The Preparation of Porous Activated Slag Granules/TiO2 Photocatalyst and Its De-NOx Performance
2021-06-14ZHULideCHENJingSIHeyangFANGYongleWANGXinyuWANGZongsenYANGLu
ZHU Lide, CHEN Jing, SI Heyang, FANG Yongle, WANG Xinyu,WANG Zongsen , YANG Lu
(1. State Key Laboratory of Silicate Materials for Architectures, Wuhan University of Technology, Wuhan 430070, China; 2. School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China; 3. Technical Supervision and Research Center of the Building Materials Industry, Beijing 100024, China; 4. Institute of Technical Information for Building Materials Industry, Beijing 100024,China; 5. Anhui Masteel K.WAH New Building Materials CO.LTD., Maanshan 243099, China)
Abstract: The porous structure and honeycombed structure of granulated blast furnace slag formed by alkali activation (AGBFS) can be used as a promising photocatalysts substrate for the photocatalytic removal of atmospheric or water pollutants. In this study, photocatalytic activated slag granules were synthesized by loading TiO2 on AGBFS with immersion method. The physicochemical properties and NOx removal performance of activated slag granules/TiO2 photocatalysts were studied by X-ray diffraction (XRD), scanning electron microscope (SEM) and photocatalytic performance test. The effects of slag particle sizes and nano-TiO2 loading concentrations on photocatalytic efficiencies of NOx removal were also investigated. It was found that the De-NOx performance of activated slag granules/TiO2 photocatalyst increased with the increasing of slag particle size in low TiO2 loading concentration situation, while increasing the TiO2 loading concentration would result in the opposite De-NOx performance as slag size increased. Nevertheless, for the same size activated slag, the photocatalytic efficiency of activated slag granules/TiO2 photocatalyst gradually improved with the increase of loading concentration of TiO2.
Key words: granulated blast furnace slag; porous structure; alkali-activation; photocatalysis; NOx
1 Introduction
Granulated blast furnace slag is the main industrial by-product of producing pig iron in steel plant. According to the statistics, in 2019, the output of pig iron in China was more than 80.9 million tons.When one ton of pig iron is produced, about 0.4-0.8 tons of slag will be discharged, and at least 32 million tons of granulated blast furnace slag will be produced[1].With the development of China’s iron and steel industry, a large amount of slag will be produced every year. If it cannot be processed in time, the accumulation of slag will not only waste land resources, but also produce harmful chemicals, resulting in severe damage to the atmosphere, soil and groundwater[2-6]. Therefore,the development of high value-added slag products can not only turn slag into treasure, reduce land occupation and pollution, but also bring huge economic and social benefits to society.
At present, granulated blast furnace slag in China is mainly obtained by water quenching and has potential cementing activity[7-9]. Granulated blast furnace slag has been used on a large scale in many fields. For example, it can be used as a raw material to produce slag cement, slag fiber and slag powder, and especially the grinding technology of slag powder has been achieved in the industrial production[10-14]. The more vitreous content in the granulated blast furnace slag there is, the better the potential hydraulicity will be. But the grindability and grinding efficiency will become worse, which will lead to a higher energy consumption. The power consumption of grinding slag is about 2-3 times more than that of producing the cement clinker[15-17]. It is necessary to develop a new method of utilizing the slag particles.
Using alkali-activated aggregates as a photocatalyst carrier for the treatment of environmental pollutants is one of the most important methods in recent years[18-21]. The granulated blast furnace slag is irregularly granular before being grinded. There is a porous structure on the surface, which makes it have a large specific surface area. After alkali activation,honeycomb structure will be formed on the slag surface,which has a potential to enhance the photocatalytic performance of loaded photocatalysts. In this case,this work studied the influence of the slag particle size, type of the TiO2photocatalysts and catalyst loading concentrations on the De-NOxperformance of activated slag granules/TiO2photocatalyst by using X-ray diffraction (XRD), scanning electron microscope(SEM), stereo microscope and photocatalytic performance test. We hope the research can provide a new method for the resource utilization of slag in environmental protection filed.
