Fabrication of Ordered Meso-macroporous HPW/TiO2 Catalyst for Efficient Heterogeneous Oxidative Desulfurization
2021-06-14SHANHailinDUYueDUXiaodiLEIJiaheng
SHAN Hailin, DU Yue, DU Xiaodi, LEI Jiaheng*
(1. School of Chemistry, Chemical Engineering and Life Science, Wuhan University of Technology, Wuhan 430070, China; 2. Institute for Advanced Materials, Hubei Normal University, Huangshi 435002, China; 2. School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China)
Abstract: In order to reduce the emission of SOx in the environment, sulfur compounds must be removed efficiently from fuels. Three-dimensional highly ordered meso-macroporous HPW/TiO2 (3DO m/M HPW/TiO2)materials were synthesized successfully by sol-gel method and applied as oxidative desulfurization catalyst for the model fuel. The characterization results displayed the existence of highly ordered meso-macroporous structures and the Keggin type of HPW was highly dispersed in TiO2 framework. The effect of catalyst on desulfurization under different reaction conditions was studied systematically. The results showed that the catalyst exhibited excellent desulfurization performance in the hydrogen peroxide oxidation system, which could be explained by the unique meso-macroporous structure of catalyst. In addition, the catalyst showed good cycling performance and the removal rate of DBT still reached 96.1% even after 6 cycles, providing a feasible method for the development and application of fuel deep desulfurization catalysts.
Key words: sol-gel; heteropoly acid; 3DO m/M HPW/TiO2; oxidative desulfurization
1 Introduction
A large number of untreated SOxgas has been discharged into the environment for ages, which leads to air pollution and the formation of acid rain, causing serious impact and incalculable loss to environment[1].Moreover, the organic sulfur compounds (OSCs) in the fuel will lead to the deactivation of catalyst and corrosion of equipment such as pipelines during the refining process[2]. Thus, the desulfurization process of fuel oil is of great importance to alleviate the environmental pressure. Traditional hydrodesulfurization technology(HDS) could effectively remove most of the organic sulfur in fuel oil such as mercaptan and disulfide. Nevertheless, the refractory aromatic sulfur compounds(ASCs) in fuel can’t be effectively removed by HDS results from its large steric hindrance effect[3], such as benzothiophene (BT), dibenzothiophene (DBT)and 4,6-dimethyldibenzothiophene (4,6-DMDBT). To gain more solution of these problems, more and more scholars have committed to research and develop new methods to replace the traditional HDS. Currently, a series of methods including adsorption desulfurization(ADS)[4], extraction desulfurization (EDS)[5], biological desulfurization (BDS)[6], atomization desulfurization and oxidation desulfurization (ODS)[7]are under consideration. Among them, the ODS is a new and clean alternative or complementary technology to HDS for deep desulfurization, which has excellent selectivity and reactivity to ASCs under the mild reaction conditions[8].
In the process of oxidative desulfurization, H2O2has been widely used as an oxidant due to its strong oxidation, high cleanliness and low price. Aromatic sulfides and their alkyl derivatives in fuel oil are oxidized into sulfones or sulfoxides compounds with stronger polarity in H2O2oxidation system, and then removed by distillation or polar solvent extraction (such as acetonitrile)
[9]. Furthermore, heteropoly acids with Keggin structure,such as phosphotungstic acid (H3PO12W40, HPW), showing excellent catalytic activity in the oxidation desulfurization system[10]. Unfortunately, the specific surface area of Keggin-type heteropoly acid is very small (just 2-10 m2/g) in the insoluble catalytic system, and it is also difficult to recycle in the soluble catalytic system[11]. Thus,using solid carrier with large specific surface area loaded heteropoly acid is an effective method to solve the above problems, including TiO2[12], SiO2[13], Al2O3[14]and CeO2[15]. Recently, several excellent reviews describing these applications are available like mesoporous HPW/TiO2[13], mesoporous HPW/SiO2[16], mesoporous HPMo/SiO2[17], HPW/TUD-1[18], and so forth. To further improve the catalytic activity of the catalyst and the mass transfer rate in the pores, researchers have focused their attention on catalysts with different pore structures[19]. It is generally accepted that the macroporous structure in the meso/macroporous catalyst can dramatically improve the mass transfer efficiency of reactants and products,and shorten their transport distance on the mesoporous wall. Meanwhile, the mesoporous structure can increase the surface area of the catalyst, providing more catalytic activity centers[20]. Identical conclusions are confirmed in subsequent catalytic studies[21-23]. In our previous work, meso/macroporous HPW/titanium-silica[24]and meso/macroporous HPW/SiO2[25,26]catalysts have been prepared and used in the process of ODS. The excellent catalytic performance exhibits that the pore structure of the catalyst is the key problem to enhance its catalytic activity.
In this paper, TiO2with high stability, low price and large specific surface area was used as a solid carrier to load phosphotungstic acid. Three-dimensional ordered meso-macroporous HPW/TiO2(3DO m/M HPW/TiO2) catalyst was prepared by sol-gel method, using PMMA microspheres as macroporous template and block copolymer F127 as mesoporous template. The morphology and structure of the catalyst were studied by a series of characterization methods. The selectivity and activity of the catalyst for S compounds in the model fuel were investigated, and the recycling ability of the catalyst was also tested. The results showed that the as-fabricated catalyst exhibited excellent catalytic activity and circulation performance in the process of oxidative desulfurization, which may benefit from its unique pore structure.
