ldentification and resistant characterization of legume sources against Meloidogyne incognita
2021-12-14PornthipRUANPANUNPrakitSOMTA
Pornthip RUANPANUN,Prakit SOMTA
1 Department of Plant Pathology,Faculty of Agriculture at Kamphaeng Saen,Kasetsart University,Kamphaeng Saen Campus,Nakhon Pathom 73140,Thailand
2 Department of Agronomy,Faculty of Agriculture at Kamphaeng Saen,Kasetsart University,Kamphaeng Saen Campus,Nakhon Pathom 73140,Thailand
Abstract Root-knot nematodes (RKNs; Meloidogyne spp.) are becoming a serious problem in legume production. This study identified Vigna genotypes exhibiting resistance to M.incognita (RKN) and characterized the modes of the resistance to M.incognita. In total,279 accessions from 21 Vigna species were screened for resistance based on a galling index (GI) and an egg mass index(EI). Seven accessions were highly resistant to RKN with GI≤25,namely JP74716 (V.mungo var. mungo; cultivated black gram),JP107881 (V.nepalensis),JP229392 (V.radiata var. sublobata; wild mungbean),AusTRCF118141 (V.unguiculata subsp. unguiculata; cultivated cowpea),AusTRCF306385 (V.unguiculata subsp. unguiculata),AusTRCF322090 (V.vexillata var. vexillata; wild zombi pea) and JP235929 (V.vexillata var. vexillata). JP229392 and AusTRCF322090 were the most resistant accessions having EI values of 18.74 and 1.88,respectively. Continuous culture of M.incognita on both JP229392 and AusTRCF322090 resulted in a weakness in pathogenic ability for this RKN. The resistance in JP229392 and AusTRCF322090 to RKN appeared to be antibiosis that was associated with reduced nematode penetration,retardation of nematode development and impeding giant cell formation. The Vigna germplasm resistance to RKN identified in this study could be utilized as gene sources for the development of RKN-resistant Vigna cultivars.
Keywords:host-plant resistance,root-knot nematode,Vigna radiata,Vigna vexillata
1.lntroduction
The genusVignais an important plant taxon comprising about 100 leguminous plant species,of which up to 10 species have been domesticated or cultivated as crops,such as mungbean and cowpea (including yardlong bean) which are of significant economic importance in Asia and Africa.SixVignaspecies are well known in Asia,namely mungbean(V.radiata(L.) Wilczek var.radiata),black gram (V.mungo(L.) Hepper var.mungo),rice bean (V.umbellata(Thunb.)Ohwi and Ohashi),azuki bean (V.angularis(Ohwi) Ohwi and Ohashi var.angularis),bambara groundnut (V.subterranea(L.) Verdc.) and cowpea (V.unguiculata(L.) Walp.var.unguiculata). The area covered by these crops is more than 20 million ha. Among these crops,particularly mungbean,black gram and cowpea are economically important in Asian countries such as India,China,Myanmar,Indonesia,Pakistan,Thailand and Bangladesh,where they are mainly grown in rotation with cereal crops,such as rice,corn and wheat. Seeds of these legumes contain about 20-25%proteins and 60-70% carbohydrates (Kumar and Pandey 2020). Seeds of mungbean and black gram are used as raw materials in the bean sprout industry. In addition,seeds of mungbean are also used in the starch industry.Due to the ability ofVignacrops to fix atmospheric nitrogen in association with soilRhizobiumbacteria,Vignacrops can improve soil fertility and reduce the nitrogen fertilizer requirement of the main crop in a crop rotation system (Luceet al.2015). Thus,they are affordable sources of nitrogen for resource-poor farmers (McDonaghet al.1995).
Root-knot nematodes (RKNs) are among the most devastating soil-borne crop nematode pests worldwide.They have a diverse host range and have spread to agricultural fields,especially in tropical areas as well all temperate areas (Caillaudet al.2008). RKNs parasitize plant root systems and thus directly affect the uptake of the water and nutrients needed for normal plant growth and reproduction. Their infection of plant roots can also act as components of disease complexes with other pathogens including vascular diseases such asFusariumwilt and root rots (Robertset al.1995).Meloidogyne incognitais the most important species that has spread throughout agricultural areas in northern,northeastern,central and southern Thailand (Ruanpanun and Khun-In 2015).
