Amelioration of metabolic disorders by a mushroom-derived polyphenols correlates with the reduction of Ruminococcaceae in gut of DIO mice
2021-05-24LiSunLiBaoDorjiPhurbuShanshanQiaoShanshanSunYangzomPermaHongweiLiu
Li Sun, Li Bao, Dorji Phurbu, Shanshan Qiao, Shanshan Sun, Yangzom Perma, Hongwei Liu,*
a State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100864, China b Savaid Medicine School, University of Chinese Academy of Sciences, Beijing 100864, China
c Beijing Shijitan Hospital, Capital Medicinal University, Beijing 100864, China
d Tibet Plateau Institute of Biology, Lhasa 850000, China
e School of Life Sciences, University of Science and Technology of China, Hefei 230026, China
Keywords: Sarcodon leucopus Polyphenolic alkaloids Metabolic syndrome Gut microbiota Ruminococcaceae
ABSTRACT A polyphenolic alkaloid-enriched extract (PAE) was prepared from the fruiting bodies of a wild edible mushroom Sarcodon leucopus.Oral administration of PAE reduced hyperglycemia, hyperlipidemia, hepatic steatosis, and LPS-related inf lammation in high fat diet-induced obese (DIO) mice.Furthermore, we show that PAE produces taxonomic and predicted functional changes in the gut microbiome of DIO mice.A signif icant decrease in the family of Ruminococcaceae, especially the secondary bile acid-producing bacteria of Intestinimonas and Anaerotruncus, is detected in the gut microbiome of PAE-treate mice.Accordingly, reductions of deoxycholic acid and lithocholic acid are found in the feces of PAE-treated DIO mice, which benef its for the intestinal integrity and the reduction of inf lammation.A gut microbiota related mechanism for the anti-metabolic syndrome effects of the PAE is proposed.We suppose the polyphenolic alkaloid extract from S.leucopus be a new and benef icial prebiotic regulating glucose and lipid metabolisms.
1.Introduction
Metabolic syndrome (MS) has attracted much public attention due to its prevalence all over the world and damage on body’s health.MS has been defi ned as a polygenetic diseases characterized by central obesity, insulin resistance, type 2 diabetes, hypertension, atherosclerosis, fatty liver disease, and low-grade chronic inflammation [1-5].An ideal anti-MS drug should possess multiple therapeutic effects on decreasing hyperglycemia and hyperlipemia, reducing body weight, and improving cardiovascular health.In recent years, accumulating evidences have indicated that changes in the structure and composition of gut microbiota are closely correlated with the occurrence and development of obesity and its associated metabolic disorders [6-8].Gut microbiota has been raised as a potential drug target for the treatment of MS [9,10].Medicines, prebiotics, and probiotics modulating gut microbiota have been confi rmed to have clinical benefi ts on patients with MS [11-14].
With diverse structural scaffolds and a wide range of bioactivities, natural products are being used as good leading compounds for the development of anti-MS agents targeting at gut microbiota.Ginseng extract was found to increase the abundance ofEnterococcus faecalisthat produced myristoleic acid and induced myristoleic acid-dependent activation of brown adipose tissue to reduce obesity [15].Oral treatment with anthocyanins fromLycium ruthenicumameliorated atherosclerosis by enriching gut commensal bacteria ofBifidobacterium,Lactobacillus,Roseburia,Akkermansia[16].In our previous work, a ganomycin I derivative was demonstrated to ameliorate obesity, hyperglycemia and hyperlipidemia through reversing gut microbiota dysbiosis [17].In another report, high molecular weight polysaccharides extract of the mushroomGanoderma lucidumhad anti-obesity effects through modulation of the composition of the gut microbiota [18].The ethanol extract ofGrifola frondosaimproved lipid metabolism by promoting the excretion of bile acids in the cecum and increasing the abundance of gut bacteria ofButyricimonas[19].These components derived from mushroom can improve the intestinal flora and MS, implying that mushroom was a good source for the development of natural products.