2 Experimental
2.1 Materials and reagents
Methanol (high performance liquid chromatography), absolute ethanol (EtOH), methyl methacrylate(MMA), hydrochloric acid (HCl, 36 wt%), titanium tetrachloride (TiCl4, ≥98%), tetrabutyl titanate(Ti(C4H9O)4, ≥98%), potassium persulfate (K2S2O8),sodium hydroxide, acetonitrile, petroleum ether (boiling range: 90-120 ℃) and hydrogen peroxide (H2O2, 30 wt%) were purchased from Sinopharm Chemical Reagent Co., Ltd. Pluronic F127 (F127, average molecular weight was 12 600), Phosphotungstic acid (H3PO12W40,HPW), dibenzothiophene (DBT), 4,6-dimethyldibenzothiophene (4,6-DMDBT) and benzothiophene (BT)were purchased from Aladdin Industrial Corporation.All the above-mentioned chemical reagents except the MMA were used as purchased without further purification in the experimental process.
2.2 Catalyst preparation
2.2.1 Pretreatment of MMA
MMA needs to be purified before the experiment.In detail, 35 mL MMA (A.R.) was distilled by rotary evaporation instrument firstly. Then, the MMA was washed by 80 mL of 0.1 mol/L NaOH solution for more than 3 times to remove the inhibitor. At this time,the high purity MMA was obtained and used in the next experiment.
2.2.2 Synthesis of PMMA microspheres
PMMA microspheres were prepared by using an emulsifier-free emulsion polymerization. In a typical synthesis method[27], 600 mL of distilled water and 27 mL of purified MMA were added into a 1 000 mL three-necked round-bottomed flask with a condensation reflux tube. Then, heated to 70 ℃ under the protection of N2and stirred continuously at 1 000 rpm for 15 minutes. At this time, 0.4 g of K2S2O8was dissolved in 20 mL of distilled water and poured it into the above reaction flask under the same conditions. When the color in the reaction flask turned blue, adjusted the stirring speed from 1 000 to 400 rpm and extended the reaction for 1 h. At the end of the reaction, the emulsion in the flask was transferred to the beaker, placed and cooled down to room temperature. Subsequently, the supernatant was removed after centrifugation at 2 000 rpm for 5 h. Finally, a highly ordered PMMA colloidal crystal template was obtained by drying at 40 ℃ for 24 h.
2.2.3 Synthesis of 3DO m/M HPW/TiO2
3DO m/M HPW/TiO2was synthesized by using block polymer F127 as mesoporous template and PMMA microspheres as macroporous template. The detailed operation was as follows: 2.1 g of Ti(C4H9O)4and 0.7 g of TiCl4was dissolved in 20 g of EtOH with gently stirring for 1 h at room temperature to obtain the mixture solution A. Simultaneously, 1.6 g of F127 was dissolved in 8 g of EtOH and stirred intensively for 0.5 h at 40 ℃ to obtain uniform solution B. Then, put solution B into A with slowly stirring and 3.06 g of HCl(nTiO2:nHCl= 2 : 17) was added to adjust the pH of the mixture to 2. After that, a certain proportion of HPW was added into the mixture and stirred for another 2 h at room temperature to obtain the precursor solution.After the reaction, the precursor solution was slowly dripped onto a certain amount of PMMA microspheres by a burette, placed for 10 minutes to ensure it complete penetration, and repeated the drop-penetration process several times until the precursor solution was exhausted. Finally, 3DO m/M HPW/TiO2was obtained by drying overnight at 40 ℃ and calcining at different temperatures in air for 10 h (under the condition of 1℃/min ramp rate and 400 mL/min air flow rate). For the purpose of comparison, a series of m/M HPW/TiO2catalysts were prepared, whose HPW contents were 0 wt% (m/M TiO2), 5 wt% (m/M 5-HPW/TiO2), 10 wt%(m/M 10-HPW/TiO2) and 15 wt% (m/M 15-HPW/TiO2), respectively.
2.3 Test methods
The Bruker D8 Advance target X-ray powder diffractometer with a CuKα (λ=1.540 6 Å) was used for X-ray diffraction (XRD) test of samples under the operating conditions of 40 kV and 50 mA. The morphology of samples was observed by scanning electron microscope (SEM) images of Hitachi S-4800 and transmission electron microscopy (TEM) of JEOL JEM-2100F. The N2adsorption-desorption isotherms of the samples were measured at 77 K on Micromeritics Tristar-II 3020 analyzer (by the way, all samples to be tested were degassed under a vacuum of 323 K for 10 h before measurement). According to the available data of N2adsorption, the surface area of samples was calculated by the Brunauer-Emmett-Teller (BET) method and the pore size distributions were derived by the Barrett-Joyner-Halenda (BJH) method. Digilab-FTS60 spectrometer was used to record the Fourier transform infrared spectrum of the samples (FT-IR) by KBr methods. Ultraviolet diffuse reflection spectrum (UV-Vis/DRS) of the samples were collected by PE Lambda 35 spectrometer with BaSO4as the background material.Moreover, the actual content of HPW in the samples was calculated by inductively coupled plasma (ICP)method, using Perkin-Elmer 3300DV atomic emission spectrometer.
