Effect of Nano Silver Modification on the Dielectric Properties of Ag@TiO2/PVDF Composites
2021-06-14DAIJinhangMENGShunliangYANGChuntianWenzhongCHENXiziYINYuhaoLIANGFei
DAI Jinhang, MENG Shunliang, YANG Chuntian, LÜ Wenzhong, CHEN Xizi,YIN Yuhao, LIANG Fei*
(1. Key Laboratory of Functional Materials for Electronic Information (B), Ministry of Education, Huazhong University of Science and Technology, Wuhan 430074, China; 2. Wenzhou Institute of Industrial Science, Wenzhou 325028, China; 3. Huawei Technologies Co. Ltd., Shenzhen 518116, China)
Abstract: To get a dielectric material with a high dielectric permittivity and suppressed dielectric loss,nano-Ag with a particle size of 20 nm and Ag@TiO2 core-shell particles with diameters of approximately 70-120 nm were embedded in polyvinylidene fluoride (PVDF) to fabricate nano-Ag/Ag@TiO2/PVDF composites. After being modified by nano-Ag with 3 vol% optimal amount, the relative permittivity (εr) at 100 Hz of 50 vol% Ag@TiO2/PVDF composites was 61, and the dielectric loss can be suppressed to 0.04, almost 96.4% lower than that of unmodified composites, and a higher frequency stability of both εr and loss has also been found. The underlying mechanism of the reduced loss was attributed to Maxwell-Wagner polarization and the Coulomb blockade effect caused by the introduction of a small amount of nano-Ag, which will block the movement of electrons between metal nanoparticles and composites. The space charge polarization and conductance loss are weakened at lower and higher Ag@TiO2 filling ratios, respectively, thus leading to a very low loss of the composites.
Key words: polymer composites; dielectric properties; AC impedance spectrum; Ag@TiO2/PVDF;coulomb blockade effect
1 Introduction
High permittivity (high k), low dielectric loss polymeric nanocomposites have been widely used in many fields including high energy storage density capacitors and high-speed integrated circuits owing to their flexibility and tailorable dielectric properties[1-3].High dielectric permittivity composites can be easily achieved by introducing other high k materials such as ceramic grains[4], core-shell structures[5-7], conductive fillers into polymers[8]. However, the composites generally exhibit high dielectric loss, which prohibits their practical application in the electronics industry.Nanoscale metal particles have some special properties,such as plasmon absorption, quantum size effects, superparamagnetism, the Coulomb blockade effect,etc.[9]. The Coulomb blockade effect of nanoscale metal particles has been used in the preparation of dielectric composites with suppressed loss in recent reports[10,11].While the dimension of the metal particle is small enough to make its capacitance with outer arrive to 10-16F, the charge capacity e2/C which is far more than the capacity KBT (KBis the Boltzmann constant) of thermal movement of an electron at low temperature,inhibits the charge transfer through the small island below a certain voltage threshold and leads to an increase in resistance[12,13]. Using nanoscale Ag to modify the ceramic particles is a common modification method,which will hinder electron transport and then suppress the loss of the composites. Huanget alfound that by introducing core-satellite nano assemblies comprising BaTiO3and Ag nanoparticles, the PVDF/BaTiO3@Ag high-k polymer composites have enhanced breakdown strength and lower dielectric loss in comparison with conventional polymer-ceramic particulate nanocomposites[14]. They also reported that high-k polymer nanocomposites with high breakdown strength and low dielectric loss were successfully prepared by introducing ultrasmall platinum nanoparticles[15]. CCTO and polymer composites are often used in the field of dielectric materials, but the CCTO-polymer two-phase system usually has high dielectric loss[16,17].Ghoshet alfound that, by functionalizing the surface of CCTO nanoparticles with an Ag coating, the PVDF/CCTO@Ag composites exhibited a higher dielectric constant(an approximately 20% enhancement) and lower loss than PVDF polymer filled with pure CCTO at the same content of filler concentration[18]. Different from the traditional percolation theory, the defects introduced by the nanoscale metal particles will not increase the conductance but can inhibit the loss. The key point of the experiment is the influence of the size and amount of the metal particles utilized in the modification. If the particle size of the metal particles is too large, the Coulomb blockade effect will be weakened so that the loss under the AC field will increase, and the effect of improving the loss property no longer occurs.
In this work, we developed a nano-Ag/Ag@TiO2/PVDF three-phase system to improve the dielectric properties of nanocomposites. Silver nanoparticles(70-120 nm) were used as the core because of their outstanding thermal and electric performance[19], and rutile TiO2was chosen as the material of shell for its excellent dielectric properties[20]. In order to further improve the dielectric properties of Ag@TiO2through the Coulomb blockade effect, the filler used in this study is Ag@TiO2decorated by 20 nm Ag particles. The major role of the larger Ag core in the core-shell structure is to increase the dielectric permittivity, while the smaller nano-Ag is used for loss reduction. PVDF was selected as the polymer matrix of these composites because of its relatively high permittivity and easy processing.