2 Experimental
2.1 Raw materials
In this paper, granulated blast furnace slag(GBFS) from Maanshan Iron & Steel Company Ltd. (MA Steel) was selected as the carrier of TiO2photocatalyst. The main chemical composition was shown in Table 1. P25 TiO2(30 nm particle size) and self-made TiO2hydrosol powder were selected as slag-loaded photocatalyst. Acetic acid (CH3COOH,analytically pure), ethanol (C2H5OH, analytically pure),sodium hydroxide (NaOH, analytically pure), titanium isopropoxide (TTIP, analytically pure) were purchased from Sinopharm Group Chemical Reagent Co., Ltd.
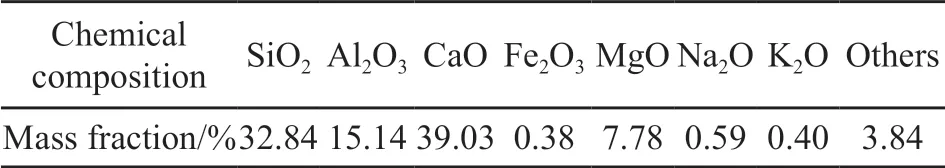
Table 1 Main chemical composition of GBFS from MA steel
2.2 Synthesis and characterization
2.2.1 Synthesis of TiO2photocatalysts
TiO2hydrosols was synthesized by the hydrolysis of TTIP[22]. The typical preparation route was as follows: 2.5 mL of TTIP was dissolved in 7.5 mL of ethanol, with stirring at 50 ℃ for 30 minutes. Then the mixed solution was added (1-2 drops/sec) to an aqueous solution of acetic acid (40 mL of deionized water and 2 mL acetic acid). After that, the obtained solution was stirred at 50 ℃ for 48 hours, and then aged at room temperature for more than 72 hours to obtain a transparent sol. Subsequently, the transparent sol was dried at the temperature of 105 °C for 24 hours to obtain a solid gel, after which the gel was grinded into the powder, and screened with a 200-mesh squarepore sieve to obtain TiO2catalyst powder.
2.2.2 Synthesis of activated slag granules/TiO2photocatalysts
Before loading the catalyst, the slag with different particle size (0.25-0.6 mm, 0.6-1.18 mm, 1.18-2.36 mm) was pretreated to obtain the honeycombed structure. The slag was firstly immersed in 5 mol·L-1NaOH for 24 hours followed by washing repeatedly with deionized water until the pH was 7-8, then the slag was dried at the temperature of 105 °C for 24 hours to obtain alkali-activated slag particles (AGBFS).
A certain amount of self-made TiO2(3 g·L-1) and P25 TiO2(3 g·L-1, 10 g·L-1) powder were dispersed respectively in 250 mL of ethanol solution and ultra about 1 hour. Then 10 g of AGBFS were added into the above solution with ultrasonic treatment for 1 hour. After then, the activated slag/TiO2photocatalytic materials was dried at the temperature of 105 ℃ for 24 hours, which are denoted as GBFST-3, GBFSP-3 and GBFSP-10 according to photocatalysts type and coating concentrations.
2.2.3 XRD
X-ray diffraction (XRD) pattern was used to determine the composition and phases of sample, which was recorded on an X-ray diffractometer produced by PANalytical B.V. of the Netherlands with Cu Kα radiation (200 mA, 40 kV, scanning speed 8°·min-1,scanning range 20°-70°).
2.2.4 SEM
The QUANTA FEG 450 environmental field emission scanning electron microscope(SEM)(voltage 15 kV, beam spot size 3, secondary electron detector) produced by the FEI was used to analyze the microscopic morphology of the samples, and the material composition was analyzed by EDS spectroscopy. Before testing, the samples were dried at the temperature of 105 ℃ for 24 hours.
2.2.5 Stereomicroscope
The SZX16 stereo microscope produced by Olympus was used to characterize the macroscopic morphology of slag surface before and after loading the photocatalyst.