2.4 Study on catalyst performance
In order to conveniently investigate the oxidative desulfurization performance of the prepared catalyst in the laboratory, we used aromatic sulfur compounds and petroleum ether (boiling range: 90-120 ℃) to simulate the relevant environment in the actual fuel. In other words, BT, DBT or 4,6-DMDBT with the same sulfur content was respectively added into the petroleum ether to obtain three different types of model fuel with sulfur concentration of 500 mg/L. Then, 10 mL of model fuel and 10 mL of acetonitrile were mixed in a 50 mL two-necked flask. The flask was placed in a constant temperature water bath and heated to the temperature required for the reaction. Next, a certain amount of catalyst was added and the required amount of H2O2was also rapidly injected (calculated according to the molar ratio of O/S) into the oil layer in the flask. The reaction started at a stirring speed of 400 rpm, and the reaction schematic diagram was shown in Fig.1. At a certain interval, 30 μL of oil phase were taken from the flask, and the concentration of S in the model fuel was analyzed by high performance liquid chromatography(HPLC). Among them, HPLC was produced by Shimadzu Company of Japan, including the LC-20A system, LC-20AT pumps, SPD20A ultraviolet detector and an ODS column (4.6 mm × 200 mm, 5 μm). After the test, the catalyst in the flask was centrifuged, washed by methanol and dried at 100 ℃ for its cycle performance test.
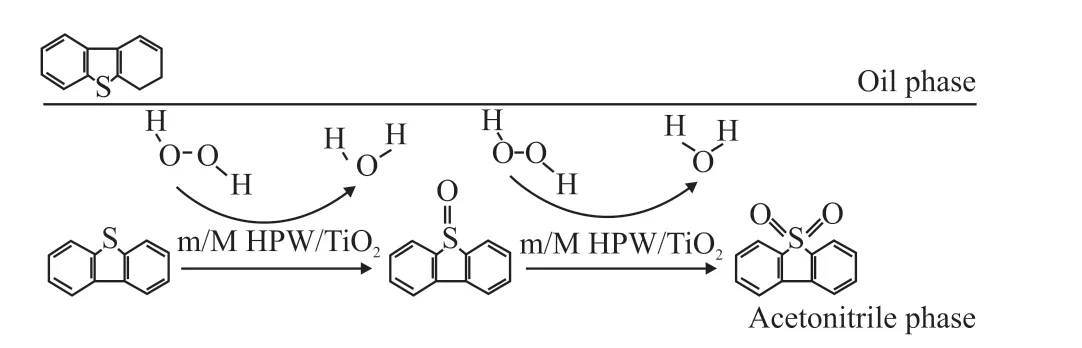
Fig.1 Schematic diagram of oxidative desulfurization
3 Results and discussion
3.1 Characterization of catalysts
The catalyst in this work was synthesized by using PMMA as the macroporous template, so that the accumulation of PMMA microspheres had a great influence on the structure of the as-synthesized catalysts.Hence, SEM was used to observe its internal information and the results were shown in Fig.2. The PMMA microspheres were packed tightly, with an average particle size of about 265 nm, indicating a high homogeneity and periodicity of PMMA microspheres.
The SEM and SEM-EDS spectra in Fig.3 showed the macropore structure information and element distribution of as-prepared catalysts. As can be seen from parts (a) and (b) and (c) of Fig.3 that there was a three-dimensional ordered network macropore structure in the sample. Meanwhile, the high magnification SEM image of the catalyst in Fig.3(a) clearly revealed that the spherical macropore structure in the sample, and the average size of the spherical macropore was about 180 nm. As compared with PMMA microspheres (265 nm, Fig.2), the macroporous structure in the sintered catalyst was slightly smaller. This difference may arise from the shrinkage of the pore wall of PMMA microspheres during the process of high temperature calcination[28]. Moreover, according to the results of SEMEDS shown in Fig.3(f), the tungsten element in the sample was evenly distributed on the surface of TiO2framework.