The effects of different amounts of nano-Ag and the optimum amount of nano-Ag on the dielectric properties of 50 vol% Ag@TiO2/PVDF were investigated,and the impedance spectra data were also analyzed.Modification experiments using different volume fractions of Ag@TiO2/PVDF with an ideal amount of nano-Ag were conducted.
2 Experimental
2.1 General consideration
Ag@TiO2nanoparticles were made through a standard sol-gel process. Nano-Ag, polyvinylpyrrolidone (PVP) and tetrabutyl titanate were purchased from Aladdin, other chemicals were obtained from National Chemicals Reagent Co. Ltd. The microstructures of the Ag@TiO2core-shell structures were measured using a field-emission scanning electron microscope (FESEM;ZEISS Gemini 300, Germany) and a transmission electron microscope (TEM; JEOL JEM-1230, Japan). The sample structures were also examined via an X-ray diffractometer (XRD; Shimadzu XRD-7000, Japan)using Cu Kα1radiation (λ = 0.154 056 nm) over the range of 20°≤2θ≤80°. The dielectric properties of the composites were determined using an impedance analyzer (Agilent 4294A, USA) at room temperature. The alternating current (AC) conductivities and impedance spectra of the composites were measured using an impedance analyzer (Wayne Kerr 6500B, UK) at 150 ℃.
2.2 Synthesis of nano-Ag/Ag@TiO2/PVDF composites
First, 0.023 mol of Ag nanoparticles (60-120 nm)with PVP (K30, 1.242 g) were dissolved in 100 mL of ethanol and ultrasonically dispersed evenly for 30 min.This solution was mixed with a solution containing 0.115 moL of tetrabutyl titanate, 5 mL of acetylacetonate, 40 mL of acetic acid, and 80 mL of ethanol under constant stirring at room temperature. After stirring for 30 min, 30 mL of diluted deionized water was added slowly dropwise into the solution to trigger the hydrolysis of tetrabutyl titanate. The mixture was stirred at 80 ℃ to evaporate the solvent. Rutile Ag@TiO2nanoparticles were prepared after calcination at 800 ℃for 2 h.
Then, the Ag@TiO2powder was modified and the composite was prepared. Nano-Ag (20 nm) and Ag@TiO2were dispersed in alcohol with ultrasonic dispersion for half an hour. Octyl phosphonic acid (OPA,C8H19O3P) used as surface modifiers were added to this dispersion while stirring[21,22], and the weight percentage of modifiers was fixed at 1 wt%. A certain amount of PVDF was added to the mixture. After stirring for 30 min, the powder was dried, crushed, and molded at 10 MPa to produce tablet samples, each with a diameter of 12 mm and a thickness of 1 mm. These samples were calcined at 200 ℃ for 2 h to form the composites.
3 Results and discussion
3.1 Characterization of Ag@TiO2 core-shell nanoparticles
Fig.1(a) shows the XRD pattern of the Ag@TiO2decorated by nano-Ag. Four peaks at 38.1°, 44.2°,64.2°, and 77.6° can be observed in the patterns, corresponding to the (111), (200), (220), and (311) of Ag,respectively. The peaks at 27.5°, 36.1°, 39.2°, 41.3°,54.4°, 56.7°, 64.1°, 69.1°, and 69.8°, clearly represent the data of rutile TiO2(JSPDS No. 21-1276). As the content of nano-Ag used for modification increases, the relative intensity of the peak corresponding to Ag in the XRD pattern is stronger. Fig.1(b) shows the TEM image of the Ag@TiO2core-shell nanoparticles, where Ag cores represent the dark gray core area that ranges from 70 to 120 nm, while the rutile TiO2shells represent the gray sheath with an area of approximately 8 to 11 nm, and revealing clear contrast between the Ag cores and TiO2shells. Fig.1(c) shows the HRTEM image of the core-shell interface and distinct lattice fringes can be used to identify the different crystallographic spacings between Ag and rutile TiO2. The fringe spacings of 0.236 and 0.325 nm match well with the Ag (111)and rutile TiO2(110) planes, respectively, further confirming the well-crystallized TiO2structure. The SEM image of the modified nano-Ag/Ag@TiO2particles is shown in Fig.1(d). The large silver particles (70-120 nm) are coated with a TiO2shell and the small silver spheres dispersed outside the TiO2shell are nano-Ag particles (20 nm). In this form, silver could be dispersed more evenly in the matrix, which is beneficial to further improve the dielectric properties of the composites.