2.2.6 Photocatalytic performance test
The photocatalytic performance of sample was tested with a standard gas reactor (ISO 22197-1: 2007).The schematic diagram of the photocatalytic test device is shown in Fig.1. During the photocatalytic test, a 300 W xenon lamp was used to irradiate the sample surface vertically. At the same time, the light intensity was adjusted to 1 mW·cm-2. The gas parameters were set as that 1 000±50 ppb NO, and the relative humidity was 50% of the air mixed gas at a flow rate of 3 L·min-1on the surface of the sample. In order to test accurately,the test sample was evenly spread on the surface of the test chamber of the reactor, and then placed in a standard gas reactor for photocatalytic performance test. The reaction is divided into three stages. First,the reactor is shaded until the concentration of the gas flowing through the reactor reached a stable level. Then a photocatalytic reaction is performed under the light illumination. After the concentration of the gas reaches a stable level in the reaction, the light is turned off to conduct a dark reaction. After the gas concentration is stabilized again, the test ends and the test is performed again on the next sample.
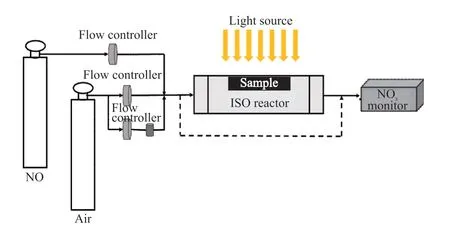
Fig.1 Schematic diagram of photocatalytic performance test device
The photonic efficiency (ξ) of the NO2generation and the removal of NO and NOx, and the nitrate selectivity (S%) are calculated by Eq.(1) and Eq.(2)respectively[23]:
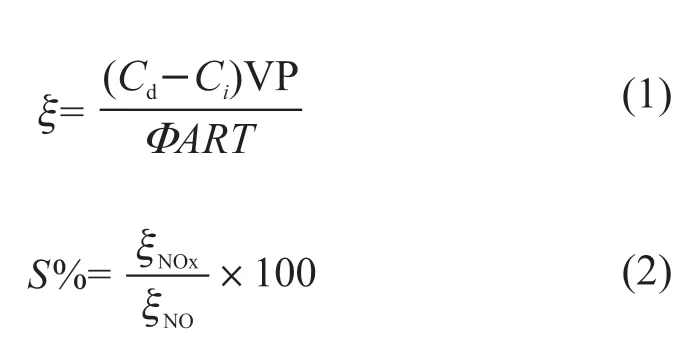
where,Cdis the gas concentration under dark conditions,Ciis the gas concentration under light conditions,Vis the rate of gas flow,Pis the atmospheric pressure,Ais the sample area of reaction,Ris the gas constant,Tis the absolute temperature, andΦis the photon flux.
3 Results and discussion
3.1 Physicochemical properties
Fig.2 shows the crystalline phase composition of slag, TiO2/activated slag and self-made TiO2photocatalyst. It can be seen that the slag mainly consists of an amorphous glass phase and contains a small amount of Åkermanite. After treated with NaOH activator, a small amount of calcium silicate hydrate (Ca2SiO4·nH2O) and calcium aluminate hydrate(Ca4Al2O7·xH2O) were generated[1]. It can also be found from the XRD pattern that the crystal structure of TiO2photocatalyst is anatase, and it can be clearly seen from the diffraction peak of GBFST that the TiO2photocatalyst has been successfully loaded on the surface of the activated slag.
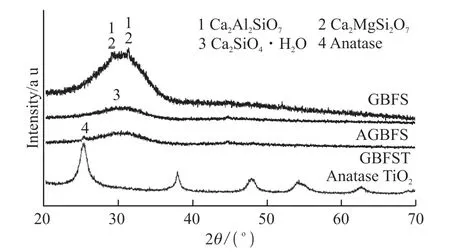
Fig.2 Crystal phase composition of GBFS, GBFST-3 and TiO2 photocatalyst

Fig.3 Macroscopic morphology of alkali-activated slag loaded with different TiO2 photocatalysts (× 32)
Fig.3 shows the macro morphology of slag(GBFS, GBFST-3 and GBFSP-3). It was found that the surface of slag presents a porous structure.After loaded TiO2particles, the surface color of both GBFST-3 and GBFSP-3 aggregates showed significant different, which indicated that the loading amounts of TiO2on GBFSP-3 was significantly higher than that of GBFST-3, which may be related to the different pH values of the two TiO2catalysts solution. Different pH loading solution may cause the difference of surface electric charge of TiO2particles and activated slag,resulting in a significant difference in load of catalyst.