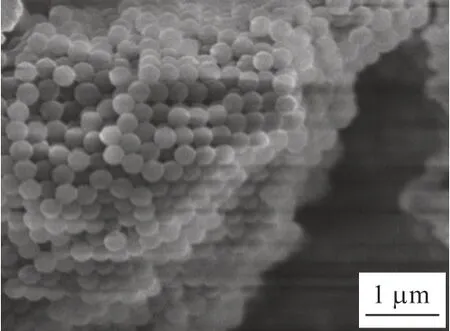
Fig.2 SEM image of PMMA microspheres
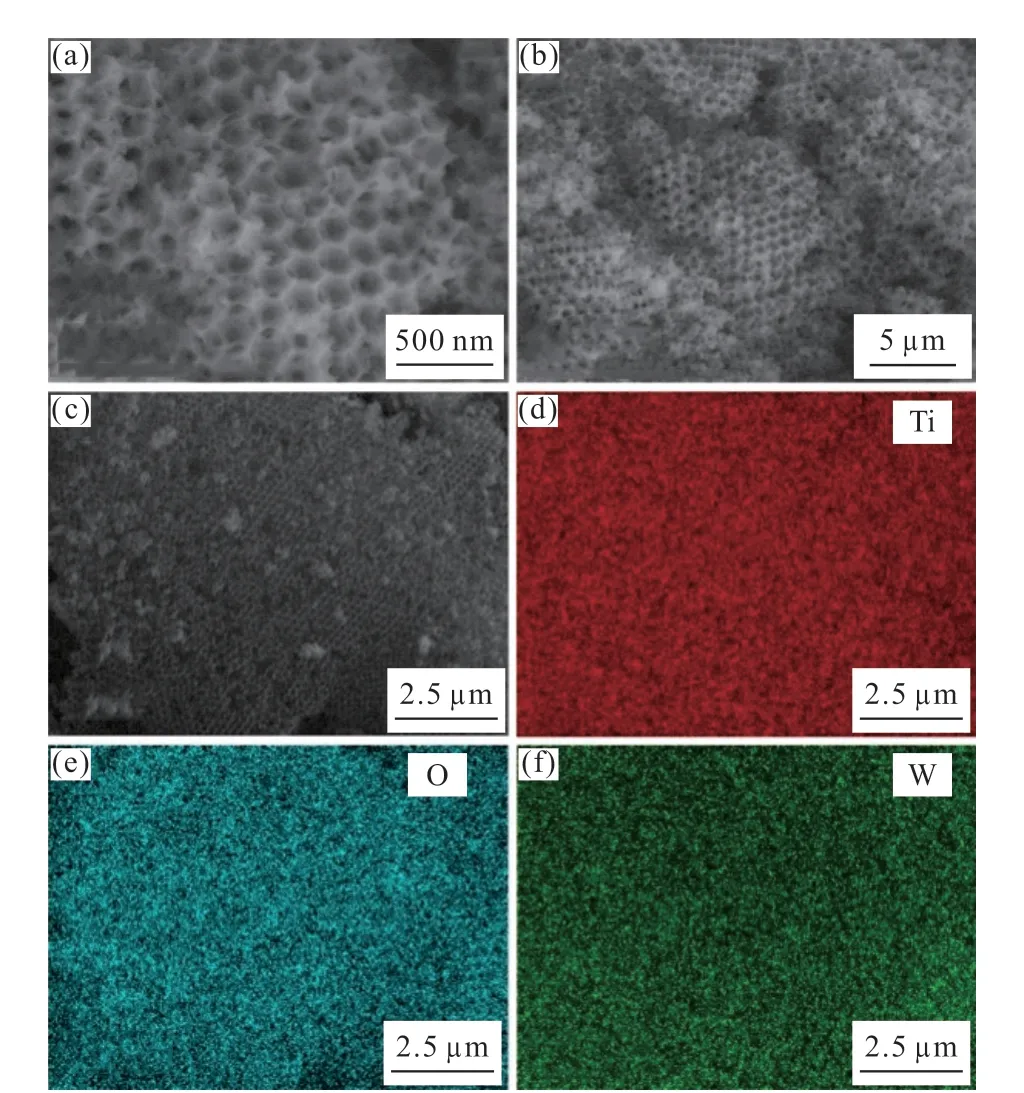
Fig.3 SEM images (a-c) and SEM-EDS spectra ((d) titanium, (e)oxygen and (f) tungsten) of m/M 10-HPW/TiO2 catalyst
For the purpose of collecting the pore structure information of the catalyst completely, TEM was used to further explore it. Fig.4(a) showed a typical low-magnification transmission electron microscopy (TEM)image of the synthesized m/M 10-HPW/TiO2catalyst,in which the macroporous structures were connected by an ordered mesoporous structure, forming a three-dimensional network. Furthermore, the macroporous structure was highly ordered and periodic, and its average diameter was about 181 nm, which was consistent with the observation from SEM. The average pore size of the mesopores in the catalyst was measured to be about 5.7 nm, as shown in Fig.4(b), an enlarged TEM image.
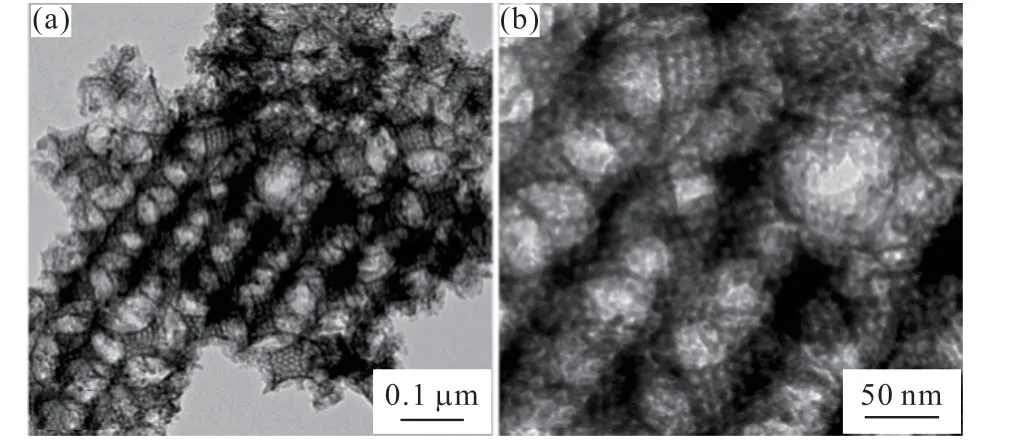
Fig.4 TEM images of m/M 10-HPW/TiO2
The X-ray diffraction was prepared to further verify the internal structure of the as-synthesized catalysts.Wide-angle X-ray diffraction (WAXD) for the catalysts in Fig.5(a) exhibited five broad peaks at 25°, 37.8°,48°, 55° and 62.7°, corresponding to (1 0 1), (0 0 4),(2 0 0), (2 1 1) and (2 0 4) of anatase[29], respectively.The characteristic diffraction peak position of anatase TiO2crystal in samples were clearly visible, proving that all the as-prepared catalysts were anatase phase.Moreover, no characteristic diffraction peaks of HPW were observed because of its lower loading content and weak crystallization, on the other hand, also implying the good dispersion of the HPW on the TiO2surface[30].As can be seen from the small angle X-ray diffraction pattern in Fig.5(b), a wide range diffraction peak could be obviously observed in m/M TiO2, m/M 5-HPW/TiO2and m/M 10-HPW/TiO2at 1.0°, indicating the existence of ordered mesoporous structure in catalysts. However, the same diffraction peak was not observed in m/M 15-HPW/TiO2, which can be ascribed to the ordered mesoporous structure in the catalyst being destroyed by excessive HPW[31]. The mesoporous structure and amorphous HPW species have been successfully introduced into the highly dispersed anatase structure.