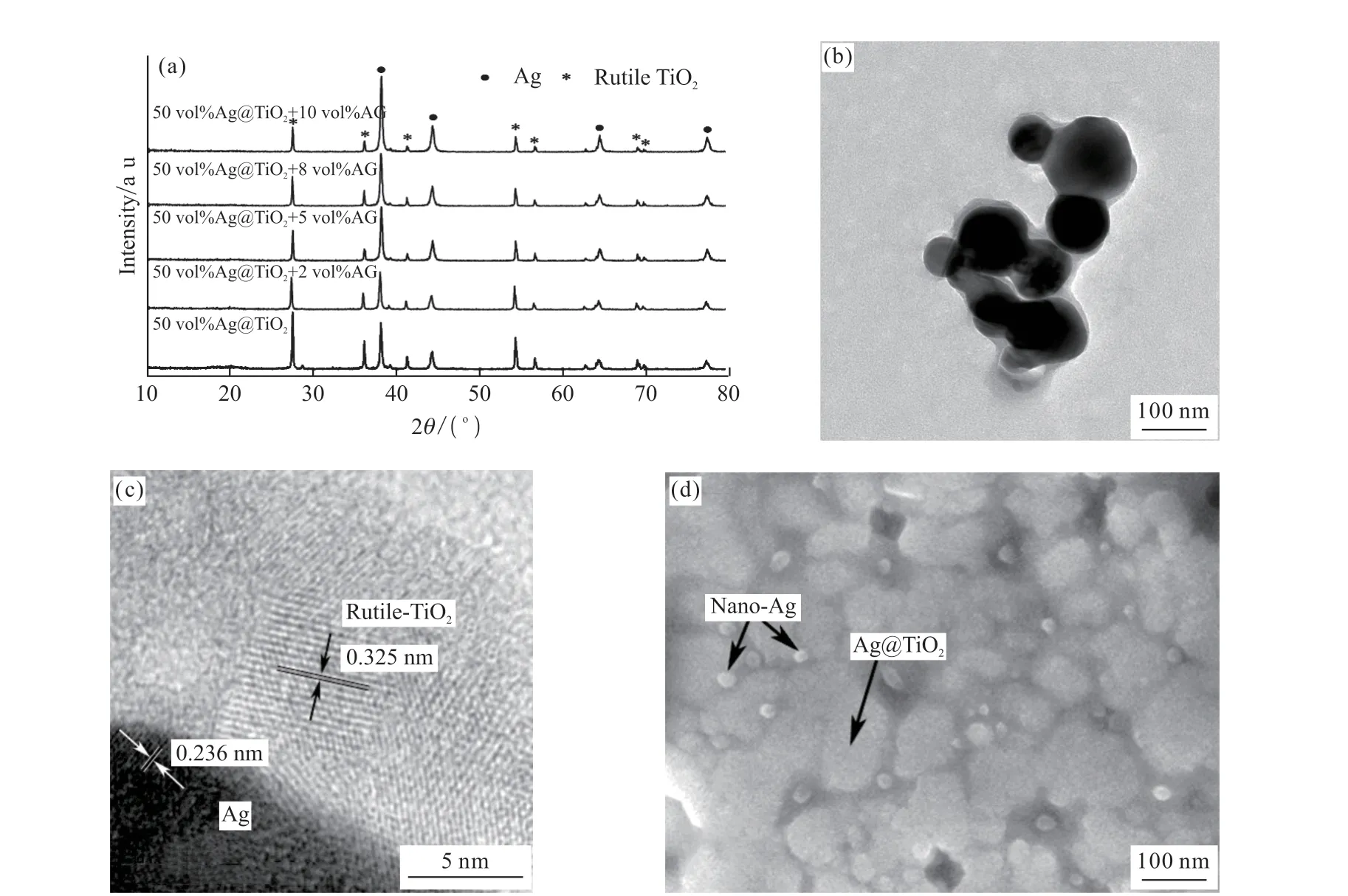
Fig.1 (a) XRD pattern of modified composites decorated by nano-Ag; (b) TEM image of Ag@TiO2 core-shell nanoparticles; (c) HRTEM image of a Ag@TiO2 nanoparticle; (d) SEM image of Ag@TiO2 and modified nano-Ag powder
3.2 Dielectric properties of nano-Ag/Ag@s TiO2/PVDF compositesn
Fig.2(a) shows the dielectric properties of the composite after the nano-Ag modification. The variation of the test curve with frequency is consistent with the literature[23]. When 2 vol% modified nano-Ag was added to 50 vol% Ag@TiO2/PVDF composite, the dielectric constant and loss of the composite decreased. As the nano-Ag continued to increase, its dielectric constant started to rise. For a clearer comparison, the dielectric constant and loss values at 100 Hz for each sample are shown in Fig.2(b). As the modified nano-Ag increases,the dielectric constant and loss both first decrease and then rise. In the traditional concept, the introduction of conductive particles leads to an increase in loss, but it cannot be explained by the traditional percolation theory due to the different properties of ultra-small Ag nanoparticles and conventional metal conductive particles. The decreased loss with the incorporation of nano-Ag particles can be explained as the Coulomb blockade effect[24]. When the amount of nano-Ag is small, they can be uniformly dispersed in the composite to form many Coulomb islands, hindering the movement of electrons and suppressing the interfacial polarization[25,26]. With the further increase in the modified nano-Ag, particles were close to each other and some of them are easy to form agglomerations. Fig.2(c)hows when the amount of nano-Ag is 10 vol%, some ano-Ag particles are agglomerated into clusters, and this changes their role from Coulomb islands to conventional conductive particles, which strengthens the interfacial polarization and conductance in the composites thus makes the dielectric permittivity and loss increase significantly. Besides, there are still some uniformly dispersed ultra-small Ag nanoparticles existing in the composites, and the combined effect of conductive particles and Coulomb islands makes the dielectric constant of 50 vol% Ag@TiO2+10 vol% nano-Ag/PVDF increase by 250% compared to that of the 50 vol% Ag@TiO2/PVDF and the loss still decreased.
