Preparation and Photocatalytic Performance of Double-Shelled Hollow W18O49@C3N4@Ti3C2 Microspheres
2021-06-14TANYaqiMAHongyuXIONGRuiWEIJianhong
TAN Yaqi, MA Hongyu, XIONG Rui,WEI Jianhong
(Key Laboratory of Artificial Micro- and Nano-structures of Ministry of Education and School of Physics and Technology, Wuhan University,Wuhan 430072, China)
Abstract: C3N4, C3N4@Ti3C2 and W18O49@C3N4@Ti3C2 hollow spheres were successfully prepared by using SiO2 template followed by gradual deposition method. The degradation of phenol solution and photolysis ability were tested to characterize its photocatalytic activity. Compared with the single-shelled C3N4 and C3N4@Ti3C2 hollow spheres, double-shelled W18O49@C3N4@Ti3C2 hollow spheres possessed larger surface area and fast charge separation efficiency, exhibiting about 8.9 times and 4.0 times higher H2 evolution than those of C3N4, C3N4@Ti3C2 hollow spheres, respectively. The photocatalytic mechanism of the W18O49@C3N4@Ti3C2 hollow spheres were carefully investigated according to the results of morphology design and photoelectric performance. A Z scheme mechanism based on the construction of heterojunctions was proposed to explain the improvement of photocatalytic performance. This new charge transfer mechanism appears to greatly inhibit the recombination of electrons/holes during the charge transfer process, while maintaining its strong hydrogen reduction ability, resulting in a higher photocatalytic performance.
Key words: W18O49@C3N4@Ti3C2 composite; double-shelled hollow structure; preparation and characterization; photocatalytic H2 evolution
1 Introduction
With the increasing concerns on energy and environmental problems caused by the limited global fossil fuels, developing sustainable green energy sources becomes one of the most urgent tasks for human beings.Semiconductor photocatalysis, which uses the inexhaustible and eco-friendly solar energy to replace the fossil fuels, has attracted more and more attention due to its potential applications in water splitting, carbon dioxide (CO2) reduction, inactivating viruses and/or degrading organic pollutants,etc[1-3]. Graphite C3N4,with a band gap of about 2.7 eV, is recognized as an attractive n-type visible-light-active semiconductor photocatalyst[4,5]. However, slow charge transfer and rapid electron-hole recombination hinders its wide application. To further improve its photoelectric conversion efficiency and photocatalytic performance, extensive work has been performed by doping[6,7], morphology control[8,9], and heterojunction construction[10-13],etc.Among them, tungsten oxides (such as WO2.9, WO2.83,WO2.72), which has well-matched band-structure with g-C3N4(Eg= 2.5-2.8 eV), and excellent electron transport(ca. 12 cm2·V-1·S-1) compared with TiO2(0.3 cm2V-1S-1)[14]. Some of them can withstand a considerable oxygen vacancies because of their non-stoichiometric properties,making them promising as photocatalysts[15,16].
Besides, MXenes, a large family of twodimensional (2D) transition-metal carbides, nitrides or carbonitrides, were confirmed to be potential cocatalysts for H2evolution after combining with semiconductor photocatalysts because of their special physical-chemical properties such as large specific surface area and adjustable conductivity,etc[17-19]. In addition, morphology is also playing important role in adjusting mass transfer and light-harvesting[20].In general, hollow shells exhibit lower density and higher light-harvesting efficiency compared with solid structures. Especially, the light utilization rate of the double-shell hollow micro-sphere is significantly improved because of the repeated reflection and refraction of the inner and outer shell[21]. At present,the solar-hydrogen conversion efficiency (STH) of particulate photocatalysts rarely exceeds 1%, which is far below the target of commercial application thresholds of 10% STH[22,23]. The main reason for it is that too much energy is consumed in the absorption of light, in the separation and transfer of charges, and in the surface reactions of carriers. How to reduce the energy consumption of the above three problems is very important to effectively improve the photocatalytic efficiency.
Herein, for the sake of achieving more satisfactory solar-energy conversion efficiency, ternary double-shelled hollow W18O49@C3N4@Ti3C2composites (DSWCT) were developed, and the structure,photocatalytic performance and mechanism were carefully researched. We expected this work can further deepen our understanding for g-C3N4-based composite photocatalysts with advanced nanostructures.