N2adsorption-desorption method was an effective means of pore structure characterization. From the N2adsorption-desorption curve of catalysts shown in Fig.6(a), the adsorption curve increased rapidly at low pressure. When theP/P0continued to rose to 0.4-0.7,the adsorption amount showed a segmented increase,suggesting that there were a large number of mesoporous structures in the catalysts[32]. The type of hysteresis ring obtained conforms to the H2type specified by IUPAC, indicating that the pore size distribution of mesopores was relatively uniform[33]. Meanwhile, an additional adsorption step was observed at high relative pressure, confirming the existence of macropores in the catalysts[34]. Furthermore, the pore size distribution of all catalysts was relatively concentrated, as shown in Fig.6(b), the curve of BJH pore size distribution. Based on the study of N2adsorption-desorption, the relevant data were summarized through calculation, as shown in Table 1. The average pore sizes of m/M 5-HPW/TiO2,m/M 10-HPW/TiO2and m/M 15-HPW/TiO2were 5.89,5.54 and 5.21 nm with pore volumes of 0.317, 0.273and 0.256 cm3/g, and the BET specific surface area(SBET) were 172.5, 164.6 and 153.5 m2/g, respectively.Because theSBETof the catalysts given by the calculated data decreased with the increase of HPW content,we concluded that theSBETof the catalysts may be affected by the HPW content. Moreover, the pore size and volume of mesoporous in catalysts decreased with the increase of HPW content, which may be related to the collapse of some mesoporous structures caused by excessive HPW[30].
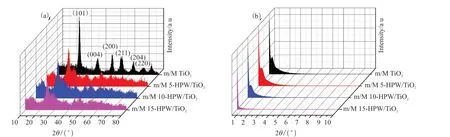
Fig.5 Wide-angle (a) and small-angle (b) XRD patterns of m/M TiO2, m/M 5-HPW/TiO2, m/M 10-HPW/TiO2 and m/M 15-HPW/TiO2
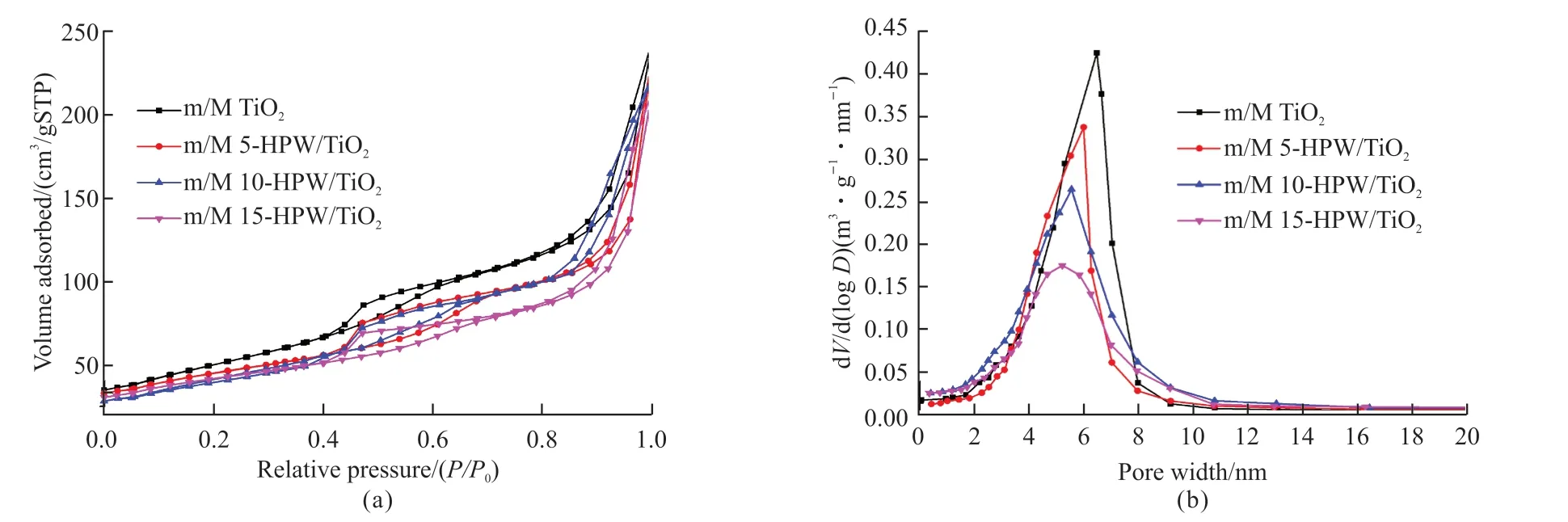