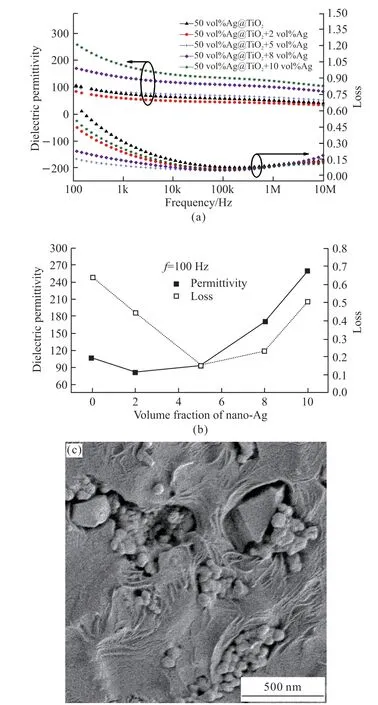
Fig.2 (a) Frequency dependence of the dielectric properties of the modified 50 vol% Ag@TiO2/PVDF composites with 2 vol%, 5 vol%, 8 vol%, 10 vol% nano-Ag; (b) the dielectric properties of the modified composites at 100 Hz; (c) SEM image of 50 vol%Ag@TiO2 +10 vol% nano-Ag/PVDF composites
Fig.3 shows the relationship between the conductivity and frequency of the composites modified with different volume fractions of nano-Ag. The conductivity of the composite increases with the rising frequency. The value at 100 Hz of 50 vol% Ag@TiO2/PVDF is less than 3.5 × 10-7S/m, indicating the insulation of this composite. When the amount of nano-Ag is 2 vol%, the change in the conductivity is low, which is related to the Coulomb blockade effect and the introduction of defects in the cross-linking network.With the increase in the amount of modified nano-Ag,more mobile charges are introduced. This phenomenon weakens the Coulomb blockade effect and leads to an increase in conductivity, but the magnitude of the increase is small, which suggests that a certain number of Coulomb islands are still functioning.
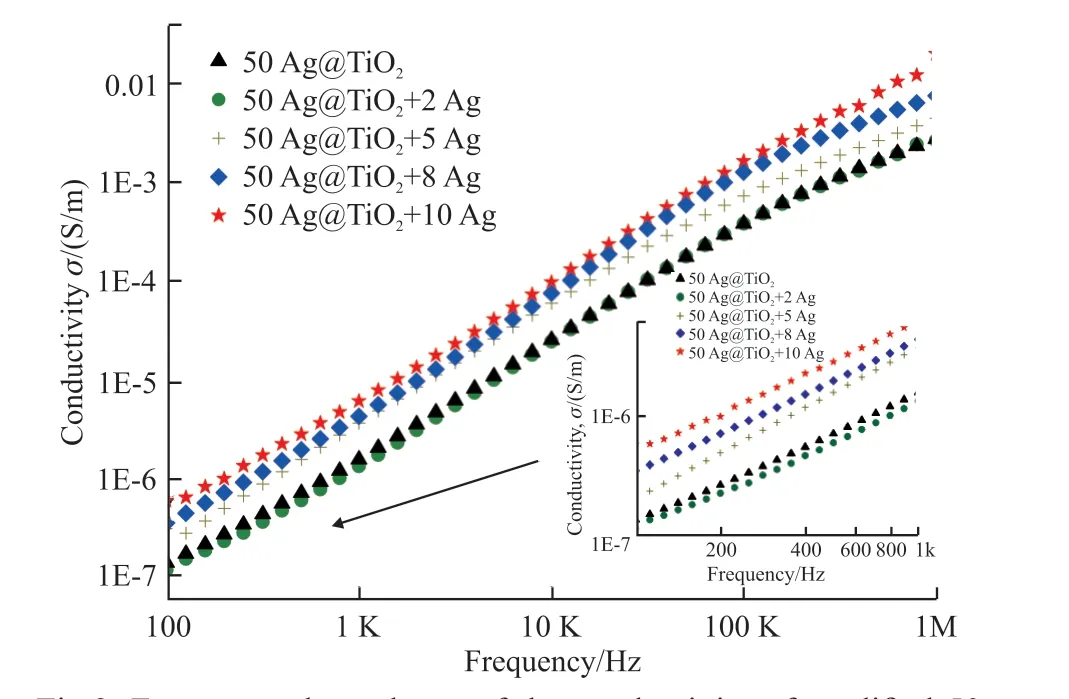
Fig.3 Frequency dependence of the conductivity of modified 50 vol% Ag@TiO2/PVDF composites with various ratio of nano-Ag
3.3 Impedance spectral analysis of the nano-Ag modified Ag@TiO2/PVDF composites
AC-IS was used to study the mechanism of different polarizations in the modified core-shell Ag@TiO2/PVDF composites. Fig.4(a) shows the impedance plot of the composite after nano-Ag modification, where the test temperature is 150 ℃. The radius of the Cole-Cole plot of the nano-Ag-modified material is larger than that of the unmodified composite material, which is inconsistent with the result of previous studies and cannot be explained by the traditional percolation theory[27]. The radius of the unmodified 50 vol% Ag@TiO2/PVDF sample curve is the smallest and when the amount of nano-Ag is 5 vol%, the radius is the largest.The radius of the circle graph first increases and then decreases when the modified nano-Ag increases from 0 vol% to 10 vol%. Fig.4(b) shows the Cole-Cole plot of the nano-Ag-modified composites at 30 ℃, which is consistent with the loss test at room temperature, and has a similar tendency that the radius changes with the nano-Ag amount