In legumes,yield losses caused by RKNs amount to about 26% worldwide (Gupta and Verma 1990; Ali 2009).Since legumes are generally grown as a major component in cropping systems,introducing susceptible or intolerant legume crops into cropping systems will support RKNs attacking the legume crops and increasing the nematode population that is subsequently carried over to the following crops. Due to cost and safety limitations on the use of chemical nematicides,alternative management strategies of RKNs are preferable including crop rotations and host-plant resistance (Robertset al.1995). Using nematode-resistant legume cultivars as a grain crop or cover crop in cropping systems can promote yields and suppress nematode populations in the soil (Robertset al.1995). However,the development of nematode-resistant cultivars is limited by the lack of efficient resistant germplasm. Thus,a major challenge appears to be the identification of resistant germplasm for one or more breeding programs. Among several crops of the genusVigna,nematode resistance has been well studied only in cowpea. There have been only a few reports on nematode resistance in otherVignaspecies.The aims of this research were:(i) to identify sources of resistance toM.incognitainVignagermplasm by screening a large number of accessions ofVignaspecies and (ii) to characterize the mode of the resistance in the resistant accessions. The resistant germplasm from this study will be useful as a gene source for breeding newVignacrop cultivars resistant to RKN.
2.Materials and methods
2.1.Plant materials
In total,279 accessions were used in this study,consisting of wild and cultivated forms of 21Vignaspecies (Appendix A).Cultivated mungbean (Vigna radiatavar.radiata) cultivar Kamphaeng Saen 2 (hereafter called as KPS2) which is highly susceptible to RKN was included as a susceptible check. Seeds from each accession were sown in a pot (6 cm in diameter and 5.5 cm high) filled with approximately 150 mL of peat moss. After germination,seedlings were thinned to one plant per pot and then transferred into a larger pot (9 cm in diameter and 7.5 cm high) filled with approximately 250 mL of nematode-free soil. When the seedlings had 5-6 trifoliate leaves,they were inoculated with 48-h eggs or juveniles ofM.incognita.
2.2.Nematode inoculum preparation
Meloidogyne incognitawas cultured in KPS2 in a greenhouse for 45 days. Root pieces with galls were collected and washed in running tap water to remove any soil. Cleaned galling roots (2-4 roots) were placed into 150 mL of 0.5%sodium hypochlorite (NaOCl) in 250-mL Erlenmeyer plastic flasks and shaken for 3.5 min for egg extraction. The eggs in NaOCl solution were passed through a sieve (200 μm aperture) to remove any non-egg component,and the solution that passed through the sieve was collected and passed through a finer sieve (30 μm aperture). The free eggs retained on the sieve were washed three times with sterile distilled water to remove any residual NaOCl. For infective second-stage juvenile (J2) preparation,eggs in water were incubated at room temperature ((25±3)°C) for 48 h or more until the eggs had hatched; the J2s were carefully removed and used in the experiment.
2.3.Screening for resistance to M.incognita
The 279Vignaaccessions were screened for resistance and KPS2 was added as a susceptible control. The screening was conducted under greenhouse conditions using a completely randomized design (CRD) with 10 replicates.In each replicate,eachVignaaccession was grown in a separate pot. Preparation of theVignaplants for screening was the same as described above.Meloidogyne incognita(1 000 J2s) were inoculated into the plant roots of each pot. The pots were left for 24 h without watering to allow theM.incognitato settle on the roots. After 45 days of inoculation,the inoculated plants were carefully separated from the soil and the roots were scored for the degree of galling. The level of galling on the roots in the total root system was classified into five classes:0=no gall,1=galling less than or equal to 25%,2=galling 26-50%,3=galling 51-75%,and 4=galling greater than 75%. Subsequently,the gall index (GI) of each accession was calculated as follows according to Barker (1985):GI=(Number of plants in class 0×0+Number of plants in class 1×1+Number of plants in class 2×2+Number of plants in class 3×3+Number of plants in class 4×4)×100/Number of plants×4. Accessions with GI≤20 were classified as resistant and repeated for resistance screening (Mukhtaret al.2017). For the repeated screening,3 000 J2s ofM.incognitawere inoculated to the resistant seedlings as described above. The GI value and the egg mass index (EI) on the root surface were also calculated in the repeated screening as:EI=(Average number of egg mass on tested accession/Average number of egg mass on the susceptible control mungbean KPS2)×100. The resistant accessions with GI≤20 and EI≤20 were selected as being representative of resistance and were used to study the host-pest interaction in the next experiments compared to the susceptible accession of each species.