Fig.4 Macroscopic morphology of GBFSP-3 and GBFSP-10 aggregates (× 32)
The macroscopic morphologies of alkali-activated slag loaded with different concentrations of TiO2(P25)are shown in Fig.4. The load of TiO2on the surface of alkali-activated slag obtained by immersing in 10 g·L-1P25 TiO2ethanol solution was significantly more than that of the catalyst by immersing in 3 g·L-1P25 TiO2ethanol solution. The micropores on the surface of GBFSP-10 were obviously blocked by TiO2nanoparticles, which may affect the photocatalytic performance of the material.
Fig.5 shows the SEM and EDS images of AGBFS and GBFST. From the SEM image, it can be seen that the alkali-activated slag surface is a honeycomb structure, which is beneficial to the loading and dispersion of TiO2particles. As can be observed by the image of GBFST, a thick layer has been coated on the activated slag surface. The EDS diagram of AGBFS and GBFST confirmed that this thick layer was TiO2nanoparticles. The thick layer and TiO2agglomerations can be explained by the weak acidity and high concentration of TiO2loading solution, which caused the high electrostatic adsorption and agglomeration chance.
3.2 Photocatalytic performance
The curve in Fig.6 shows the concentrations change of the nitrogen oxides during the photocatalysis process. The entire curve can be divided into three parts: (1) Before illuminating the sample, the gas concentration remains stable; (2) When starts to illuminate the sample, the catalytic reaction occurs, and the gas concentration gradually reaches a stable state;(3) After illuminating the sample, the gas concentration returns to the level before being illuminated. It can be seen from Fig.6 that the concentration of NO drops rapidly after being