at 150 ℃. The conductivity at room temperature is influenced by both the Coulomb blockade effect and the tunneling effect. With the increase of temperature, the thermal motion of molecules is intensified while the Coulomb blockade effect is weakened,therefore the conductivity of the composites at high temperature mainly comes from the leakage current generated by the tunneling effect. That explains the difference in radius distribution under different nano-Ag ratios in Fig.4(a) and Fig.4(b).
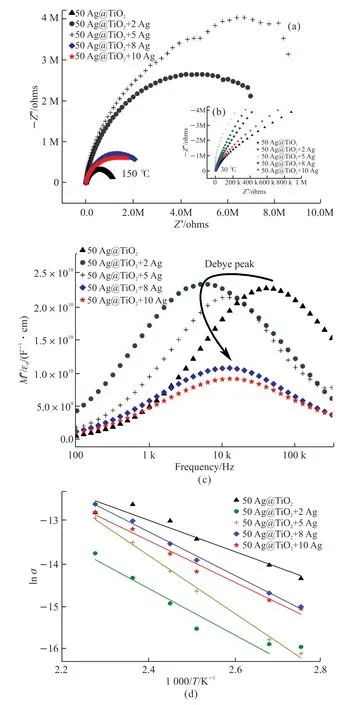
Fig.4 Impedance analysis of the 50 vol% Ag@TiO2/PVDF composites after modification with various nano-Ag loadings:(a) Cole-Cole plot at 150 ℃; (b) Cole-Cole plot at 30 ℃;(c) Imaginary part of the electric modulus with frequency at 150 ℃; (d) ln(σ) versus 1 000/T curves for the composites after modification (The straight lines were used to fit the Arrhenius law)
Complex electric modulus is a powerful tool to reveal the phenomenon of dielectric relaxation and charge transfer in dielectric materials. Fig.4(c)shows the relationship between the imaginary part of the electrical modulus and the frequency of the nano-Ag-modified composite at 150 ℃. With the increase in frequency,M’’ is not monotonous, which corresponds to different relaxation processes, and one segment is interface polarization and another is dipolar polarization[28]. The position of the Debye peak moves to the low frequency and then to the high frequency as the amount of nano-Ag increases. This result indicates that the interfacial polarization in the composite is first weakened and then strengthened, and the turning point should be between 0 and 5 vol%. The weakening of interface polarization can be explained by a small number of nanoparticles that can act as the crystal nuclei to increase the crystallinity of α-phase PVDF, resulting in the decrease of the interface density between the crystalline and amorphous regions of PVDF. In addition,the combined action of the PVDF matrix, coupling agent and nano-Ag particles increases the density of the deep level trap in the composites, and further inhibits the injection, migration, and accumulation of space charge. When excessive nano-Ag particles are introduced, nanoparticles and their associated defects and agglomerations increase the interfacial polarization intensity rapidly.