2.4.Pathogenicity of continuous culture of M.incognita
Cultivated mungbean accession JP229392 andV.vexillatavar.vexillata(wild zombie accession AusTRCF322090),as representatives of RKN resistant accessions,and cultivated mungbean accession JP240384 andV.vexillatavar.vexillataaccession TVNu760,as representatives of RKN susceptible accession,were planted in plastic pots (16 cm in diameter and 20 cm high) under the greenhouse conditions.When the plants had 5-6 trifoliate leaves,they were inoculated with approximately 1 000 J2s ofM.incognita. The plants were maintained for 60 days,when the egg masses of each accession were isolated from the roots and used to prepare J2 inocula for the next culture. Where there was no egg mass,the plant roots were chopped and mixed with soils for culturing. After the second cultivation,all egg masses from the roots of each accession were isolated and counted.Hatching juveniles were gathered and inoculated onto 10 seedlings (replicates) of each accession. Experimental pots were arranged in a CRD. The number of galls and the egg mass were counted and evaluated in all culturing.
2.5.Development of M.incognita in roots of resistant and susceptible Vigna accessions
Seeds of the RKN-resistant accessions (JP229392 and AusTRCF322090) and the RKN-susceptible accessions JP240384 and TVNu760 were each sown in 10 pots,with one pot containing one seedling. When each seedling had 5-6 trifoliate leaves,approximately 1 000 J2s ofM.incognitawere inoculated into each pot and the plants were maintained under greenhouse conditions. Seedlings from 10 pots were harvested each day (days 1-30) after inoculation,and soil was gently washed off the root systems with tap water. The roots were removed from the pots and washed under running tap water. Nematodes in the roots were stained with NaOCl-acid fuchsin following the method described by Byrdet al.(1983). Stained roots were observed under a stereomicroscope. The numbers in the juvenile stage of RKN in the root were determined (Moenset al.2009).
2.6.Histological experiment
The RKN-resistant accessions (JP229392 and AusTRCF322090) and the susceptible accessions(JP240384 and TVNu760) were each grown in 10 pots under the greenhouse conditions as described above. Each pot(replicate) was inoculated with 3 000 J2s ofM.incognita.Ten root tips with galls (50 mm in length) from each pot were harvested randomly from the resistant and susceptible plants at 15 and 30 days post inoculation (dpi). For inclusion in resin,the root sections were prepared following the method described by Silvaet al.(2013). The root tissues were observed under a light microscope. Microphotographs were taken to elucidate differences in host-plant interactions between the resistant and susceptible accessions. The width and length of giant cell diameters were measured at 15 and 30 dpi using a stage micrometer. The number of giant cells and the number of nuclei in giant cells were also determined. In addition,any anatomical change in the plant tissue and the stages of nematode development were also examined.
2.7.Statistical analysis
In the experiment on the pathogenicity of continuous culture ofM.incognita,the number of egg mass and number of root galls were subjected to analysis of variance (ANOVA)atP<0.05. Mean separation was conducted using Tukey’s test (P<0.05). In the experiment on the development ofM.incognitain the roots of resistant and susceptible accessions,means of the size of giant cells,the number of giant cells/nematode and the number of nuclei/giant cell were compared between resistant and susceptible accessions of the same species using at-test (P<0.01 andP<0.05). All data were analyzed using the R-Program 3.4.1 Software (R Core Team 2017).