illuminated, and then rises slightly to reach a relative stable state. At the same time, the concentration of NO2changes accordingly. When the light is turned off, the NO2concentration rapidly decreases to zero, and the NO concentration returns to the level before the illumination. According to the curve in Fig.6 and Eqs.(1) and (2), the photonic efficiency and nitrate selectivity of photocatalytic removal of NOxby the photocatalyst can be calculated.
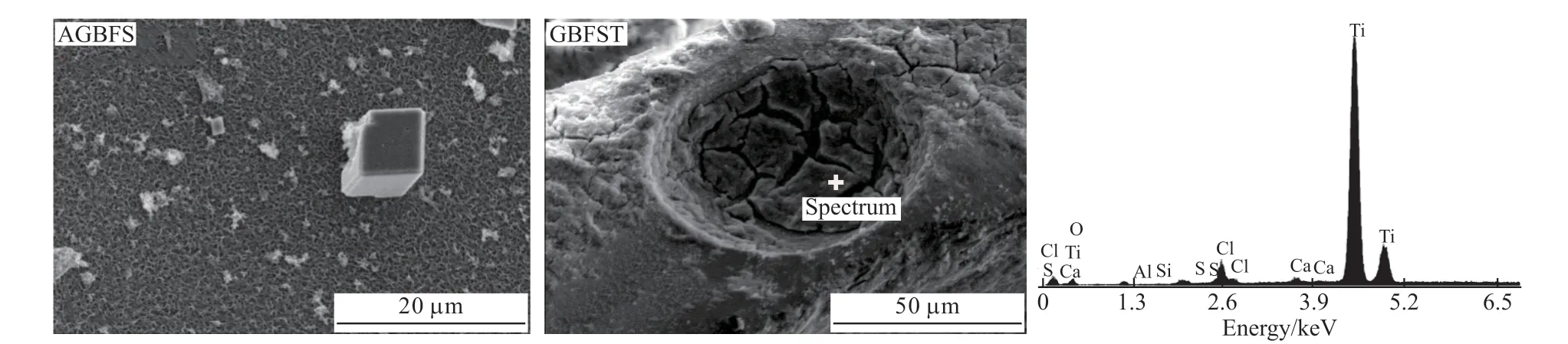
Fig.5 SEM images and EDS diagram of AGBFS and GBFST
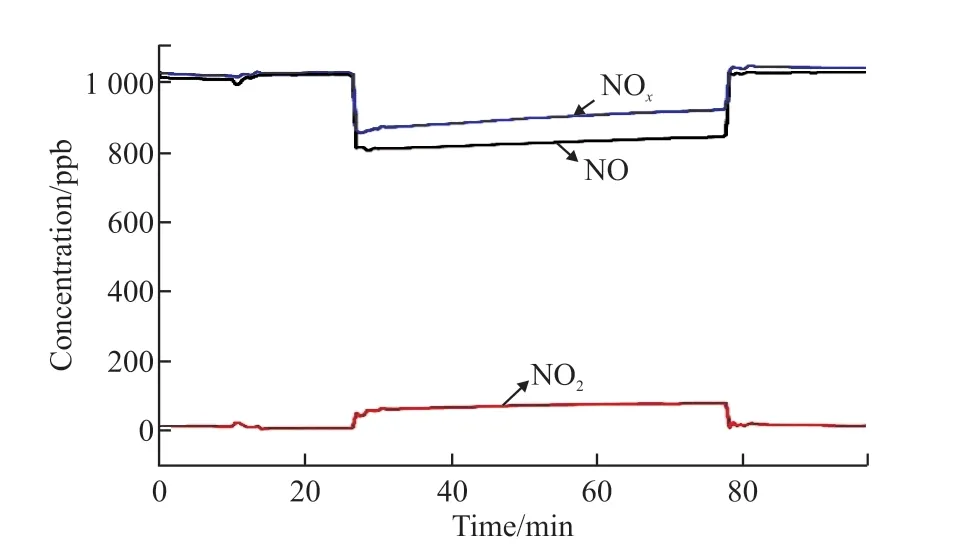
Fig.6 The curve of nitrogen oxide gas concentration in the process of photocatalysis
The photonic efficiency (ξ) and nitrate selectivity(S%) of NO, NOxremoval and intermediate product NO2formation of GBFST-3 photocatalysts with different particle sizes are compared, as shown in Fig.7. It can be seen that compared with the activated slag without TiO2, GBFST-3 has a better photocatalytic effect. As the particle size increases, the De-NOxefficiency of activated slag/TiO2photocatalytic material increases. This is because that the pores amount and loading areas can be increased by increasing the particle size of slag, which is beneficial to the loading and the photocatalytic reaction of TiO2particles, and the utilization ratio of irradiated light also can be enhanced. However, from Fig.7, it also can be observed that the nitrate selectivity (S%) of GBFST-3 slightly decreases as particle size increases, which may be caused by the increase of the number of Si-O-Ti bonds between the TiO2and activated slag as the increase of particle size, since the previous research have shown that an increase in the concentration of Si-O-Ti bonds would cause a decrease inS%[23].
