmortem muscle quality traits. Adenosine triphosphate (ATP) is an energy source and a key substrate of phosphorylation which provides the phosphatase groups to proteins in the presence of protein kinases. However,in postmortem muscle,the effects of ATP content on phosphorylation are poorly studied. The study investigated the effect of ATP on protein phosphorylation and degradation in postmortem ovine muscle. The ground muscle with/without additional ATP were treated/control groups and stored at 25 and 4°C,respectively.The ATP content led to different changes of pH value between the ATP-treated and control groups. The phosphorylation level of myofibrillar proteins was higher (P<0.05) in ATP-treated group compared to the control group at both temperatures,which suggested that ATP played a vital role in postmortem protein phosphorylation. A slower degradation rate of μ-calpain,desmin and troponin T was observed in the ATP-treated group which showed that there was a negative relationship between ATP level and the degradation of proteins. These observations clearly highlighted the role of ATP on the development of meat quality by regulating the phosphorylation and degradation of myofibrillar proteins in postmortem ovine muscle.
Keywords:ATP,postmortem ovine muscle,phosphorylation,protein degradation
1.lntroduction
Protein phosphorylation is involved in postmortem biochemical processes and plays its role in the development of meat quality (Huanget al.2014; LiXet al.2015; Gaoet al.2017; LiMet al.2018; Chenet al.2019). The phosphorylation level of myofibrillar proteins might be related to meat rigor mortis through fast pH decline and the degradation of structural proteins such as troponin,desmin and tropomyosin (Huanget al.2012; LiZet al.2018). Besides,results of phosphoproteomics revealed that the phosphorylation of structural proteins and glycolytic enzymes contributed to their fragmentation and activities in the tough and tender muscle (D’Alessandroet al.2012;Chenet al.2016).
Numerous studies showed effect of protein phosphorylation on meat quality such as tenderness,color and so no.However,in postmortem muscle,how the phosphorylation level forms and which factors can regulate protein phosphorylation are not available. In our previous study(Renet al.2019),we analyzed the effect of temperature,pH and adenosine triphosphate (ATP) content on protein phosphorylation. The result of correlation analysis indicated that the rate of ATP consumption at different temperatures might be the most important one among three factors in the formation of phosphorylation levels in postmortem muscle. In phosphorylation,ATP provides phosphate groups to proteins in the presence of protein kinases,whereas phosphatases remove phosphate groups and catalyze dephosphorylation. The two-direction reversible process with phosphorylation and dephosphorylation regulates the function of proteins (Gannonet al.2008).ATP has been widely reported to be correlated with the development of rigor mortis by regulating the postmortem glycolysis (Hamm 1977; Watabeet al.1991). In the scope of complicated postmortem processes,a variety of biochemical reactions need ATP for activation such as the disassociation of myosin and actin (Huff-Lonerganet al.2010). Based on ATP metabolite,Batlleet al.(2001)suggested that 2-h postmortem was the optimal sampling time to detect exudative pork meat. Moreover,the results of Liet al.(2012) showed that the improved rate of ATP consumption and increased tenderness occurred in beef muscle treated with electrical stimulation. Thus,ATP may impose its influence on postmortem meat quality through some biochemical pathways including phosphorylation.
Based on previous studies,we hypothesized ATP content was the key factor that could influence the phosphorylation level of proteins in postmortem muscle. In the present study,the ground ovine muscle was stored at 25°C (hot meat is always sold at this temperature in China) and 4°C(traditional refrigeration temperature). The phosphorylation level of myofibrillar proteins in ground muscle with/without additional ATP was evaluated,and the degradation of μ-calpain,desmin and troponin T was measured. This study may advance our understanding about the mechanism of postmortem phosphorylation and the relationship between ATP metabolism and protein degradation.
2.Materials and methods
2.1.Sampling
Six sheep (carcass weight (25.73±0.94) kg and 8 months of age) from Small-tail Han sheep and Mongolia sheep crossbred were transported about 1.5 h to the slaughterhouse. All animals had the same gender,feeding strategies and pre-slaughter conditions and were slaughtered on the same day. Both sides of thelongissimus thoracis lumborum(LTL) muscle were collected within anhour postmortem. The visible fat and connective tissue were removed. LTL muscles were ground by using one electric grinder and then 50 g of muscle was placed in a culture dish.About 3.5 mL of 0.1 mol L-1ATP (purity≥99%,A7699,Sigma-Aldrich,St.Louis,MO,USA) solution (dissolved in 10 mmol L-1MgCl2) was immersed to 50 g of ground muscle as ATP-treated group. The ground muscle with 3.5 mL of 10 mmol L-1MgCl2solution was the control group. Within 1 h,ATP-treated group and control group were divided into two storage treatments:(1) stored at 25°C (hot meat is commonly sold at room temperature in China) for 0 h,0.75 h,2 h,12 h,and 1 d; (2) stored at 4°C (meat sold at supermarket) for 0 h,0.75 h,2 h,12 h,1 d,2 d,3 d,5 d,and 7 d. At each time point,muscle samples were collected,frozen and stored at -80°C until analysis.