3.Results
3.1.Resistance screening
Of the 279Vignaaccessions initially screened for resistance toM.incognitaand classified into five GI levels (0-20,21-40,41-60,61-80 and >80; Table 1),seven accessions had a low GI (0-20) and were classified as highly resistant,namely JP74716(V.mungovar.mungo),JP107881 (V.nepalensis),JP229392 (V.radiatavar.sublobata),AusTRCF118141(V.unguiculatasubsp.unguiculata),AusTRCF306385(V.unguiculatasubsp.unguiculata),AusTRCF322090(V.vexillatavar.vexillata) and JP235929 (V.vexillatavar.vexillata). To confirm the resistance,these accessions were subjected to repeated screening; the results showed that all these accessions had the same GI levels as in the initial screening. The accessions JP229392 and AusTRCF322090 were considered the most resistant accessions having the lowest GI (<20) and EI (<20) values and were selected for characterization of the resistance (Fig.1; Table 2).
3.2.Pathogenicity of continuous culture of M.incognita on resistant Vigna accessions
The resistant accessions JP229392 and AusTRCF322090 and two susceptible accessions,JP240384(V.radiatavar.radiata) and TVNu760(V.vexillatavar.vexillata),were selected for continuous RKN culture. The number of egg masses of the accessions JP229392,AusTRCF322090 and TVNu760 decreased after the second cycle of cultivation. The rates of egg mass decrease of JP229392,AusTRCF322090 and JP240384 were 51.00%,100% (no egg mass) and 12.37%,respectively. The number of galls in the resistant accessions JP229392 and AusTRCF322090 decreased significantly. The rates of root gall decrease from the 1st to 3rd cycles of JP229392 and AusTRCF322090 were 84.57 and 100% (no galling),respectively. In contrast,the numbers of galls in the susceptible accessions were very high. The rates of root gall increase in JP240384 and TVNu760 were 158.57 and 117.32%,respectively (Table 3).
3.3.Development of M.incognita in the roots of resistant and susceptible Vigna accessions
Juvenile stages ofM.incognitain the roots of the resistant accessions JP229392 and AusTRCF322090 and the susceptible accessions JP240384 and TVNu760 were observed each day from day 1 to day 30 after inoculation.At day 3,J2s were detected in the roots of the susceptible accessions but were not found in the resistant accessions.At day 5,J2s were found in the roots of allVignaaccessions(Appendix B). Nevertheless,the numbers of J2s in the roots of the susceptible accessions were higher than those of the resistant accessions. The mean numbers of RKN in each stage of each accession at 15 and 30 dpi with J2s are presented in Table 4. Overall,the numbers of juveniles in the roots of the susceptible accessions were higher than those of the resistant accessions. At day 15,in the roots of susceptible accessions,the number of third-stage juveniles(J3s) was the greatest followed by forth-stage juveniles(J4s) and J2s,respectively. In contrast,in the roots of resistant accessions,J2s and J3s were found in roots of AusTRCF322090,while J2s,J3s and J4s were found in JP229392. The numbers of each juvenile stage in the resistant accession were significantly lower than those in the susceptible accession of the same species. However,the numbers of J4s in JP229392 and JP240384 were not significantly different. At day 30,the numbers of juveniles in the roots had the same trend as those at day 15. J2s,J3s,J4s,adult females and adult males were detected in the roots of all accessions. The numbers of adult females in the roots of the resistant accessions were significantly lower than those of the susceptible accessions. The numbers of all juvenile stages in each resistant accession were significantly lower than those for the susceptible accession of the same species.
3.4.Effect of M.incognita on giant cell formation in resistant and susceptible Vigna accessions
Giant cell formation in the roots of accessions that were RKN resistant (JP229392 and AusTRCF322090) and susceptible(JP240384 and TVNu760) and inoculated with J2s ofM.incognitawere observed at 15 and 30 dpi. The size of the giant cells (based on the width and length) of the resistant accessions was significantly smaller than that of susceptible accessions of the same species (Fig.2). Furthermore,the number of giant cells per nematode in the roots of resistant accessions was less than that of the susceptible accessions at both 15 and 30 dpi. However,the number of nuclei per giant cell was not significantly different between the resistant and susceptible accessions of the same species (Table 5).
4.Discussion

Fig.1 Roots of Vigna spp.inoculated with second-stage juveniles (J2s) of Meloidogyne incognita. A,resistant accession JP229392.B,AusTRCF322090. C,susceptible accession JP240384.