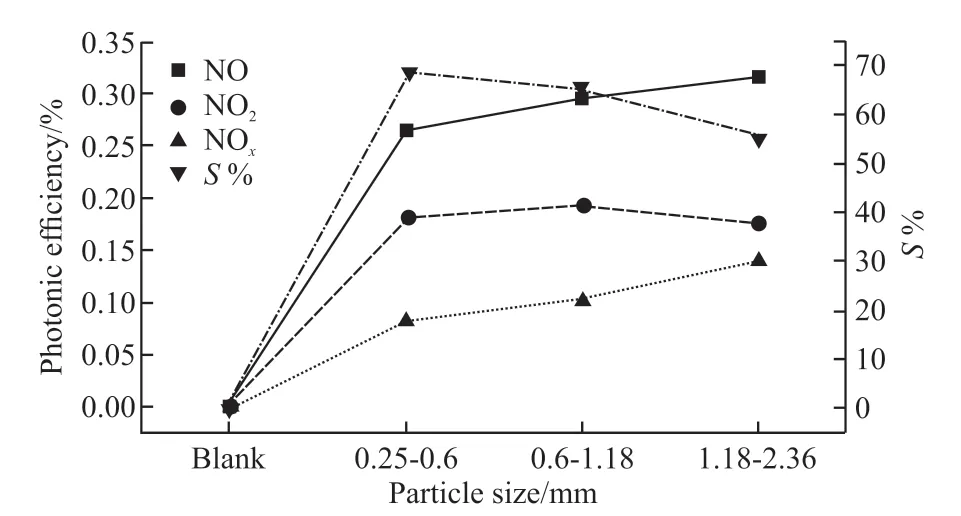
Fig.7 Photonic efficiency (ξ) of the NO2 generation and the removal of NO and NOx, and nitrate selectivity (S%) of different particle sizes GBFST-3 and activated slag without TiO2
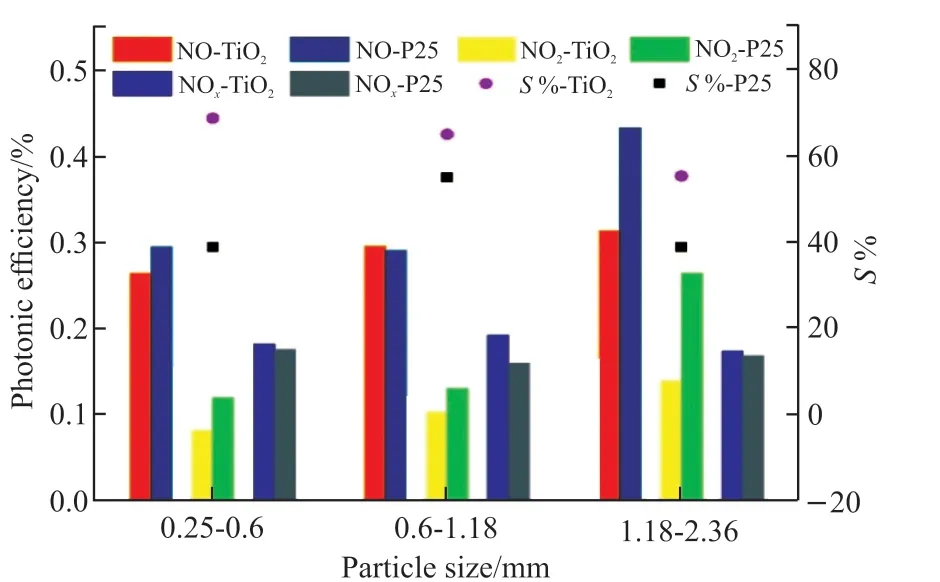
Fig.8 Photonic efficiency (ξ) and nitrate selectivity (S%) of NO,NOx removal and intermediate product NO2 formation of slag loaded with different photocatalyst
Fig.8 shows the photonic efficiency (ξ) and nitrate selectivity (S%) of NO, NOxremoval and intermediate product NO2formation of GBFST-3 and GBFSP-3. It appears that both GBFST-3 and GBFSP-3 exhibit good photocatalytic performance, and as slag particle size increases, the photocatalytic efficiency increases. Since the size of slag particles increases, the number of pores on the surface increases, which leads to the increase of loaded TiO2amount. This finding is also consistent with Figs.3 and 4. For photocatalysts modified slag, comparing the catalytic performance of the activated slag loaded with TiO2hydrosol and P25 TiO2, it can be found that the overall photocatalytic De-NOxefficiency of GBFSP-3 is better than that of GBFST-3. Especially when the slag particle size is 1.18-2.36 mm, the NO removal efficiency of GBFSP-3 is about 1.38 times higher than that of GBFST-3, which may be caused by the difference in the loading environment of different TiO2.