2 Experimental
2.1 Synthesis of Ti3C2 nanoparticles (NP)
Scheme 1 shows the overall synthetic procedure of DSWCT composites by using SiO2nanospheres as templates. Firstly, Ti3C2was prepared by immersing Ti3AlC2in 49% HF at 333 K for 20 hours as described in previous report[24]. The as-prepared sample was washed repeatedly with de-ionized water, centrifugally separated and dried in vacuum at 343 K for 8 hours to obtain a laminated Ti3C2. 100 mg of as prepared Ti3C2was added into 100 mL of ethanol absolute and subjected to ultra-sonication for 5 hours, followed by centrifugation at 10 000 RPM. After removal of the precipitates, a homogeneous dispersion of Ti3C2NPs in the supernatant was obtained.
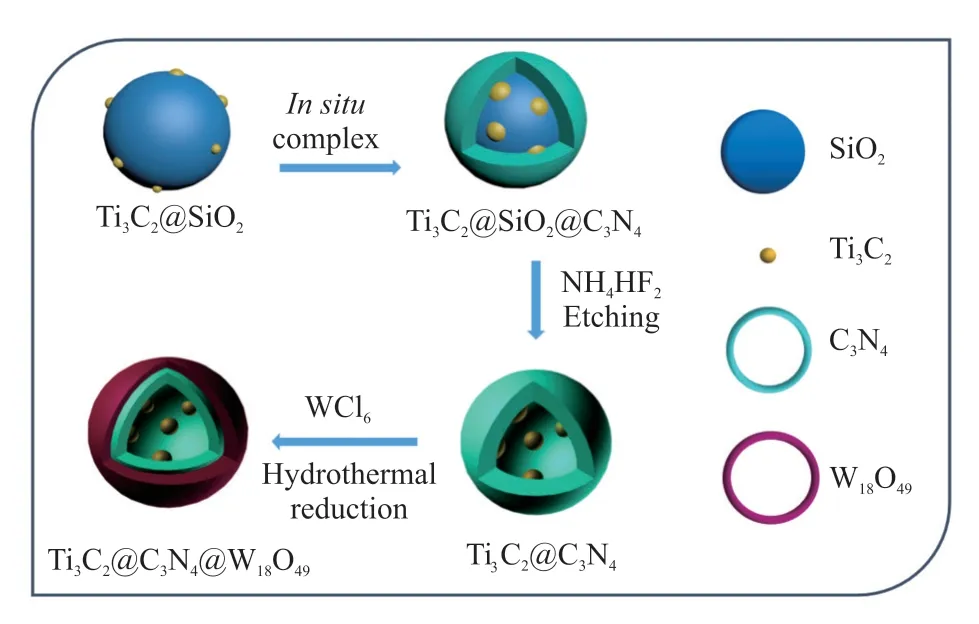
Scheme 1 Synthesis schematic of hollow DSWCT composites
2.2 Synthesis of Ti3C2 NPs /SiO2 microspheres
The monodisperse SiO2templates were synthesized according to the Stöber method. In a typical synthesis, 3.42 mL of aqueous ammonia and 10 mL of deionized water were added to the 73.68 mL of the above alcohol solution containing Ti3C2NPs. After stirring for 30 min at 30 ℃, 5.6 mL of TEOS was added to the above mixture with vigorous stirring and was left stationary for 1 h to yield uniform nonporous silica spheres. A mixture of TEOS and C18TMOS was then added dropwise to the above solution with magnetic stirring to create a thin mesoporous silica shell around the dense silica core. The mixed solution was then kept at ambient temperature for 3 h without stirring to promote the cohydrolysis and condensation of the TEOS and C18TMOS on the nonporous SiO2beads.The nanostructured silica was centrifuged, dried at 70oC and calcined at 550 ℃ for 6 h in air. The as-prepared monodisperse SiO2templates were neutralized with a 1-M HCl solution and then dried at 80 ℃ overnight to obtain the Ti3C2/SiO2microspheres.
2.3 Synthesis of core-shell C3N4 @Ti3C2 NPs microspheres
2 g of the Ti3C2NPs /SiO2microspheres was added to 10 g of cyanamide under ultrasonication treatment at 60 ℃ for 2 h. After that, the reation mixture was kept stirring at 60 ℃ for 12 h, which was then centrifuged, dried, and calcined at 550 ℃for 4 h. The obtained powder was further treated with 2 M NH4HF2to remove the silica template, then centrifuged, washed with deionized water and ethanol three times and finally dried at 80 ℃ in a vacuum oven for 12 h. The obtained yellow colk-shell microspheres were denoted as C3N4@Ti3C2.