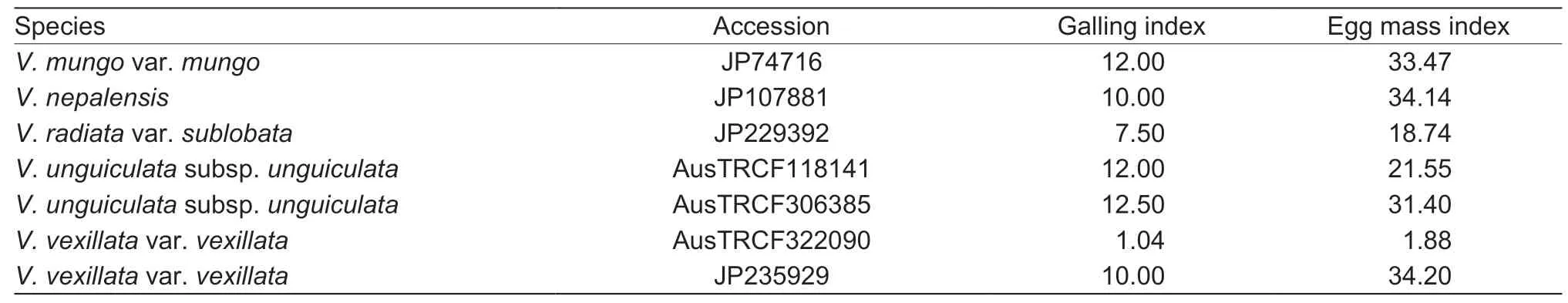
Table 2 Galling index and egg mass index in highly resistant Vigna accessions infected with Meloidogyne incognita
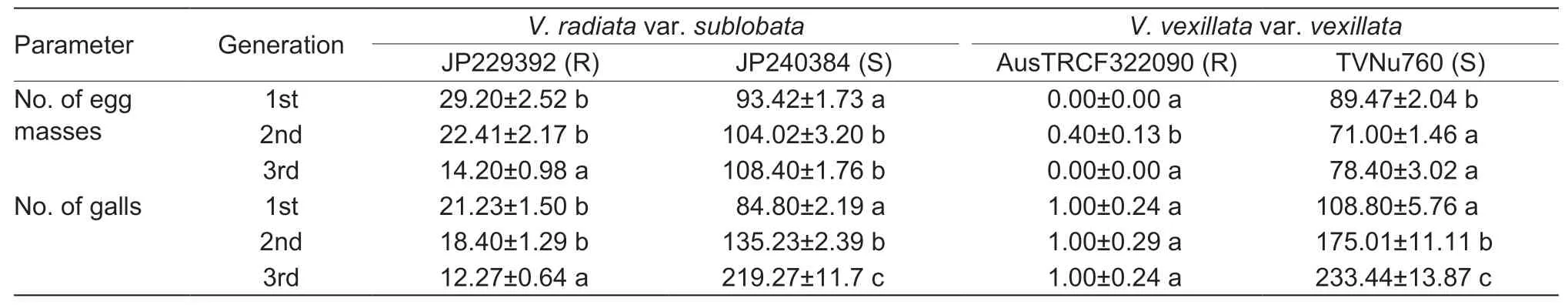
Table 3 Egg masses and galls in selected resistant (R) and susceptible (S) accessions

Table 4 Number of each stage of Meloidogyne incognita in roots at 15 and 30 days post inoculation (dpi)
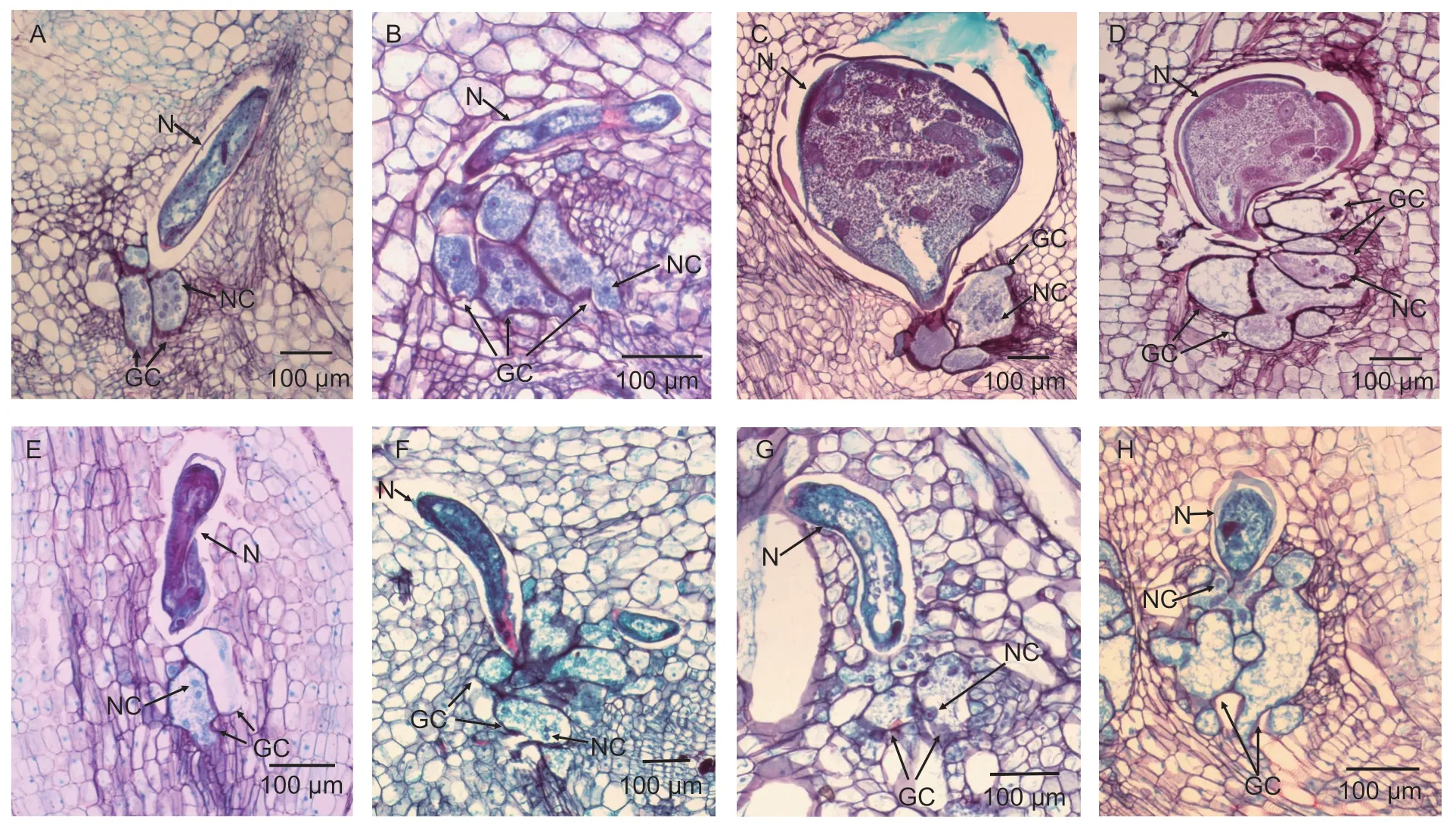
Fig.2 Light micrographs of root tissues of resistant and susceptible Vigna accessions infested by Meloidogyne incognita. Roots of V.radiata var.sublobata accession JP229392 (resistant) and V.radiata var.radiata accession JP240384 at 15 days post inoculation (dpi) (A and B,respectively) and 30 dpi (C and D,respectively) with Meloidogyne incognita,and of V.vexillata var.vexillata accessions AusTRCF322090 and TVNu760 at 15 dpi (E and F,respectively) and 30 dpi (G and H,respectively). GC,giant cell; N,nematode; NC,nucleus.