Fig.4(d) shows the relationship of ln(σ)versus1 000/Tfor the 50 vol% Ag@TiO2/PVDF composites after modification by 0-10 vol% nano-Ag. Arrhenius relationships can be written as Eq.(1):
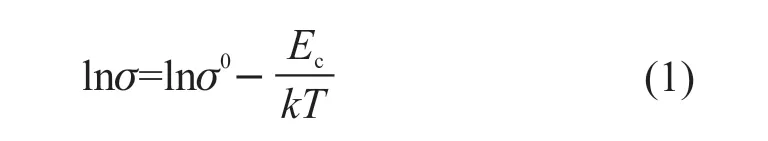
whereEcis the conductance activation energy,kis the Boltzmann constant, and σ0is a constant[29]. According to the slope of fitting lines, the calculated conductance activation energy of modified composites with 0 vol%, 2 vol%, 5 vol%, 8 vol%, 10 vol% nano-Ag are 0.316, 0.469, 0.585, 0.419, 0.440 eV respectively. The changing trend ofEcwith the addition ratio of nano-Ag is consistent with the changing trend of the peak frequency ofM’’ and the radius of the Cole-Cole plot. The composites with 5 vol% nano-Ag have the highestEc,and this suggests that the charges need more activation energy to move from one site to the neighboring site through the tunneling effect.
3.4 Optimum amount of nano-Ag Used in the modification
From the analysis in the previous sections, it is known that the optimum amount of nano-Ag used in the modification of Ag@TiO2/PVDF should be between 0 and 5 vol%. To obtain a suitable dosage value,a series of modification experiments were performed in the range of 0 to 5 vol% at intervals of 1 vol% in 50 vol% Ag@TiO2/PVDF composites. The test results of the dielectric permittivity and loss are shown in Fig.5(a) and a series of test values at 100 Hz were chosen to plot for Fig.5(b). The loss of the sample in the low frequency band first decreases and then increases when the modified nano-Ag increases from 0 vol% to 5 vol%. When the amount of nano-Ag is 3 vol%, the loss is minimal. The changing trend of the dielectric permittivity is consistent with the loss. Similarly, 3 vol.% is a turning point. As shown in Fig.5(a), when the amount of nano-Ag is 3 vol%, the dielectric constant of simples is 61 at 100 Hz and relatively stable over the 100 Hz to 10 MHz frequency band, and in the frequency band,its loss is small with a relatively stable value of 0.04.Therefore, it is concluded that 3 vol% of nano-Ag (20 nm) is a suitable amount for the modification of Ag@TiO2/PVDF.
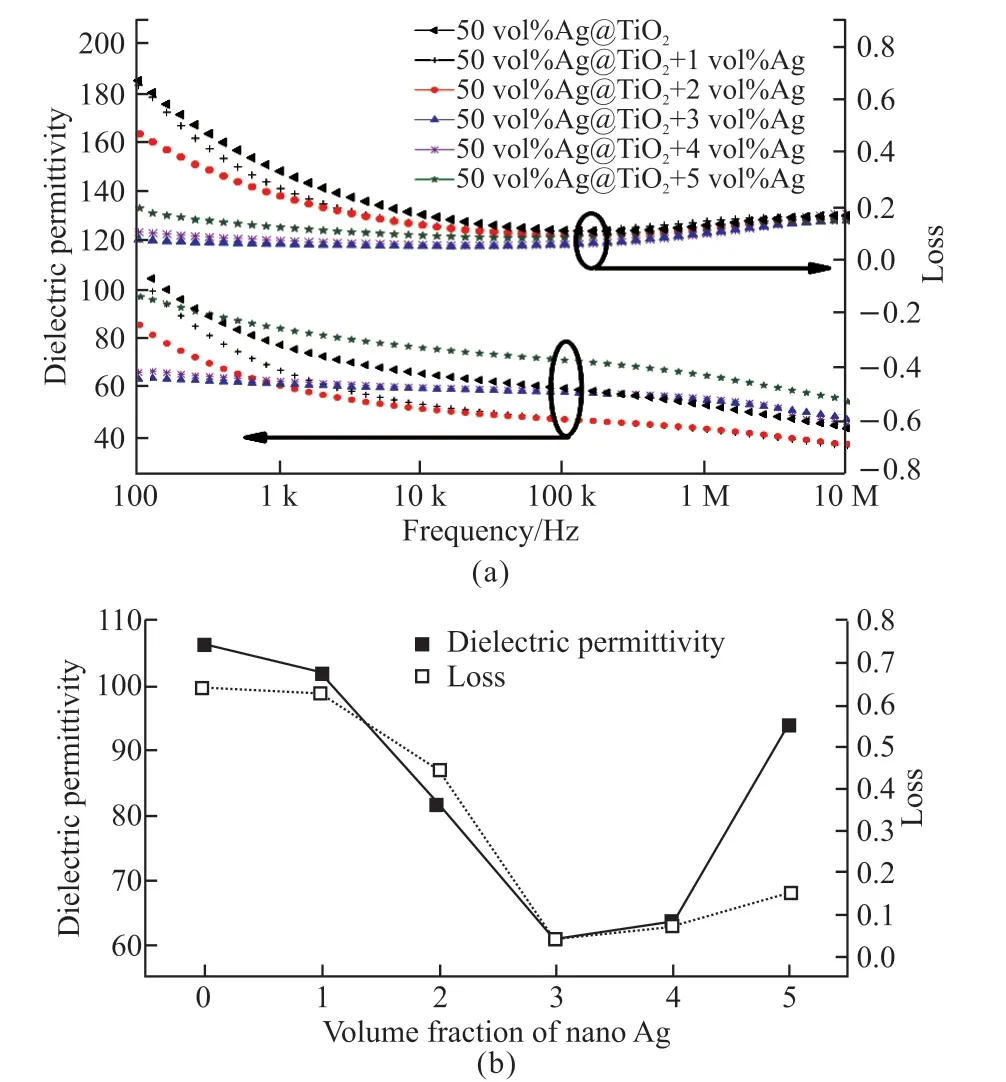
Fig.5 (a) Frequency dependence of the dielectric properties of the modified composites with 0 vol%, 1 vol%, 2 vol%, 3 vol%,4 vol%, 5 vol% nano-Ag; (b) the dielectric properties of the modified composites at 100 Hz
To further explore the effect of 3 vol% nano-Ag on the other volume fraction Ag@TiO2/PVDF composites,the dielectric properties of 10 vol%-50 vol% Ag@TiO2/PVDF after 3 vol% nano-Ag modification were tested.In Fig.6(a), with the increase in the Ag@TiO2volume fraction, the dielectric permittivity of the unmodified composite becomes larger and decreases rapidly as the frequency increases. The permittivity increases with increasing filler loading because the permittivity of Ag@TiO2filler (more than 10 