2.4 Synthesis of double-shelled hollow W18O49@C3N4@Ti3C2 microspheres
0.2 g of YS-TCN and 0.4 g of WCl6were dissolved in 50 mL of absolute ethanol with intense stirring until a translucent blue solution formed.Subsequently, the resulting solution was then loaded into a Teflon-lined autoclave and heated in an oven of 180 ℃ for 12 h, followed naturally cooled to room temperature. The powder product was collected,washed with ethanol and distilled water several times to remove ions and possible remnants and then dried at 70℃ for overnight. The obtained double-shelled hollow W18O49@C3N4@Ti3C2microspheres were denoted as DSWCT microspheres .
3 Results and discussion
The morphology of the as-synthesized samples were investigated by SEM and TEM, as shown in Fig.1.As indicated, SiO2samples possess regular spherical structure with uniform diameters of about 400 nm(Fig.1(a)). Compared to SiO2particles, C3N4covered in the surface of SiO2particles resulting in the larger diameter of the C3N4@SiO2than that of unmodified SiO2(Fig.1(b) and 1(c)). The shell thickness of C3N4is about 80 nm, which can be adjusted by reaction time and reactant concentration,etc.After the SiO2colloidal core is removed, some broken particles clearly indicate that the morphology of C3N4@SiO2changes into hollow microspheres, and the center color of the microspheres gets into lighter, as indicated in Fig.1(d) and 1(e).Besides, the microspheres are still quite uniform but getting denser and with a smaller diameter of 300 nm. Fig.1(f) shows TEM images of ternary doubleshelled WCT microspheres. The average diameter of hollow spheres is about 320 nm. Moreover, Ti3C2nanoparticles with an average size of 20 nm (inset of Fig.1(f)) are evenly distributed on the internal surface of the C3N4and W18O49. In addition, the outer casing of the DSWCT shows some wrinkles which are most likely due to the asymmetric shrinkage of the precursor during heat treatment. Moreover, the TEM image of the DSWCT also exhibits a contrast between the darker periphery and the brighter area. Combined with the preparation process, the darker one is regarded as C3N4,and the lighter one is W18O49. The thicknesses of the inner C3N4shell and outer W18O49shell are estimated to be around 80 and 20 nm, respectively. Fig.1(f) also indicates close contact between Ti3C2NPs and C3N4shell, which facilitates charge transfer and improves photocatalytic efficiency.
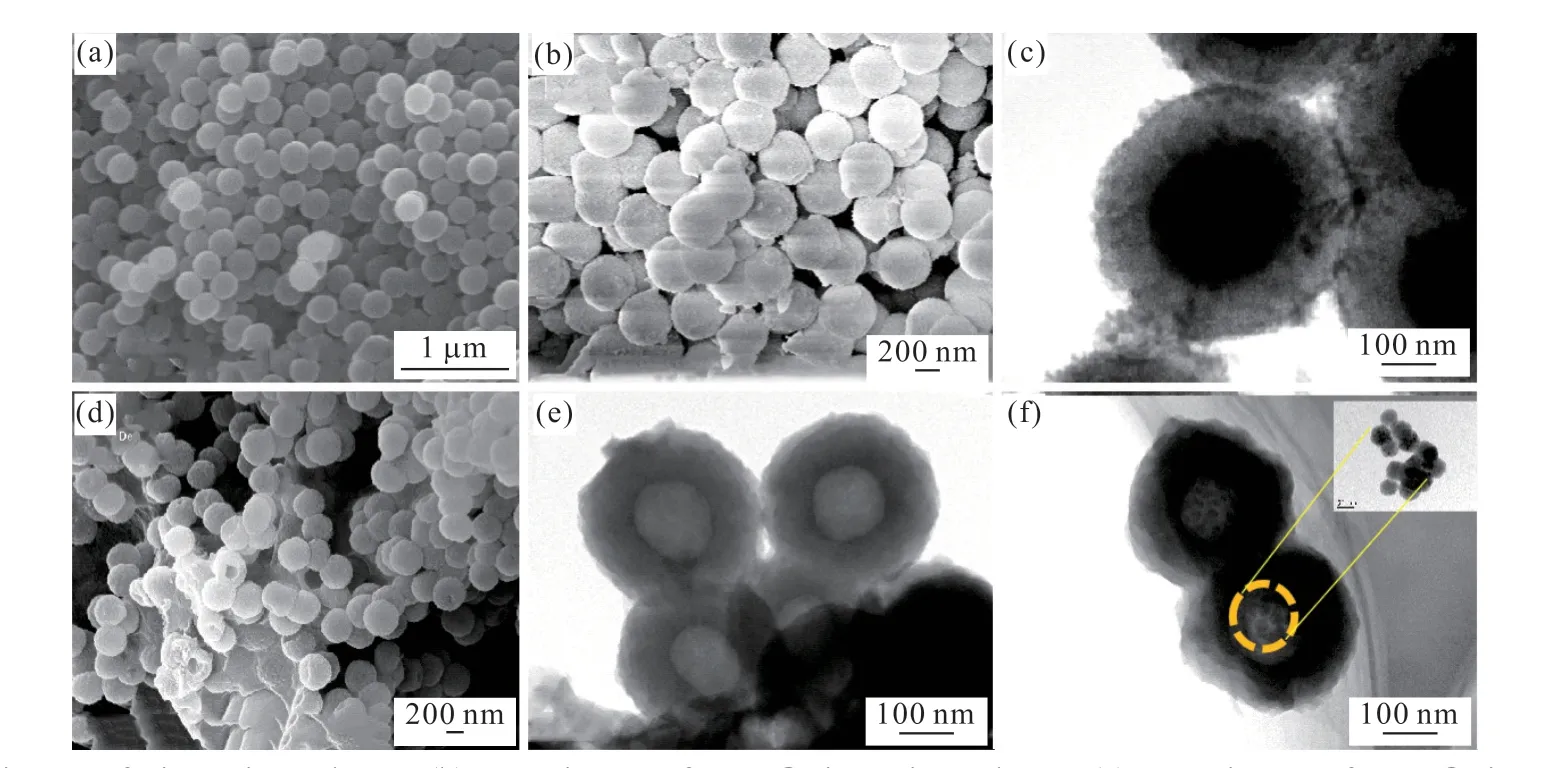