Polyphenols were very important class of natural antioxidants that are rich in fruits, vegetables and mushrooms, and were safein vivo[20-23].In addition, polyphenols were reported to have immunomodulatory [24], anti-inflammatory [25], hepatoprotective [26], antihypertensive [27], and hypoglycemic [28]activities.Recently, the intake benefits of polyphenols on lipid and glucose metabolism disorders have been causatively ascribed to their impact on gut microbiota [29,30].Sarcodon leucopus, belonging to theSarcodongenus in the Bankeraceae family, is a popular edible and medicinal mushroom in Tibet, China.In our early work, we reported tenp-terphenyl polyphenols including two new phenolic alkaloids, episarcoviolinβ(1) and sarcoviolinβ(3) from the ethyl acetate extract ofS.leucopus[31], which indicates potential health-promoting effects ofS.leucopus.However, up to date, thein vivobioactivities have not been investigated for this edible mushroom.In present study, we prepared a polyphenolic alkaloid-enriched extract (PAE) from the fruiting bodies ofS.leucopusand evaluated therapeutic effects of PAE on metabolic disorders in diet-induced obese (DIO) mice, moreover, elucidated its action mechanism associated with gut microbiota.Oral treatment with PAE significantly improved glucose and lipid metabolism and alleviated hepatic steatosis in DIO mice.These beneficial therapeutic effects are associated with the changes in the composition of gut microbiota, especially with the decrease of secondary bile acid producing bacteria in the family of Ruminococcaceae.These results confirmed the therapeutic potential of PAE as a functional food or prebiotics for the regulation of lipid and glucose metabolism.
2.Materials and methods
2.1 Materials
Fruiting bodies ofS.leucopusused in this study were collected from Lizhi County (Tibet, China) and identified by Professor Tiezheng Wei (Institute of Microbiology, CAS).The characteristic features of the pileus, stipe, context, and spores in the specimen were fully consistent with the morphological description forS.leucopus.The fungal specimen was deposited in Mycological Herbarium of Institute of Microbiology, Chinese Academy of Sciences (HMAS No.252750).
2.2 Preparation of the PAE from S.leucopus
Two hundred grams dried and crushed fruiting bodies ofS.leucopuswere extracted three times by anhydrous ethanol (2 L × 3) under ultrasonication at 20 °C for 30 min.The combined solvents were concentrated to dryness under vacuum and re-dissolved with 0.5 L of ethanol/water (70/30,m/m).The obtained solution was loaded onto a D-101 macroporous adsorption resin column (50 mm × 500 mm) and then eluted with distilled water, 20% ethanol in water, and 80% ethanol in water (each 2 L).The eluents from 80% ethanol in water was combined and dried under vacuum to give PAE.PAE was weighed and stored at -20 °C until use for animal experiments.With the standard compounds obtained in the previous report [31], the HPLC analysis of PAE was performed on YMC-pack ODS-A column (250 mm × 2.1 mm, 3 μm, each) eluted with 50% acetonitrile in water containing 0.1% trifluoroacetic acid.Column temperature was 35 °C, the flow rate was 1 mL/min and run time was 30 min.The injection volume was 10 μL.
2.3 Quantitative analysis of episarcodonin α (2) in PAE
Episarcodonin α (2) was purified from PAE by semi-preparative HPLC using the Kromasil C18column (250 mm × 10 mm, 5 μm, each) eluted with 50% acetonitrile in water containing 0.1% trifluoroacetic acid at the flow rate 2 mL/min (Rt.= 18.2 min; purity > 99%).Episarcodonin α (2) was dissolved in methanol and successively diluted to obtain concentrations in the range of 0.15-2.5 mg/mL.The dried PAE sample was dissolved in methanol to yield a solution of 0.40 mg/mL.The content of episarcodonin α (2) in PAE was calculated via the calibration curve.The standard curve of episarcodonin α (2) was obtained with a correlation coefficient (R2) greater than 0.9999 and a line equation ofy= 22 420x-1 042.4 in the concentration range from 0.15 mg/mL to 2.5 mg/mL, in whichxandyrepresented episarcodonin (2) concentration and the integral area under its spectrum curve respectively.
2.4 Animals and animal care
All animal protocols were approved by the Institute of Microbiology, Chinese Academy of Sciences (IMCAS) of Research Ethics Committee (permit APIMCAS2017023), and performed in accordance to the standards of the Department of Health and Human Services.C57BL/6J mice (8 weeks) were obtained from the Experimental Animal Center, Chinese Academy of Medical Sciences.Mice were housed in a room with controlled temperature at 20-25 °C and humidity of 40%-60% and a 12 h light/dark cycle with free access to normal water and chow.Mice were fed with a normal chow diet (NCD, 13.5% calories from fat) or a high-fat diet (HFD, 60 kcal% fat, 20 kcal% proteins and 20 kcal% carbohydrates, Cat.D12492i, Research Diet, New Brun-swick, NJ, USA).Body weight and blood glucose were measured weekly.