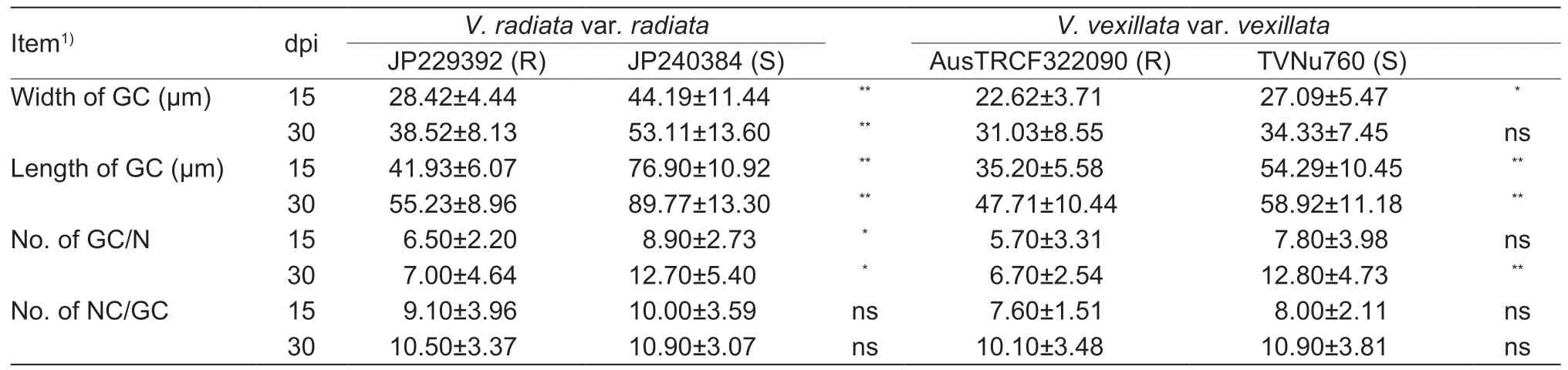
Table 5 Size and number of giant cells and number of nuclei in giant cell in roots of selected resistant (R) and susceptible (S)accessions of Vigna radiata var.radiata and V.vexillata var.vexillata at 15 and 30 days post inoculation (dpi) with Meloidogyne incognita
In this study,host suitability was examined againstM.incognitain a germplasm set of 279 accessions of diverseVignaspecies. This set ofVignagermplasm had varying degrees of resistance againstM.incognita. Similarly,Kushidaet al.(2013) presented the results with their testedVignavarieties showing different responses to infection withHeterodera glycines. The GI and EI values were used as measures to select forM.incognita-resistantVignain the current study. Both parameters are important standards for RKN infection and have been previously applied in screening forM.incognitaresistance of various crops (Talwanaet al.1997; Cervantes-Floreset al.2008; Mudiopeet al.2012).Strong and positive correlations between the GI and number of eggs were reported by Karuriet al.(2017). The GI and EI are considered good guides to evaluate resistanceviathe measurement of nematode establishment and reproduction in the host,respectively (Hadisoeganda and Sasser 1982;Sasseret al.1984; Marcheseet al.2010; Gomeset al.2015; Mukhtaret al.2017). Based on the current evidence,resistantVignavarieties in the current experiment had low GI and EI values. Hence,the experiment was successful in the selection of RKN-resistantVignavarieties (Mukhtaret al.2017).
The loss of resistance in plant-parasitic nematoderesistant plant cultivars and resistant-breaking nematode species and races is a problem associated with the continuous planting of resistant plant cultivars over a long period (Young 1992). Continuous use ofM.incognitaresistant tobacco in North and South Carolina was believed to have caused a species shift of RKN fromM.incognitarace 1 and 3 toM.arenariaandM.javanica(Barker 1989;Johnson 1989). A study conducted by Young (1994)found that the female index of soybean cyst nematode(SCN) increased from 10 to 77 with continuous culturing of the SCN-resistant soybean cultivar Cordell for 10 to 14 generations as well as the race of the SCN changed from race 5 to 14. In the current study,culturing RKN on the resistantVignaaccessions JP229392 andAusTRCF322090 and the susceptible accessions JP240384 and TVNu760 for 2 mon resulted in significant decreases in the numbers of egg masses and galls in JP229392 and no significant changes in these two parameters in AusTRCF322090,while,in contrast,the numbers of galls in both JP240384 and TVNu760 significantly increased. These results suggested that it is difficult for RKN to overcome the resistance in the resistant accessions JP229392 andAusTRCF322090.However,further continuous culturing of RKN on these two accessions should be conducted to confirm its resistance durability.