Fig.1 (a) SEM image of SiO2 microspheres; (b) SEM image of C3N4@SiO2 microspheres; (c) TEM image of C3N4@SiO2 microsphere; (d)SEM image of hollow C3N4 microspheres; (e) TEM image of hollow C3N4 microspheres; (f) TEM image of DSWCT microsphere(inset is TEM image of Ti3C2 nanoparticles)
The XRD patterns of the as-synthesized samples were shown in Fig.2(a). The strongest characteristic diffraction peaks of Ti3AlC2at 39.46o(which is indexed as (104) in JCPDS 52-0875) sharply reduced after etched by HF. Meanwhile, the other diffraction peaks such as (002) and (004) peak belonged to Ti3AlC2were broadened and obviously shifted to lower degrees,suggesting the Ti-Al bond was broken and Ti3C2was successfully produced[25,26]. In Fig.2(b), the pure g-C3N4pattern exhibits a strong diffraction peak at 27.49°,which indicates the accumulation of conjugated double bonds, and is indexed as a graphite phase structure of the (002) peak in JCPDS 87-1526. Both the diffraction peaks of Ti3C2and C3N4are detected in the Ti3C2@C3N4pattern, suggesting the coexisting of Ti3C2and C3N4in the Ti3C2@C3N4composites. The W18O49(also named as WO2.72) sample appears as monoclinic structure based on JCPDS card No. 71-2450. Aside from the Ti3C2and C3N4characteristic peaks, two diffraction peaks corresponding to the (010) and (020)of monoclinic lattice structure W18O49were observed in the XRD pattern of the DSWCT sample, however, the relative intensities of Ti3C2and C3N4diffraction peaks were further weakened because of the encapsulation by W18O49.
Fig.2(c) shows the UV-vis diffuse reflectance spectra of the different samples. As indicated, the undoped C3N4sample possessed photo-absorption from the UV to visible light, and the absorption threshold was located at ca. 460 nm corresponding to the bandgap of 2.69 eV. Obviously, W18O49shows much stronger absorption than that of undoped C3N4sample when the wavelength is longer than 450 nm, which maybe due to the existence of oxygen vacancies. Its absorption threshold is located at approximately 450 nm corresponding to a band gap of 2.76 eV. Benefit from the introduction of Ti3C2and(or) W18O49, both samples of C3N4@Ti3C2and DSWCT exhibit stronger absorption in the region of visible light than that of undoped C3N4, and the DSWCT exhibits the strongest visible-light absorption among all of the samples when the wavelength is longer than 450 nm because of the co-doping of Ti3C2and W18O49.