2.2.pH value measurement
For the measurement of pH,the ground muscle was put into 5 mL centrifuge tubes for convenient operation. The glass electrode of a calibrated pH meter (Testo205 pH meter,Lenzkirch,Germany) was directly inserted into the ground muscle in the middle of the tube for three times.
2.3.ATP content
The ATP content of ground muscle was measured by a commercial kit (MAK190-1KT,Sigma-Aldrich,St.Louis,MO,USA) with a spectrophotometer (SpectraMax®190,Molecular Devices,USA). Briefly,0.4 g minced muscle was homogenized in 4 mL ATP assay buffer with an Ultra Turrax T10 basic S25 (T10 basic,IKA Labortechnik,Staufen,Germany) and then centrifuged for 30 min at 9 000×g. About 3 mL of supernatant was taken by using 10 kDa MWCO spin filter (Merck Millipore Ltd.,Darmstadt,Germany) in order to remove macromolecular proteins (for 15 min,at 7 500×g). The filtrate was collected for the detection of ATP content. According to the manufacturer’s instruction,the ATP standard solution for colorimetric detection was added into a 96-well plate. The 7 μL ATP filtrate and other reagents were mixed to set up the reaction and incubated at 25°C for 30 min at a dark place. The ATP contents were detected at 570 nm in triplicates.
2.4.Protein extraction
Proteins in ground muscle were extracted referring to literature (Lametschet al.2006; LiMet al.2017) with minor modification. A total of 1 g of ground muscle was homogenized in 6 mL ice-cold buffer (0.1 mol L-1Tris,0.01 mol L-1DTT,pH 8.3,complete protease inhibitors and phosphatase inhibitor PhosStop (Roche,Mannheim,Germany)) for 30 s and repeated for 3 times with an interval of 15 s. Homogenate was centrifuged by a Neofuge 15R(Heal Force,Shanghai,China) at 10 000×g for 30 min. The supernatant containing sarcoplasmic proteins was collected.The sediment,myofibrillar proteins,was dissolved in 5%SDS buffer (20 min,60°C of water bath). Sarcoplasmic and myofibrillar proteins were diluted 20 times with ultrapure water for detecting the protein concentration by using a BCA Assay Kit (Pierce Chemical Company,Rockford,IL,USA).
2.5.Gel electrophoresis and phosphorylation level
By adopting the methods of Li Cet al.(2015) and Chenet al.(2016),sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed. The concentration of sarcoplasmic and myofibrillar proteins was adjusted to 2 μg μL-1. Sarcoplasmic and myofibrillar proteins were mixed respectively with the equal volume of loading buffer (0.1 mol L-1Tris-HCl,pH 6.8,40 g L-1SDS,1 g L-1bromophenol blue,250 g L-1glycerin). The mixtures were heated on boiling water for 5 min and centrifuged for 2 min.Then,12% separation gels and 4% stacking gels were prepared,and 5 μL of the samples were loaded in triplicates onto every well. Proteins’ markers (Thermo,Rockford,IL,USA) were loaded on the first well to point out the molecular weight of proteins. As a standard and for in-gel calibration,a sample (control group,4°C,0 h) was loaded on every gel. The gels were run at 70 V for separating gels,and then the voltage was increased to 110 V for stacking gels.After SDS-PAGE,phosphorylated proteins were stained for 80 min by Pro-Q Diamond Dye (Invitrogen,Eugene,OR,USA),and total proteins were stained overnight by SYPRO Ruby Dye (Invitrogen,Eugene,OR,USA). The fluorescently labeled proteins were scanned by using the ChemiDoc™MP Imaging System (Bio-Rad,Hercules,CA,USA). The phosphorylation levels were calculated by dividing the intensity of bands on Pro-Q Diamond image to the intensity on SYPRO Ruby image of one gel (Schulenberget al.2003;Silverman-Gavrilaet al.2009). The intensity of protein bands was analyzed by Quantity One Software (ver.4.6.2,Bio-Rad,Hercules,CA,USA).