Characterization of the RKN resistance in the resistant and susceptible accessions revealed that nematode penetration differed between the susceptible and resistant accessions. J2s were not found in the roots of JP229392 and AusTRCF322090 roots at 3 dpi but were detected in the roots of JP240384 and TVNu760. The inability of the nematodes to enter the resistant roots may have been due to the presence of toxic or antagonistic chemicals,or to the presence of barriers to penetration in the root tissue(Bendezu and Starr 2003; Anthonyet al.2005; Yeet al.2017). The smaller number of nematodes and the smaller gall and giant cell sizes,the higher number of adult males and the delay in development of the nematodes and in the roots of JP229392 and/or AusTRCF322090 indicated that these two were not the suitable hosts forM.incognita. It was possibly because they could not provide sufficient nutrients to complete the nematode life cycle (Dhandaydhamet al.2008; Yeet al.2017). Moreover,it was possible that the environmental conditions in resistant (JP229392) hosts could induce juveniles to become male rather than female.
Normally,the success of nematode parasitism depends on the establishment of feeding sites in the vascular parenchyma (giant cells). These cells act as a specialized sink ensuring uninterrupted supply of sufficient nutrition for the nematodes until reproduction occurs (Abadet al.2009). During giant cell development,neighboring cells also proliferate in the vascular cylinder,and cortex cells become hypertrophied forming as root galls (Wysset al.1992). In RKN-resistant mungbean cultivars,resistant genes block or suppress giant cell formationviainterfering with one or more of the important steps required for successful parasitism of nematodes (Mukhtaret al.2017).
Our results indicated that mungbean accession JP229392 andV.vexillatavar.vexillataaccessionAusTRCF322090 wereM.incognita-resistant cultivars. Resistance to the nematode in both accessions was related to obstruction of J2s penetration,delay of nematode development and suppression of giant cell formation. These results can aid in the identification of germplasm resistance toM.incognitaregarding genetic improvement for the resistance ofVignaspecies and may lead to a better understanding of the resistance mechanism of plants to this pest. JP229392 is a wild mungbean (V.radiatavar.sublobata). Wild mungbean is highly cross-compatible with cultivated mungbean (V.radiatavar.sublobata) and partially crosscompatible with cultivated black gram (V.mungovar.mungo)(Tomookaet al.2002). Thus,the mungbean accession JP229392 will be a useful gene source for the development of RKN-resistant cultivars in mungbean and black gram.AusTRCF322090 is a wild zombi pea (V.vexillatavar.vexillata). Although it is not cross-compatible with otherVignaspecies,successful hybridization has been reported between wild zombi pea and cultivated cowpea with the aid of embryo culture (Gomathinayagamet al.1998). Thus,the zombi pea accession AusTRCF322090 may be useful for the development of RKN-resistant cowpea cultivars.
5.Conclusion
In this study,a large number ofVignagenotypes were evaluated for resistance toM.incognita. Mungbean(JP229392) and zombi pea (AusTRCF322090) were highly resistant toM.incognita.Meloidogyne incognitahad less ability in penetrating,developing in plant cells and inducing giant cell formation in the resistant than in the susceptible species. The promising genetic resources found in this study will be useful for breedingVignacultivars resistant to the root-knot nematode.
Acknowledgements
This work was supported by the Thailand Research Fund,Thailand (MRG6080274).
Appendicesassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- The dynamic impact of income and income distribution on food consumption among adults in rural China
- Driving factors of direct greenhouse gas emissions from China’s pig industry from 1976 to 2016
- ATP regulates the phosphorylation and degradation of myofibrillar proteins in ground ovine muscle
- Use of two-stage dough mixing process in improving water distribution of dough and qualities of bread made from wheatpotato flour
- lmpact of climate change on maize yield in China from 1979 to 2016
- Estimating daily actual evapotranspiration of a rice-wheat rotation system in typical farmland in the Huai River Basin using a two-step model and two one-step models