Fig.2(d) shows the nitrogen adsorption-desorption isotherms (BET) of different samples. Obviously, all prepared samples showed a type IV isotherm indicating the presence of mesopores (2-50 nm). The isotherm at the higher relative pressure range (in the range of 0.8-1.0) shows an H2hysteresis loop indicating the presence of slit-like holes[27]. The BET surface area of C3N4,C3N4@Ti3C2and DSWCT microspheres were calculated to be 52.7, 52.4 and 75.9 m2/g respectively.Since Ti3C2NPs are covered by C3N4shell, its effect on specific surface area is not obvious. After further covering C3N4@Ti3C2microspheres with W18O49,the specific surface area was significant increased,which may be due to a lot of air void created during W18O49in situgrowth on the surface of C3N4@Ti3C2microspheres. Besides, the large amount of oxygen vacancies of W18O49also make an important contribution to the enhancement of specific surfaces.According to the inset of Fig.4(b), all samples showed a pore distribution from 2 to 100 nm with two peaks at a few nanometers or about 30 nm, indicating the presence of mesopores and macropores in the samples.The presence of these pores is attributed to surface buildup and air voids formed during heat treatment andin situgrowth.
Fig.3(a) shows the X-ray photoelectron spectroscopy (XPS) of different samples to investigate their surface properties. As indicated in Fig.3(a), C 1s and N 1s are clearly shown in full XPS spectrum for C3N4and C3N4@Ti3C2. Because of being covered by C3N4shell, the peak of Ti 2p attributed to Ti3C2can hardly be found in the full XPS spectrum for C3N4@Ti3C2[28].For DSWCT microspheres, the O1s, C 1s, N 1s, W 4f and W4p,etcare clearly seen in its full XPS spectrum,indicating the success fabrication of W18O49@C3N4@Ti3C2(DSWCT) composite, which agrees well with the results of XRD. In addition, a broader O1s peak (at ca.530.4 eV) can be divided into two peaks, at 530.2 and 532.1 eV, corresponding to lattice oxygen and hydroxyl oxygen species, respectively (Fig.3(b)). It confirms the presence of oxygen vacancies on the surface of DSWCT after W18O49coverage.

Fig.2 Typical XRD patterns of (a) Ti3C2 and Ti3AlC2; (b) DSWCT, SSCT, C3N4 and W18O49; (c) UV-Vis reflectance spectra; (d) N2 adsorption desorption isotherms of DSWCT, SSCT, C3N4 and W18O49, respectively (upper left inset in (d) are the corresponding pore size distribution plots)
The photocatalytic performance of as-prepared samples were evaluated by H2evolution (Pt cocatalyst)and photodegradation organic polluants (phenol)under visible light irradiation (λ>420 nm) (Fig.3(c)).The original Ti3C2MXene is inactive in the pollutant photodegradation and H2evolution because of its very narrow bandgap[29,30]. As demonstrated in Fig.3(c), W18O49has no obvious visible light induced photocatalytic H2evolution activity. However, distinct H2production of 29.8, 66.2, 265.3 μmol at 4 h were observed over the samples of C3N4, C3N4@Ti3C2and DSWCT, respectively. The highest H2evolution rate of DSWCT reaches 265.3 μmol, which is almost 4.0 times that of C3N4@Ti3C2and 8.9 times that of pure C3N4, inferring vital synergy role of different components, heterojunction and morphology for the visible light photocatalysis. Moreover, with such strong redox abilities, DSWCT photocatalysts also exhibited enhanced photodegradation performance, as shown in Fig.3(d). Compared with unmodified counterparts or C3N4@Ti3C2composites, the reaction rate of DSWCT were 5.3, 3.0 and 1.78 times higher as that of C3N4,3C3N4@Ti3C2, and W18O49,respectively.