2.6.Western blot analysis of protein degradation
The degradation of proteins was detected by using Western blot (Duet al.2018; LiZet al.2018; Liet al.2019). The concentrations of separation gels for μ-calpain,desmin and troponin T were 8,12 and 15%,respectively. The 4%stacking gels were used to prevent the diffusion of protein bands. The concentration of sarcoplasmic and myofibrillar proteins was adjusted to 8 and 2 μg μL-1,respectively. The loading volume of sarcoplasmic and myofibrillar proteins was 5 μL. After SDS-PAGE,proteins on gels were transformed to polyvinylidene fluoride membranes (Millipore,Billerica,MA,USA). Wet transfer apparatus (Bio-Rad Laboratories)was used for desmin (100 V,60 min) and μ-calpain (100 V,100 min). Half-dry transfer apparatus was used for troponin T(25 V,2.5 A,9 min). After blotting,membranes were washed with TBS buffer (0.01 mol L-1Tris-HCl,0.15 mol L-1NaCl,pH 7.5) for 2 min,and washing was repeated for 3 times.The membranes were blocked for 2 h in blocking buffer(0.05% Tween 20 and 3% bovine serum albumin in TBS buffer). Anti-desmin (D1033,Sigma,dilution 1:1 000 with blocking buffer),anti-troponin T (ab130003,Abcam,dilution 1:1 000 with blocking buffer) and anti-μ-calpain (MA3-940,Thermo Scientific,dilution 1:800 with blocking buffer) were respectively incubated overnight at 4°C. The membranes were washed with TBS1 (TBS buffer containing 0.1% Tween 20) for 20 min (3 times) and subsequently incubated with HRP-conjugated secondary antibodies (A9044,Sigma,St.Louis,MO,USA; dilution 1:2 500 with blocking buffer)for 80 min. After washing of 10 min for 3 times in TBST2(0.05 mol L-1Tris-HCl,0.15 mol L-1NaCl,pH 7.5,0.1%Tween 20),the blots were showed using Clarity western ECL substrate (Bio-Rad,Hercules,CA,USA) and exposed by ChemiDoc™ MP Imaging System of Image Lab 5.1 software.
2.7.Data analysis
One-way ANOVA was performed by using SPSS Statistic 21.0 Software (SPSS Inc.,Chicago,IL,USA) for comparisons of group. Differences among means were compared using Duncan’s multiple range test (P<0.05). Comparisons among pH value,ATP content and phosphorylation levels of different groups were made using independent samplest-test(P<0.05). The ATP content,temperature and post-mortem time points were considered as fixed effect,meanwhile animals as random effect. All data were expressed as mean±SD (standard deviation).
3.Results and discussion
3.1.pH value
As shown in Fig.1,the pH value decreased with the increasing time in both ATP-treated and control groups.Moreover,the rate of pH decline at 25°C was faster than that at 4°C in two groups. The ultimate pH value at 25°C was about 5.33,which was lower than the normal ultimate pH value of intact muscle,which suggested that the rate of glycolysis was faster in ground muscle than in intact muscle. Naturally,the lower ultimate pH value appeared at high temperature rather than low temperature (Kimet al.2014),which is coincident with the result in this study that the ultimate pH value was higher at 4°C than at 25°C.
At 25°C,no significant difference (P>0.05) was noted between ATP-treated group and control group,except at 0.75 h where the pH value of ATP-treated group was significantly higher than the control group. At 4°C,the pH value of ATP-treated group was significantly higher than that of the control group at 0.75 and 2 h,and opposite changes were measured during 1 to 7 d of storage (P<0.05). The activity of phosphofructokinase and phosphorylase had a dominant role in glycolysis,and the acceleration of ATP consumption enhanced the glycolysis rate (Hamm 1977).Besides,ATP is an inhibitor of phosphofructokinase and glycogen phosphorylase (Hudsonet al.1993; Englandet al.2014). Adenosine monophosphate (AMP,one of the catabolites of ATP) is also linked with the activity of phosphofructokinase (Aberle and Merkel 1968). Regarding to ATP-treated group at 4°C,in the early stage of storage,abundant ATP content would restrict the glycolysis rate due to the inhibition of some of glycolytic enzymes,which caused the additional increase of pH in ATP-treated group compared with others. However,with ATP consumption,the inhibition of glycolysis was gradually reduced. More H+ions were produced by ATP hydrolyzation,and the pH value in ATP-treated group were lower than that of the control group in later stage of storage. In addition,it is possible that higher temperature might weaken the inhibition of ATP on glycolysis through the fast ATP consumption.