After 8 weeks, the body weight of high fat DIO mice was increased to about 20% of the normal chow diet mice.DIO mice were randomly divided into 4 groups with 10 mice in each group on the basis of the body weight and blood glucose as follows: the model group (DIO), 20.0 mg/kg PAE (PAE-H), 10.0 mg/kg PAE (PAE-L), 10.0 mg/kg acarbose positive group (ACAR).Ten C57BL/6J mice fed with NCD were treated with the vehicle solvent as the control group (CON).Body weight, free diet blood glucose was recorded weekly for seven weeks.Meanwhile, the feces of each group were collected after 4 weeks of administration and stored at -80 °C.At the end of the study, blood samples from the portal and cava veins was obtained after a 12 h fasting and centrifuged at 4 000 r/min for 15 min at 4 °C, then the plasma was frozen at -80°C.After exsanguination, mice were anesthetized by 10% chloral hydrate and sacrificed.The liver, cecum, and small intestines were dissected, washed, dried, and fixed with 4% paraformaldehyde or quickly frozen at -80°C.
2.5 Oral glucose tolerance test (OGTT) and insulin tolerance test (ITT)
Fifty mice in 5 groups were fasted for 12 h, and then orally administrated with glucose (2.0 g/kg) at 42th day for OGTT assay.For ITT assay, an intraperitoneal injection of insulin (0.4 IU/kg) was given to mice after a 12 h fasting at 46th day.Blood glucose was measured before glucose/insulin load and at 30, 60, and 120 min after loading with a glucometer (Accu Check, Roche, Switzerland).Blood samples were collected from the tip of the tail vein.The area under the curves (AUCs) generated from the OGTT or ITT collected data was calculated by Graphpad 6.0.
2.6 Biochemical analyses
Plasma and liver were obtained at the sacrifice of the mice.Total cholesterol (TC), triglycerides (TG), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), glutamic oxalacetic transaminase (AST), glutamicpyruvic transaminase (ALT), superoxide dismutase (SOD), and malondialdehyde (MDA) in plasma were measured by a commercial kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).Plasma insulin, glycated hemoglobin, tumor necrosis factor (TNF-α), interleukin (IL-6), interleukin-1β (IL-1β) concentrations were determined using a commercial ELISA kit (HuaBoDeYi, Beijing, China).Plasma lipopolysaccharide (LPS) quantification was determined using a commercial kit based on the use of a Limulus amaebocyte extracts (YEASEN, Shanghai, China).The insulin sensitivity index (ISI) was calculated from fasting blood insulin (FBI, in mU/L) and the values of fasting blood glucose (FBG, in mg/dL).ISI = 1/1000 (FBI × FBG).The 10% (m/V) liver homogenate prepared with physiological saline was centrifuged at 3 000 r/min for 10 min at 4 °C.The supernatant obtained was used for determination of hepatic TC, TG, LDL-C.Each procedure was performed according to the manufacturer’s protocols.
2.7 Histological analysis
The liver fixed with 4% paraformaldehyde for 24 h were embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin/eosin (H&E) [32].The small intestine tissues treated similarly was stained with alcian blue periodic acid Schiff (AB-PAS) as described previously [33].
2.8 Real-time qPCR analysis
Total RNA was extracted from ileum tissue using the TRIzol reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA).RNA concentration and quality were checked by running 1 μL of each sample using an Agilent 2100 Bioanalyzer (Agilent RNA 6000 Nano Kit, Agilent).cDNA synthesis was achieved using a cloned AMV first strand cDNA synthesis kit (Tiangen, Beijing, China).The primers used for real-time qPCR are given in Table 1.Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene to normalize the expression of the target genes.PCR amplification was performed using Perfecta SYBR green super mix (KAPA).
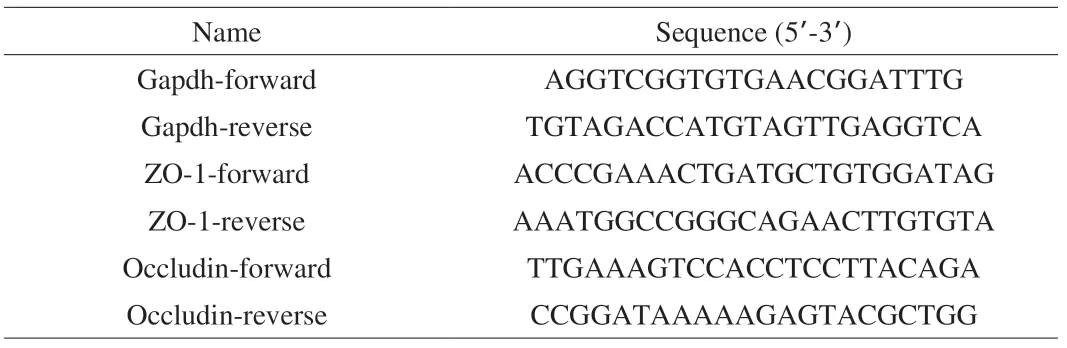
Table 1 Primers used in this study.