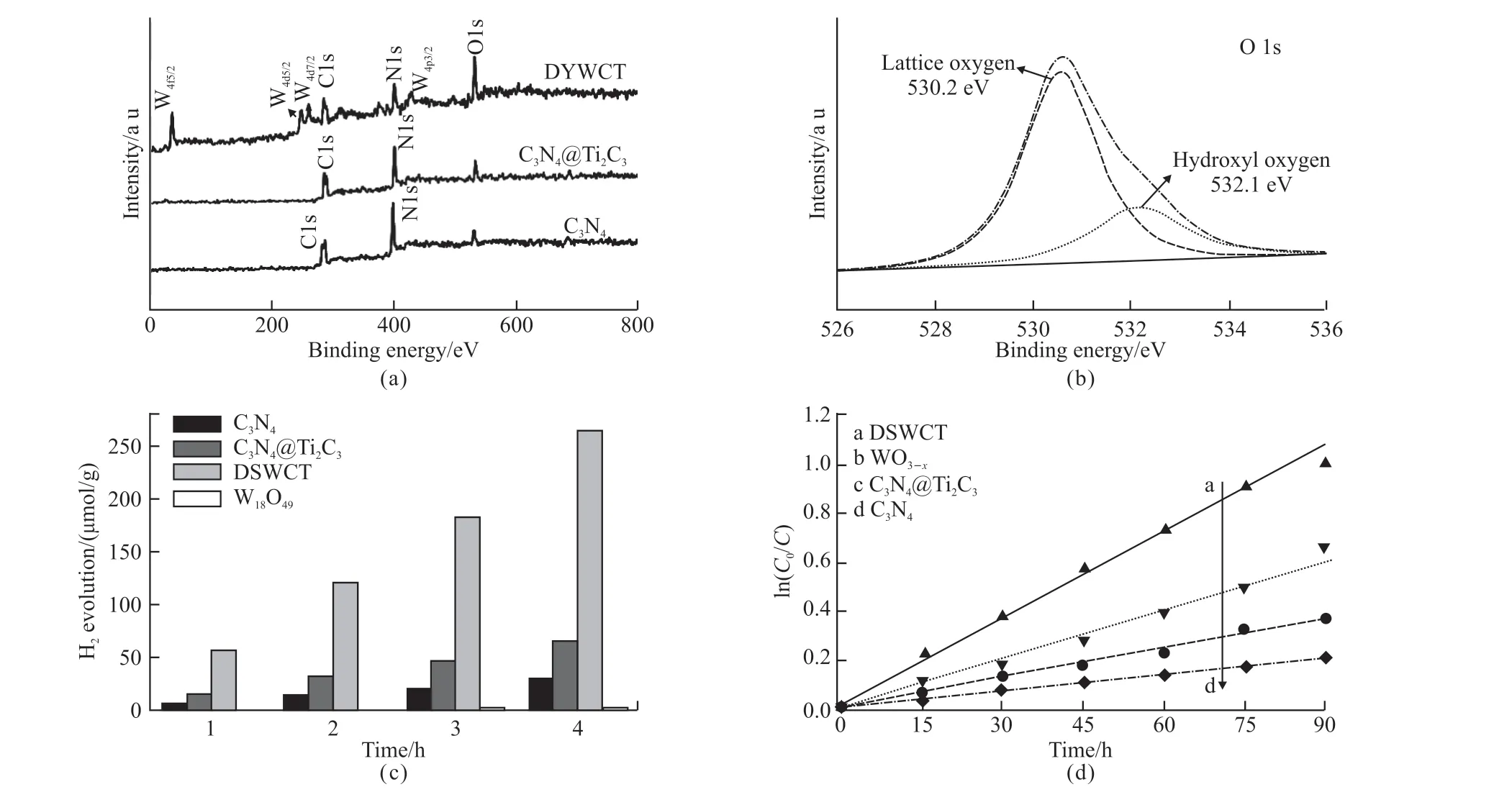
Fig.3 (a) XPS spectra for survey spectrum; (b) O1s spectrum of DSWCT; (c) Photocatalytic hydrogen evolution from water under visible light irradiation (λ > 420 nm) for different samples; (d) Visible light-driven photocatalytic degradation of phenol using as-prepared samples
As is well known, the photocatalytic performance of semiconductor materials is closely related to the separation and transfer process of carrier. To put across the photoreactivity mechanisms of DSWCT, transient photocurrent experiments and photoluminescence spectroscopy (PL) measurements,etcwere carried out (Fig.4). As indicated in Fig.4(a), DSWCT shows the highest current density of 9.8μA/cm2, which is about 2.5, 2.0, 1.3 times that of W18O49, C3N4, and C3N4@Ti3C2microspheres, respectively. Significant enhancement of DSWCT in photocurrent indicates more efficient separation and less photoinduced charge recombination at its interface[31,32].