Fig.1 The pH value of adenosine triphosphate (ATP) treated and control groups in the ground ovine muscle at 25°C (A) and 4°C (B). a-f,represent significant difference within the same group (P<0.05). X and Y,represent significant difference between ATP-treated and the control groups at the same storage time (P<0.05).
3.2.ATP content
Generally,the ATP content was decreased with the increase of storage time in both ATP-treated and control groups(Fig.2-A and B). After slaughtering,the aerobic glycolysis termination and anaerobic glycolysis initiation made less ATP synthesis,which led to the depletion of ATP content in postmortem muscle. The ATP metabolism affected the ongoing biochemical mechanisms in postmortem muscle(Scheffler and Gerrard 2007).
The ATP content in ATP-treated group was higher (P<0.05)than that in the control group except for 1 d at 25°C. The reason for the slight difference at 1 d between two groups at 25°C could be that the ATP content was unable to supply sufficient ATP for consumption. The ATP content at 25°C were almost completely consumed at 1 d,however the ATP content was available still at 7 d of 4°C,which suggested ATP consumption was slower at the lower temperature (LiCet al.2015); and the ATP content could maintain longer time because of suppressed metabolic processes.
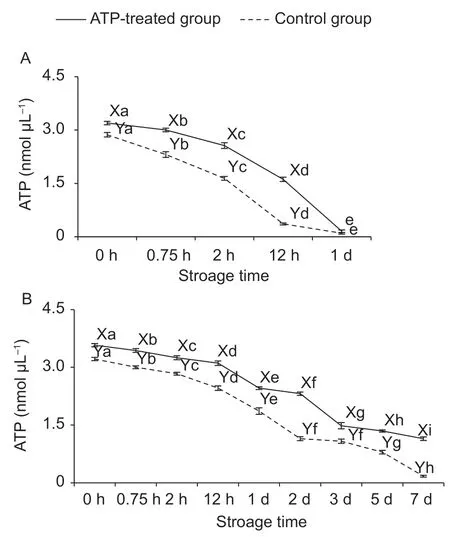
Fig.2 The changes of adenosine triphosphate (ATP) content of ATP-treated and control groups in the ground ovine muscle at 25°C (A) and 4°C (B). a-I,represent significant difference within the same group (P<0.05). X and Y,represent significant difference between ATP-treated and control groups at the same storage time (P<0.05).
3.3.Phosphorylation levels
In phosphorylation post-translational modification,the role of ATP is to provide phosphate groups to proteins in the presence of protein kinases (Humphreyet al.2015). Phosphorylation depends on the concentration of ATP,but dephosphorylation can happen without ATP.Images of gels of phosphorylated myofibrillar proteins and total myofibrillar proteins at 25 and 4°C were shown in Fig.3-A and B,respectively. Selected 18 bands were analyzed for the calculation of total phosphorylation level of myofibrillar proteins (Fig.4-A and B). In ATP-treated group at 25°C,phosphorylation levels attained the highest value at 2 h and then decreased. While in the control group at 25°C,phosphorylation levels were reduced significantly after 0.75 h. The result demonstrated that insufficient phosphate groups in the control group at 25°C contributed to lower phosphorylation level compared with ATP treatment groups. Meanwhile,dephosphorylation was unaffected on decreasing ATP content. However,changes of phosphorylation levels at 4°C were different when compared with 25°C. In the control group at 4°C,phosphorylation levels were firstly increased and then decreased significantly after 3 d. This indicated that the ATP content could help to maintain sufficient phosphate groups until 2 d. However,later the phosphorylation was weakened,and the dephosphorylation played a dominant role. Thus,the rate of ATP consumption was restricted at refrigeration temperature resulting in that more phosphate groups could be utilized in postmortem muscle. No matter 25 or 4°C,the phosphorylation level of myofibrillar proteins in the ATP-treated group was higher than that in the control group (P<0.05),which testified the important effect of exogenous-added ATP on protein phosphorylation.