000[23], calculated from previous studies) is much higher than that of PVDF (11.5),which can be explained by the micro capacitor model of the core-shell structures[30]. When an electric field is applied, a large number of free electrons in the silver core accumulate at the insulating TiO2shell, forming an electric dipole moment and polarization. When the filling ratio is high, the adjacent Ag particles can be regarded as the electrode of the capacitor, and the TiO2shell is equivalent to the dielectric. Countless micro capacitors generated further improve the dielectric constant of the composites. When a high ratio of Ag@TiO2is added into PVDF, the number of interfaces and the intensity of interfacial polarization are greatly increased, making the dielectric permittivity of composites become much higher at low frequency and become more frequency dependent with increasing volume fraction.
After the modification by 3 vol% nano-Ag, the dielectric permittivity is remarkably decreased at low frequency while slightly improved at high frequency portion, and the dielectric permittivity of the modified composites becomes more stable over the entire frequency range. This can be attributed to the interaction of the intensity of interfacial polarization and the number of micro capacitors. When the content of nano-Ag particles increased from 0 vol% to 2 vol%, nano-Ag particles were well dispersed and the interfacial polarization was progressively weakened, making the low-frequency dielectric constant decrease significantly. At the same time, the dielectric constant also decreased in high frequency, which can be explained by the Coulomb blockade effect inhibiting the formation of micro capacitors between filler particles. It is considered as the main mechanism for core-shell fillers to improve the dielectric constant of composites. When the content of nano-Ag is 3 vol%, the interfacial polarization is weakened to make its influence almost minimal,so that the low-frequency dielectric constant and loss of the composites reach the minimum value. Furthermore,some of the nano-Ag particles agglomerate into electrodes, improving the probability of forming micro capacitors, and increasing the overall dielectric constant.The interaction of the two results in the good frequency stability of the dielectric properties of 3 vol% nano-Ag in the test frequency band.
From Fig.6(b), the loss of the composites modified by 3 vol% nano-Ag is significantly suppressed at the low frequency band compared with the unmodified.This can be explained by the nano-Ag suppressing the space charge accumulation in the interface between the crystalline and amorphous regions of PVDF. Therefore,the weakened interface polarization reduces the dielectric constant and loss at low frequencies, and as the frequency increases, the difference between these values before and after the modification becomes smaller.