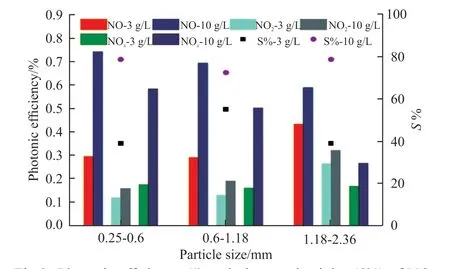
Fig.9 Photonic efficiency (ξ) and nitrate selectivity (S%) of NO,NOx removal and intermediate product NO2 formation of GBFSP-3, GBFSP-10
Fig.9 shows the photonic efficiency (ξ) and nitrate selectivity (S%) of NO, NOxremoval and intermediate product NO2formation of GBFSP-3 and GBFSP-10.As shown in the figure, for the same particle size, the photocatalytic De-NOxefficiency of the activated slagbased photocatalytic material obtained by soaking in 10 g·L-1P25 ethanol solution (GBFSP-10) is significantly better than that of GBFSP-3. Because the amount of photocatalyst loaded on the surface of GBFSP-10 is much more than that of GBFSP-3. As the slag particle size increases, the photocatalytic efficiency of activated slag based photocatalytic material obtained by immersing in 3 g·L-1P25 ethanol solution increases,while the photocatalytic efficiency of the activated slag based photocatalytic material obtained by immersing in 10 g·L-1P25 ethanol solution decreases. When the concentration of P25 ethanol solution is low, the particle size of the activated slag increases, which leads to the increase of the loaded amount of TiO2,and eventually the photocatalytic De-NOxefficiency increase. However, when the concentration of P25 ethanol solution is high, the amount of TiO2loaded on the activated slag surface will reach saturation. In this case, the micropores on the surface will be blocked by TiO2particles, and will reduce its surface area for photocatalysis. Therefore, the optimal TiO2loading on activated slag surface is important for different sizes of activated slag in order to achieve the best photocatalytic De-NOxefficiency and improve the using economic efficiency of photocatalysts.
4 Conclusions
The porous structure on the surface and the honeycomb structure formed after alkali activation of the irregular granulated blast furnace slag particle are beneficial to the loading of TiO2photocatalyst. This paper prepared the TiO2/activated slag photocatalysts and mainly discussed three main factors effecting on the photocatalytic efficiency of prepared samples including particle size of slag, type of photocatalyst and loading concentration of nano-TiO2. The conclusions obtained are as follows:
a) When the loading concentration of nano-TiO2is 3 g·L-1, with the increase of slag particle size, the De-NOxphotocatalytic performance of TiO2/activated slag eventually enhances. When the loading concentration of nano-TiO2is 10 g·L-1, with the increase of slag particle size, the loading amount of TiO2would reach saturation, resulting the decrease of surface area of catalysis, which leads to the reduce of De-NOxphotocatalytic performance of TiO2/activated slag.
b) The loading ability of 3 g·L-1P25 TiO2ethanol solution is better than that of 3 g·L-1self-made TiO2ethanol solution, which depends on the loading pH value. The pH difference of these two TiO2loading solution is the main factor causing the different loading behavior. Consequently, GBFSP-3 shows better photocatalytic removal efficiency.
c) As the loading concentration of nano-TiO2increases, the photocatalytic De-NOxefficiency of TiO2/activated slag increases. The particle size of activated slag has a significant effect on the photocatalytic performance of TiO2. In practice, the optimal loading amount of TiO2should be determined according to the particle size of activated slag in order to achieve the optimal photocatalytic performance and economic efficiency.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- In Vitro Angiogenic Behavior of HUVECs on Biomimetic SF/SA Composite Scaffolds
- Synthesis and Performance Characterization of a Low Adsorption Clay-resistant Polycarboxylate Superplasticizer
- Strength and Microstructural Analysis of Geopolymer Prepared with Recycled Geopolymer Powder
- Preparation of Phlogopite-based Geopolymer and Its Surface Nonpolar Modification
- Properties and Structure of PEO Treated Aluminum Alloy
- Effects of Strain Rate on the Mechanic Performance of Lattice Materials