Besides the direct effect of phosphate groups provided by ATP,the pH value and postmortem time could also affect the phosphorylation level. In postmortem muscle,a large proportion of sarcoplasmic proteins were glycolytic enzymes,and their phosphorylation statuses were regulated by different pH decline rates which would affect their activities (Huanget al.2011). Regarding to myofibrillar proteins,their phosphorylation status mainly involved in muscle contraction (Lametschet al.2011; Chenet al.2016). Muroyaet al.(2007) reported that the myosin regulatory light chain was double-phosphorylated during rigor formation. The increasing phosphorylation level of myosin light chain 2,desmin and actin were measured in aged muscle (Gannonet al.2008). However,the phosphorylation status of much more stable structural proteins included in myofibrillar proteins were mainly related to postmortem time rather than the rate of pH decline (Huanget al.2012). Combined with the result of the present study,we speculated that the ATP consumption with postmortem time in ground muscle played key roles in the phosphorylation level of myofibrillar proteins.Thus,ATP content might play an important role in muscle contraction and meat tenderness through regulating the phosphorylation level of myofibrillar proteins.

Fig.3 The images of Pro-Q Diamond staining (A) and SYPRO Ruby staining (B) of myofibrillar proteins. MW,molecular weight.
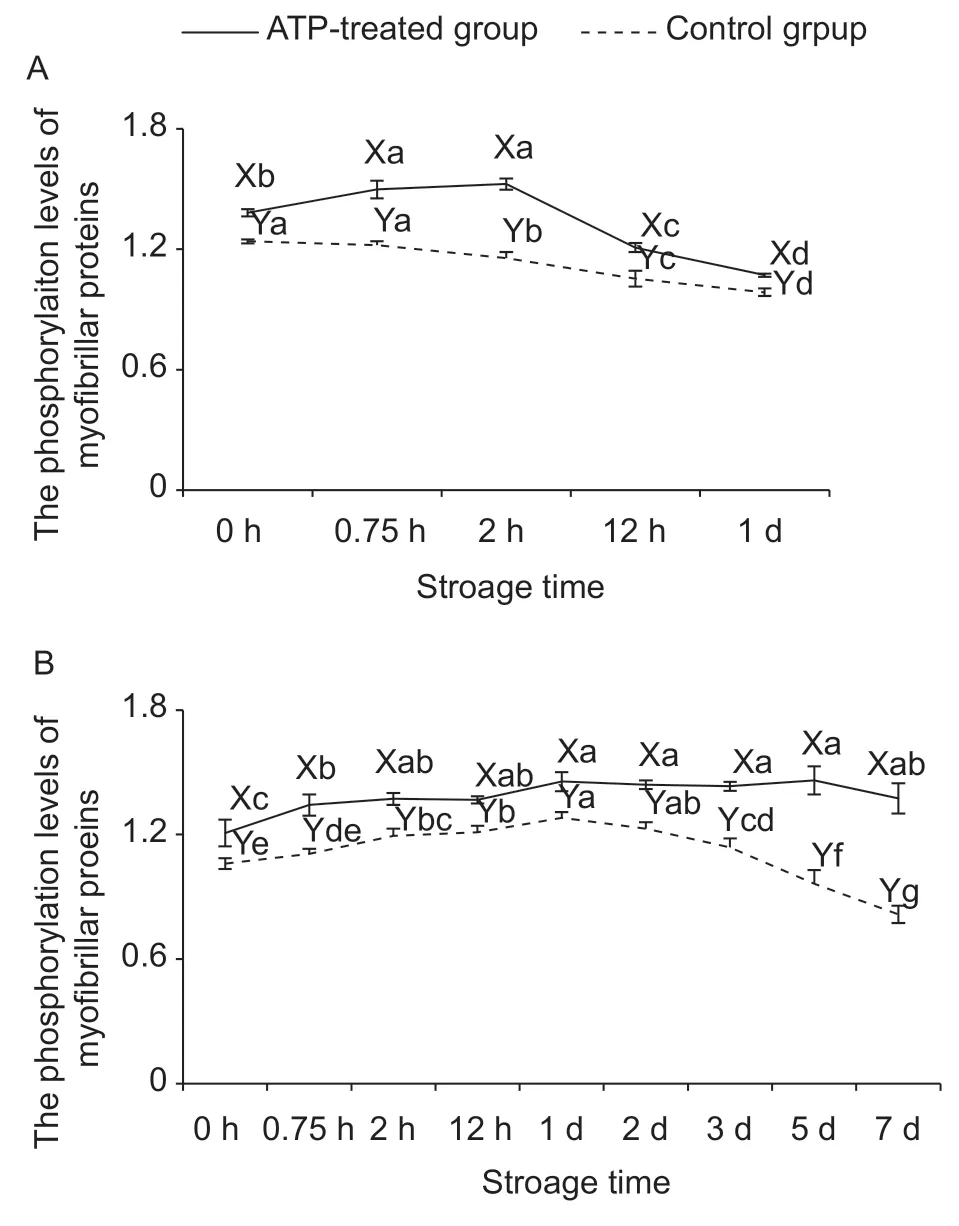
Fig.4 The changes of the phosphorylation levels of myofibrillar proteins in the ground ovine muscle at 25°C (A) and 4°C (B)of adenosine triphosphate (ATP)-treated and control groups.a-g,represent significant difference within the same group(P<0.05). X and Y,represent significant difference between ATP-treated and the control groups at the same storage time(P<0.05).