Fig.6 N2 adsorption-desorption isotherm (a) and pore size distribution (b) of various samples calcined at 380 ℃ in air for 10 h
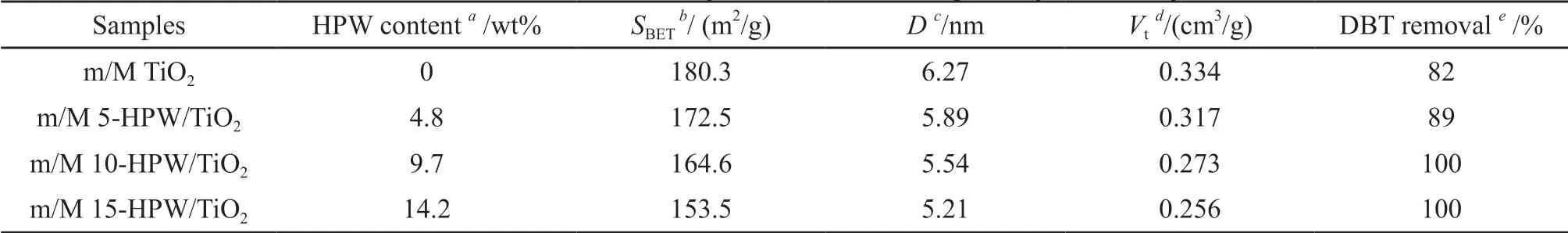
Table 1 Structural information of samples obtained from N2 adsorption-desorption studies
FT-IR analysis was further carried out to verify the information of HPW in the catalysts. It is well established that the HPW with Keggin-type has four infrared characteristic absorption peaks in the range of 700-1 300 cm-1, which can be respectively attributed to 1 079 cm-1[vas(P-O) in the central PO4tetrahedron],983 cm-1[vas(W=O) in the exterior WO6octahedron],889 cm-1[vas(W-Ob-W) in corner shared octahedron]and 805 cm-1[vas(W-Oc-W) in edge shared octahedron][17]. As shown in Fig.7, the four characteristic diffraction peaks attributed to HPW were observed in all catalysts, implying that HPW had been successfully introduced into TiO2matrix and the Keggin structure had been completely retained[18].
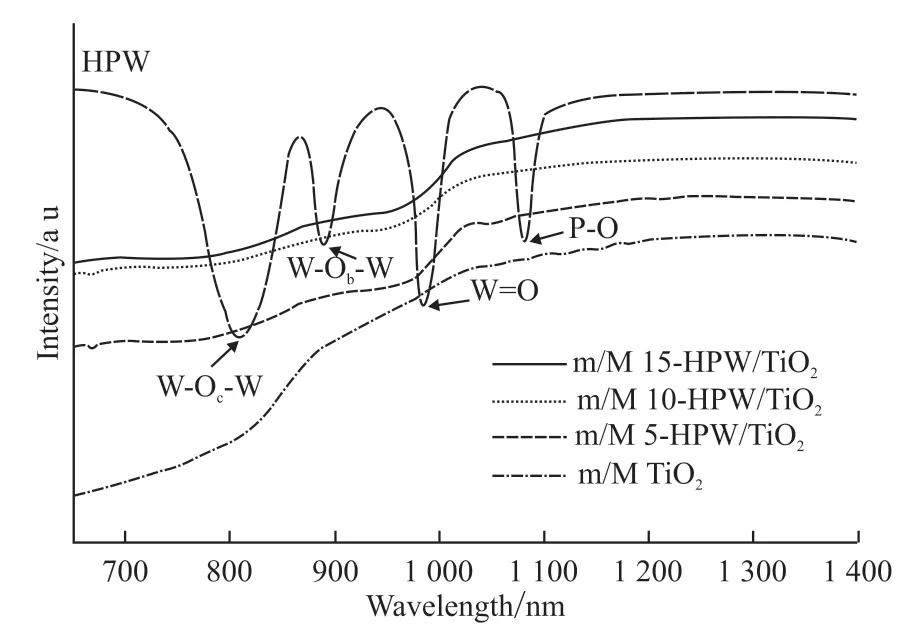
Fig.7 FT-IR spectra of samples
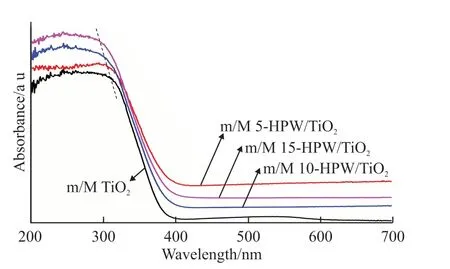
Fig.8 UV-Vis spectra of samples
UV-Vis diffuse reflectance spectra were used to gain a better understanding of the charge transfer in the m/M HPW/TiO2catalysts. As reported, bulk HPW exhibits the absorption bands at 260 and 320 nm[13],respectively. TiO2showed a strong absorption in the range of 230 to 330 nm, given that the transition of electrons from O2pto Ti3dwas the key, indicating that the octahedron of TiO6existed in TiO2[35]. It was noteworthy in Fig.8 that the position of the maximum absorption peak in m/M HPW/TiO2also changed with the increase of HPW content. This result can be attributed to the chemical interaction between TiO2and HPW influencing the coordination environment of TiO2octahedron.