The volume fraction dependence of dielectric constant at 100 Hz of Ag@TiO2/PVDF composites can be studied by fitting the experimentalεrvalues with several appropriate models such as the traditional effective medium theory (EMT) model (Eq.(2)):
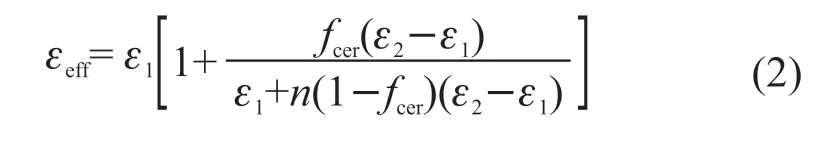
whereεeff,ε1,ε2are the dielectric permittivity of the composite, polymer, and ceramic, respectively.fcerstand for the volume fraction of ceramic filler. We regard Ag@TiO2as a hybrid ceramic filler in the EMT model.nis the filler morphology fitting factor. As shown in Fig.6(c), the fitting result of the EMT model matches well with the experimental data of Ag@TiO2/PVDF withε1=11.5,ε2=10 500 andn=0.12. This indicates that Ag@TiO2can be considered as a single phase in the composite system as a core-shell particle with a complete structure. The introduction of silver nanoparticles increases the complexity of the composite system. It seems like the percolation model or the effective medium percolation theory (EMPT) model can fit the experimental results after adding nano-Ag modification. But in these models, the significant decline of the dielectric constant at 50 vol% loading should be explained as that volume fractions of the metallic filler at this ratio has exceeded the percolation thresholdfc. According to previous studies on core-shell/polymer composite like Al@Al2O3/epoxy[31], Ag@/MSA/epoxy[32], Ag@/C/epoxy[33], the decrease in dielectric constant at high filling ratios was attributed to pores forming and generally accompanied by an increase in loss, which is contrary to the sharp decrease in loss observed in the experiment at 50 vol% loading. The average distanced(Eq. (3)) between neighboring filler particles in the PVDF matrix affects the electromagnetic coupling between particles:
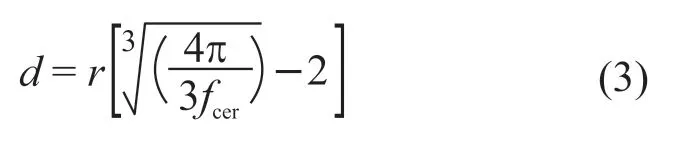
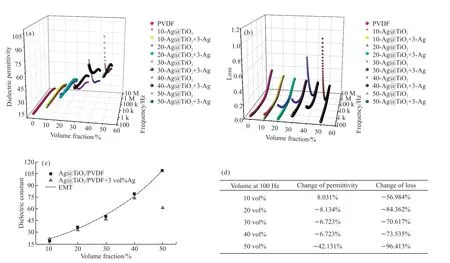
Fig.6 The dielectric properties of the 10 vol%-50 vol% Ag@TiO2/PVDF composites modified by 3 vol% nano-Ag: (a) Dielectric constant; (b)Loss; (c) Experimental and fitted values of dielectric constant of composites at 100 Hz; (d) percentage change of dielectric properties at 100 Hz
whereris the average radius of Ag@TiO2and almost 55 nm measured from the SEM picture. Whenfcer<0.4, the average spacing is more than 10 nm, making the tunneling of electrons between the uncontacted particles cannot be generated[34], therefore the interface polarization between the crystalline and amorphous regions of PVDF is the main factor that determines the dielectric constant and loss of composites. The interparticle distance is only 1.7 nm whenfcer= 0.5, and the small spacing hinders the growth of PVDF grains and reduces the density of boundary in nanocomposites. In addition, with increasing offcer, the density of nano-Ag particles at the interface also rises, which enhances the Coulomb blockade effect, thereby inhibiting the charge transfer between Ag@TiO2particles. This can explain the significant decline of the dielectric constant and loss after modification at 50 vol% loading (Fig.6(d)). In general, 3 vol% nano-Ag inhibits the loss of Ag@TiO2/PVDF, reduces the dielectric constant at low frequency,and improves the frequency stability of both.
4 Conclusions
In summary, Ag@TiO2/PVDF composites were prepared by sol-gel method, and modified by adding 20 nm nano-Ag particles. The incorporation of a small amount of nano-Ag into the Ag@TiO2/PVDF composite motivates the Coulomb blockade effect, which will hinder the movement of electrons at the interface, and then contributes to the loss reduction. By analyzing the imaginary part of the sample’s electrical modulus,it was found that a small amount of modified nano-Ag can suppress interfacial polarization in the low frequency band, making the dielectric constant and loss decreases. A suitable amount of nano-Ag is 3 vol%, at this point, the dielectric constant of modified 50 vol%Ag@TiO2/PVDF simples is 61, and its loss is small with a relatively stable value of 0.04, 96.4% lower than unmodified composites. The Coulomb blockade effect caused by ultra-small nano-Ag particles can be an effective approach to reducing the loss of polymer-based composites at low frequency.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Preparation and Photocatalytic Performance of Double-Shelled Hollow W18O49@C3N4@Ti3C2 Microspheres
- Effects of Cracks on the Mass Transfer of Polymer Electrolyte Membrane Fuel Cell with High Performance Membrane Electrode Assembly
- Refinement of TiB2 Powders with High-speed Planetary Mill and Its Effect on TiB2 Sinterability
- Fabrication of Ordered Meso-macroporous HPW/TiO2 Catalyst for Efficient Heterogeneous Oxidative Desulfurization
- The Preparation of Porous Activated Slag Granules/TiO2 Photocatalyst and Its De-NOx Performance
- Effects of Magnetization on Thermoelectric Transport Properties of CoSb3 Material