3.4.Protein degradation
The degradation of μ-calpain,desmin and troponin T was measured by using Western blot and the result was shown in Fig.5. With the increase in postmortem time,μ-calpain is the most well-characterized type of calpains and can show more proteolysis through autolysis (Huff-Lonerganet al.1996). Compared with other intact muscle,the degradation of μ-calpain was faster in the ground muscle (Pomponioet al.2010; Liet al.2017a). At 25°C in ATP-treated group,the bands of 80 kDa were completely disappeared showing that μ-calpain was degraded to lower molecular weight(78-76 kDa) after 12 h of storage,while at 2 h in the control group. Similarly,at 4°C,the degradation of 80 kDa was faster in the control group than the ATP-treated group,which indicated the earlier autolysis of μ-calpain was activated in the control group. Therefore,the ATP content was negatively related to the degradation of μ-calpain through increased phosphorylation level of μ-calpain (Duet al.2017). On the other hand,the faster exhaustion of ATP in the control group led to the early release of Ca2+which attributed to the earlier autolysis of μ-calpain (Huanget al.2012). Besides,one of the reasons also might be the earlier decrease of autolysis and activity of μ-calpain with faster pH decline at early storage time in the control group (Pomponioet al.2010).

Fig.5 Degradation of μ-calpain (A and B),troponin T (C and D) and desmin (E and F) in adenosine triphosphate (ATP)-treated and control groups at 25 and 4°C,respectively.
Desmin and troponin T,related to meat tenderness,are structural proteins of the sarcomere and can be degraded by μ-calpain during postmortem (Huff-Lonerganet al.2010).Desmin is localized at the periphery of the myofibrillar Z-disk in skeletal muscle and degrades during postmortem storage(Huff-Lonerganet al.1996). Troponin T is strongly related to the shear force and regulates the thin filament during muscle contraction through its interaction with tropomyosin(Lehmanet al.2001). The bands of desmin in ATP-treated group (25°C) were degraded to lower molecular weight(approximately 38 kDa) after 12-h storage,and more rapid degradation occurred in the control group. The degradation trend of troponin T was similar to that of desmin. At 4°C,the degradation of desmin and troponin T were quicker in the control group than in the ATP-treated group. So,an obviously slower degradation of desmin and troponin T was showed in ATP-treated group where the autolysis of μ-calpain was inhibited as well. The reason was that the phosphorylation of myofibrillar proteins reduced the susceptibility of proteolytic degradation by μ-calpain (Liet al.2017b). Thus,ATP might contribute to meat tenderness by affecting the phosphorylation and controlling the protein degradation.
4.Conclusion
ATP is a key factor in phosphorylation formation and could effectively increase the protein phosphorylation level of myofibrillar proteins in postmortem muscle. The exogenous ATP content might affect the glycolytic process in postmortem ground muscle. ATP content affects meat quality traits through pH change and protein degradation like desmin and troponin T. Moreover,high ATP content might inhibit the autolysis of μ-calpain through phosphorylation.
Acknowledgements
The authors thank the financial support from the National Natural Science Foundation of China (31771995),the earmarked fund for China Agriculture Research System(CARS-38),and the Agricultural Science and Technology Innovation Program,Chinese Academy of Agricultural Sciences (CAAS-ASTIP-IFST).
杂志排行
Journal of Integrative Agriculture的其它文章
- The dynamic impact of income and income distribution on food consumption among adults in rural China
- Driving factors of direct greenhouse gas emissions from China’s pig industry from 1976 to 2016
- Use of two-stage dough mixing process in improving water distribution of dough and qualities of bread made from wheatpotato flour
- lmpact of climate change on maize yield in China from 1979 to 2016
- Estimating daily actual evapotranspiration of a rice-wheat rotation system in typical farmland in the Huai River Basin using a two-step model and two one-step models
- Optimization of water and nitrogen management for surge-root irrigated apple trees in the Loess Plateau of China