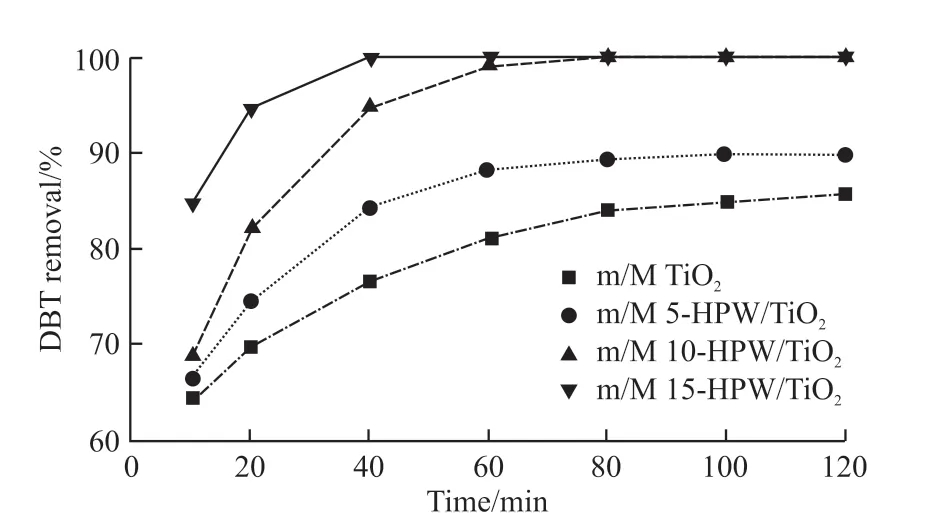
Fig.9 Effect of four samples on DBT removal (catalyst dosage =0.03 g, T = 60 ℃, O/S = 8)
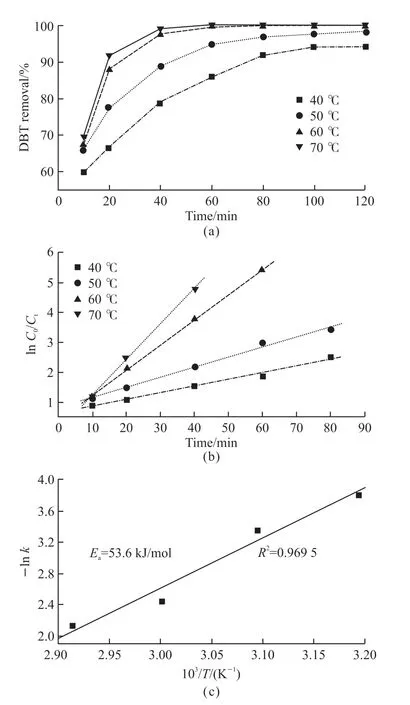
Fig.10 (a) Effect of the reaction temperature on the DBT removal with m/M 10-HPW/TiO2; (b) Pseudo-first-order rate constants for oxidation of DBT reaction at different temperature and (c) the apparent activation energy for DBT oxidation (catalyst dosage = 0.03 g, O/S = 8)
3.2 Effect on oxidative desulfurization
In order to investigate the catalytic activity of the as-prepared catalysts, the interaction between DBT and the catalysts with different HPW content was studied.As shown in Fig.9, the DBT removal efficiency of m/M TiO2was poor, while that of HPW loaded catalysts was significantly improved. Moreover, the removal efficiency of DBT improved with the increase of HPW content owing to the number of catalytic active species per unit surface area increasing with the increase of HPW content[30]. Under the reaction conditions with an O/S molar ratio of 8, a reaction temperature of 60 ℃ and a catalyst dosage of 0.03 g, the DBT in model fuel could be almost completely removed by m/M 10-HPW/TiO2within 60 min, while that of DBT could be reached by m/M 15-HPW/TiO2within 40 min. However, the DBT removal rate of m/M 5-HPW/TiO2at 60 min was only 88% under the same reaction conditions. Even if the reaction time was extended to 120 min, the removal rate of DBT was still only 90%. This result can be explained by the fact that the number of catalytic active centers per unit specific surface area in the catalyst was less with the low HPW loading[36]. Therefore, m/M 10-HPW/TiO2was selected as the catalyst for subsequent research to balance the relationship between reaction time and DBT removal efficiency.
Fig.10 displayed the effect of m/M 10-HPW/TiO2catalyst on DBT removal at different reaction temperatures. When the reaction temperature was 40 or 50 ℃,the change of DBT removal rate with temperature was not obvious. This result can be supported by the reaction kinetics of DBT at low temperature[30]. Nevertheless, the removal rate of DBT increased significantly at 60 and 70 ℃, which was in good consistent with the result of DBT oxidation rate at high temperature controlled by high temperature diffusion[37]. In addition, the reaction kinetics was an important means of investigating catalytic reactions. Fig.10(b) reflected the linear analysis results of the reaction rate at different reaction temperatures, which proved that the oxidation reaction of DBT was a pseudo first-order kinetic reaction.Meanwhile, the result of linear fitting by the Arrhenius equation in Fig.10(c) showed the apparent activation energy was 53.6 kJ/mol and theR2of fitting was 0.969 5. These kinetic data provided a theoretical basis for the excellent catalytic activity of the oxidation desulfurization system composed by m/M HPW/TiO2and H2O2.