The PL spectra were recorded to further investigate the photogenerated charge properties, as shown in Fig.4(b). It can be seen that the unmodified C3N4and W18O49and their corresponding composite samples exhibit strong PL signal centering at 450-460 nm, which is closely related with the optical band-gap energy of the C3N4(2.69 eV) and W18O49( 2.76 eV), according to the widely accepted equationEg= 1 240/λ. Noticeably,the PL intensity of C3N4is significantly decreased after coupling with a certain amount of Ti3C2, and further decreased after coupling with a proper amount of W18O49, and the DSWCT exhibits the lowest PL signal.Since the PL signal results from the recombination of band-gap-energy-excited charge carriers, the obviously decreased PL signals clearly demonstrate that the photogenerated charge recombination processes in the samples are significantly reduced. The PL results are in good agreement with the aboveI-tresults.
According to the test result of Mot-shottky junction (Fig.4(c)), the conduct band (CB) position of W18O49and C3N4were -0.09 and -1.09 eV,respectively. Combined with the bandgap of C3N4(2.69 eV) and W18O49(2.76 eV) as shown in Fig.2(c),a possible photocatalytic mechanism for DSWCT were proposed. As shown, the CB bottom of W18O49is located at -0.09 eV, meaning it has poor ability to reduce H+to H2. And vice, the conduct band bottom of C3N4is located at -1.09 eV, meaning it has strong ability to reduce H+to H2. As far as DSWCT sample is considered, now that it exhibits stronger H2evolution performance than corresponding single component and W18O49@C3N4, the H2evolution site shouldn’t locate at the CB position of W18O49, and it’s reasonable to speculate that it should be located at the CB position of C3N4or Ti3C2. According to the above analysis, aZ-scheme mechanism was proposed(Fig.4(d)). In this case, because of the strong “electron sink” effect of Ti3C2NPs[33], the CB electrons of C3N4maybe first transfer to Ti3C2, and correspondingly, the electrons in the CB of W18O49are easily to combine with the holes in the VB of C3N4. This kind of charge transfer process greatly suppressed electron/hole recombination, simultaneously maintained its strong hydrogen reduction ability, and thus resulted in higher photocatalytic H2evolution performance. In addition, because of its specific hollow structure, more photogenerated carriers were produced due to multiple reflections of light within the cavity. Meanwhile, direct interfacial contact between different components reduces the diffusion length of carriers and provides more active sites for the reactants. As a result, DSWCT exhibits the optimum photocatalytic performance.

Fig.4 (a) Photocurrent transient responses at a constant potential of 0.5 V for as-prepared samples; (b) PL spectra of as-prepared samples; (c)Mott-Schottky Junction of different samples; (d) Postulate schematic diagram of the separation and transfer of photogenerated charges in the DSWCT nanocompsosites
4 Conclusions
In summary, mesoporous double-shelled W18O49@C3N4@Ti3C2hollow microspheres (DSWCT) were successfully prepared through a template synthesis combined with hydrothermal treated method. The hollow DSWCT sample exhibited optimum visible light photocatalytic activity in the H2evolution and phenol degradation compared with the pure C3N4,C3N4@Ti3C2and W18O49samples under visible-light irradiation. The high photocatalytic activity of DSWCT can be ascribed to the synergistic interaction between the different components, which effectively promotes photogenerated electrons and holes separation and restrained their recombination, meanwhile preserved the strong oxidizing and reducing ability. In addition,its special double-shell hollow structure effectively increases the use of light sources and provides more surface charge carriers, correspondingly resulting in enhanced photocatalytic performance.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- In Vitro Angiogenic Behavior of HUVECs on Biomimetic SF/SA Composite Scaffolds
- Synthesis and Performance Characterization of a Low Adsorption Clay-resistant Polycarboxylate Superplasticizer
- Strength and Microstructural Analysis of Geopolymer Prepared with Recycled Geopolymer Powder
- Preparation of Phlogopite-based Geopolymer and Its Surface Nonpolar Modification
- Properties and Structure of PEO Treated Aluminum Alloy
- Effects of Strain Rate on the Mechanic Performance of Lattice Materials