2.9 Gut microbiota analysis
DNA was extracted from about 100 mg of the cecal contents in the vehicle-treated and the PAE-treated DIO mice under sterile conditions.Then, the V3-V4 region of 16S rRNA was amplified using the primers 341F (CCTAYGGGRBGCASCAG) and 806R (GGACTACNNGGGTATCTAAT).The purified amplicons were constructed for single-end sequencing based on the IonS5TMXL sequencing platform.The reads were cut, filtered and then clustered into operational taxonomic units (OTUs) according to 97% similarity.The diversity index and the relative abundance of each bacterial taxon were calculated; the principal component analysis (PCoA) and the analysis with linear discriminant analysis effect size (LEfSe) were performed by previously reported methods [34-36].Raw 16S rRNA data was public on gcMeta [37]with project ID NMDC10011311 (https://gcmeta.wdcm.org/opendata/opendataDetail/66dbacb6345d11 ea92a7b49691092464/) which can be access through ftp://bio-mirror.im.ac.cn/public_data/d20200110_liuhongwei.
2.10 Bile acid analysis
One hundred milligram of feces were freeze-dried and dissolved in 1 mL methanol.The supernatants were obtained by centrifugation at 6 000 r/min for 15 min.The concentrations of total bile acids (TBAs) in feces were measured by a commercial kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).The supernatants obtained were detected on a Waters Alliance e2695 separation module with 2424 ELSD Detector using C8column (5 μm, 4.6 mm × 250 mm, Phenomenex Gemini) with a flow rate of 1.0 mL/min.Analysis condition: solvent A, MeCN; solvent B, water with 1% methane acid; linear gradient: 0-35 min, 25%-100% A in B.BAs were identified by comparing the retention time with that of standards.The levels of individual BAs were calculated from the peak area in the chromatogram relative to that of cholic acid in the vehicletreated DIO mice.
2.11 Statistical analysis.
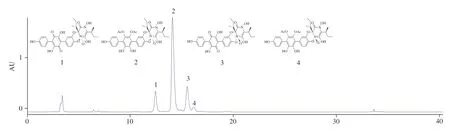
Fig.1 The high-performance liquid chromatography (HPLC) analysis of PAE.
Groups were compared by analysis of variance, followed by Tukey’s multiple comparison test with GraphPad Prism version 6.0 (GraphPad Software Inc., La Jolla, CA).Data were expressed as mean ± standard error of mean (SEM).Spearman’s correlation analyses were performed to identify relationships between variables using SPSS 22.0 software.Differences withPvalues < 0.05 were considered to be statistically significant.
3.Results and discussion
3.1 Preparation and detection of polyphenolic alkaloids in PAE
In this study, approximately 3.86 g PAE was obtained from 200 g of the dried fruiting bodies ofS.leucopus.The process of compound separation and structural analysis through1H-NMR,13C-NMR, and mass spectrometry have been published in previous studies [31].In this experiment, the inclusion of episarcoviolin β (1), episarcodonin α (2), sarcoviolin β (3), and sarcodonin α (4) in PAE was determined by comparing the standard with the HPLC of PAE (Fig.1).Moreover, the 4 compounds were stable at 100 °C for 30 min.The content of episarcodonin α (2) in PAE was determined to be 542.8 mg/g.
3.2 PAE treatment reduced HFD-induced obesity, hyperglycemia and insulin resistance
DIO mice were used to evaluate the hypoglycemic and hypolipidemic effects of PAE.Notably, oral administration of PAE for 7 weeks significantly decreased body weight in DIO mice (Fig.2A, 2B).The mean body weight of PAE-H group was decreased to (30.73 ± 1.28) g (Fig.2B), corresponding to a difference of 3.10 g or 9.16% of body weight (P< 0.05) from that of model group.Oral administration of PAE did not influence the total food intake of DIO mice, whereas acarbose significantly decreased the total food intake (Fig.2C).It was also observed that PAE significantly decreased the levels of HbA1c, insulin and blood glucose (Fig.2D-2H).Treatment with PAE at 20 mg/kg and 10 mg/kg achieved an elevation of 51.10% and 40.16% in ISI, respectively, as compared to the model group (Fig.2F).Treatment with PAE at 20 mg/kg exhibited a comparable effect on glucose tolerance and insulin resistance to that of acarbose.Results of the OGTT and ITT, and the corresponding area under curve (AUC) further confirmed that PAE treatment notably ameliorated the impaired glucose tolerance and insulin resistance in DIO mice (Fig.2I-2L).