In order to optimize the reaction conditions of as-prepared catalysts for removing DBT in model fuel,m/M 10-HPW/TiO2was used as the catalyst to investigate the effects of catalyst cycle performance, dosage,substrates and O/S molar ratio on DBT removal rate,respectively. The removal efficiency of catalyst dosage on DBT was shown in Fig.11(a). When the dosage of m/M 10-HPW/TiO2catalyst was less than 0.03 g, the maximum removal rate of DBT was only 99.8% in 2 h.However, the DBT can be almost completely removed within 1 h when the usage was 0.03 g. As the dosage increased from 0.03 g to 0.05 g, the removal rate of DBT was 99.2% in 40 min. What’s more, the desulfurization rate increased with the increase of catalyst dosage, which was due to the increase of catalyst concentration increasing the number of active centers in the reaction system[38]. Considering comprehensively, 0.03 g was selected as the best dosage of catalyst for further exploration.
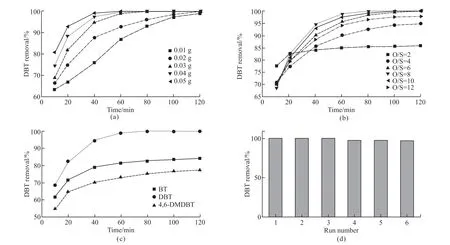
Fig.11 Effects of (a) different catalyst dosage, (b) different O/S, and (c) different substrates, and (d) the recycles on the DBT removal of m/M 10-HPW/TiO2 catalyst (catalyst dosage = 0.03 g, O/S = 8, T = 60 ℃)
Moreover, Fig.11(b) showed the effect of O/S molar ratios on DBT removal with m/M 10-HPW/TiO2. It can be concluded from the results that the removal rate of DBT exhibited a trend of increasing first and then decreasing with the increase of O/S ratio. The removal rate of DBT was only 86% at stoichiometric O/S molar ratio (O/S=2), which can be attributed to the reduction of H2O2concentration caused by the thermal decomposition of a part of H2O2during the reaction process.When the molar ratio of O/S increased to 8, the desulfurization rate reached 99.1% within 60 min. Once the molar ratio of O/S was greater than 8 and increased to 10 and 12, the removal rate of DBT decreased instead.This was because the catalyst surface in the system was occupied and wrapped by a large amount of H2O molecules decomposed from excessive H2O2[37,39]. Thus, the O/S molar ratio of 8 was used as the reaction condition for the subsequent exploration experiments.
Under the optimal reaction conditions, the removal effect of the catalyst on BT, DBT and 4,6-DMDBT was investigated to avoid the single selectivity of the as-prepared catalysts to DBT. As can be seen from Fig.11(c) that the oxidative activity of DBT was much higher than BT, which was consistent with the conclusions obtained in the study[39]. The removal reaction of BT and DBT was an electrophilic reaction, and its reaction activity was related to the electron density on the S atom. The charge density on S atom of BT,DBT and 4,6-DMDBT was 5.739, 5.758 and 5.760 respectively[40,41]. Thus, the lowest ODS performance of 4,6-DMDBT can be explained by the steric hindrance effect of 4 and 6-methyl group in its molecule[35].
In addition, the durability of the catalysts also played an important role in practical applications[42].Fig.11(d) showed the results of repeated ODS test of m/M 10-HPW/TiO2after centrifugal separation, methanol washing and drying overnight. When the number of ODS repeated tests reached 6, the removal performance of the catalyst for DBT decreased only from 100% to 96.1%. This may be related to the complex physicochemical interaction between HPW and TiO2matrix,indicating an excellent durability of the as-prepared catalysts.
4 Conclusions
A variety of m/M HPW/TiO2catalysts were successfully prepared by Sol-gel method in this work. The as-prepared catalysts possessed a 3DO mesoporous and macroporous layered structure. The introduced HPW species were highly dispersed in TiO2matrix and still retained its Keggin structure. In the oxidation desulfurization system of H2O2, m/M 10-HPW/TiO2catalyst exhibited a high catalytic activity and an excellent desulfurization performance under the reaction conditions of catalysts dosage of 0.03 g, reaction temperature of 60 ℃ and the O/S molar ratio of 8. This was because the unique pore structure characteristics of the prepared catalyst, which not only improved the mass transfer efficiency of sulfide but also enhanced the activity of oxidative desulfurization. The kinetic study displayed that the removal reaction of DBT was analogous to the first-order reaction, and the apparent activation energy was 53.6 kJ/mol. In addition, the catalyst also displayed good recyclability and the desulfurization rate of DBT after 6 cycles was still close to that of the fresh catalysts, making it had a great prospect of industrial application.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Effect of Nano Silver Modification on the Dielectric Properties of Ag@TiO2/PVDF Composites
- Preparation and Photocatalytic Performance of Double-Shelled Hollow W18O49@C3N4@Ti3C2 Microspheres
- Effects of Cracks on the Mass Transfer of Polymer Electrolyte Membrane Fuel Cell with High Performance Membrane Electrode Assembly
- Refinement of TiB2 Powders with High-speed Planetary Mill and Its Effect on TiB2 Sinterability
- The Preparation of Porous Activated Slag Granules/TiO2 Photocatalyst and Its De-NOx Performance
- Effects of Magnetization on Thermoelectric Transport Properties of CoSb3 Material