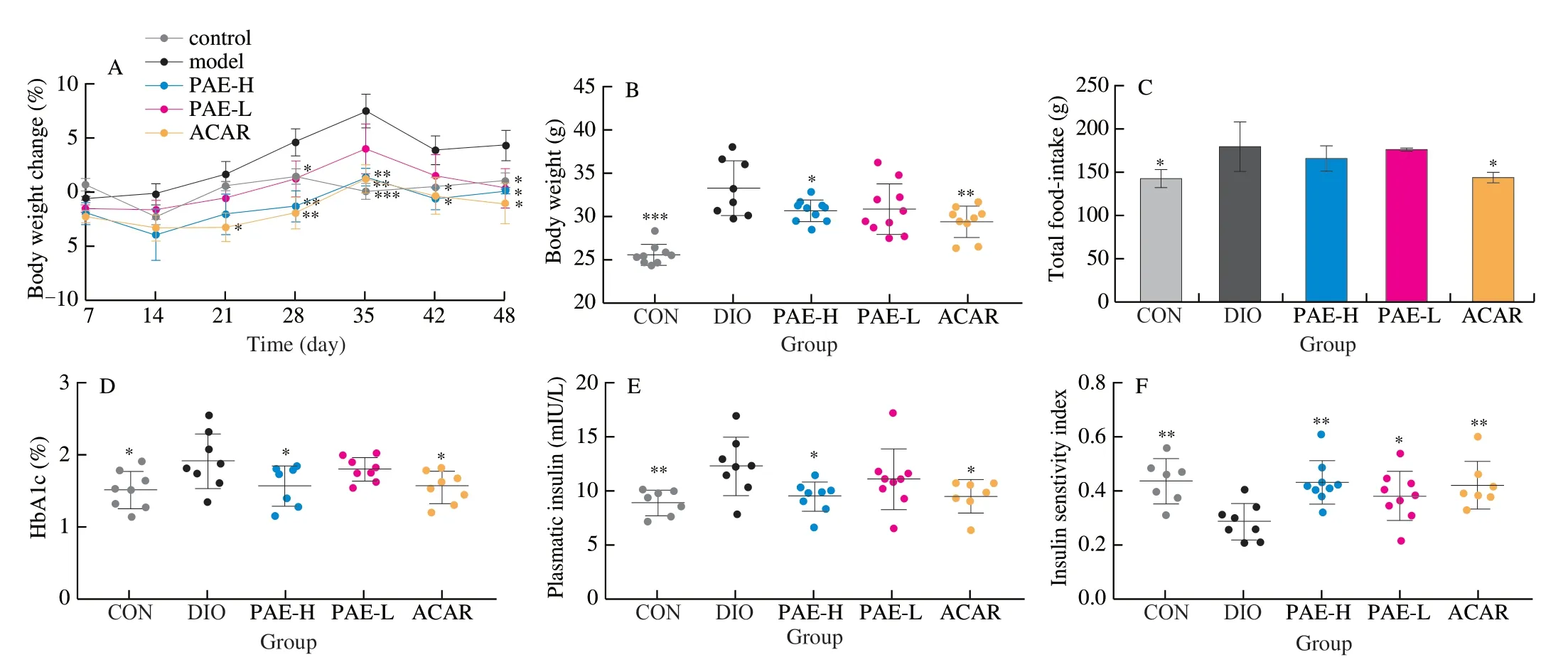
Fig.2 Effected of PAE on body weight, blood glucose level and insulin resistance.(A) Body weight change (%); (B) Body weight after 48 days of treatment; (C) Total food intake after 48 days of treatment; (D) HbA1c (%) in DIO mice; (E) Plasma insulin; (F) Insulin sensitivity index (ISI) ; (G) Free diet glucose after 48 days of treatment; (H) Fasted diet glucose after 48 days of treatment; (I) OGTT and (J) AUC after 42 days of treatment; (K) ITT and (L) AUC after 46 days of treatment.Data are presented as the mean ± standard error of the means (SEM).n = 7-10 mice per group.Statistical analysis was done using one-way ANOVA followed by the Tukey post hoc test for B, J, L and two-way ANOVA followed by the Bonferroni post hoc test for A, I, K.*P < 0.05; **P < 0.01; ***P < 0.001 vs DIO.
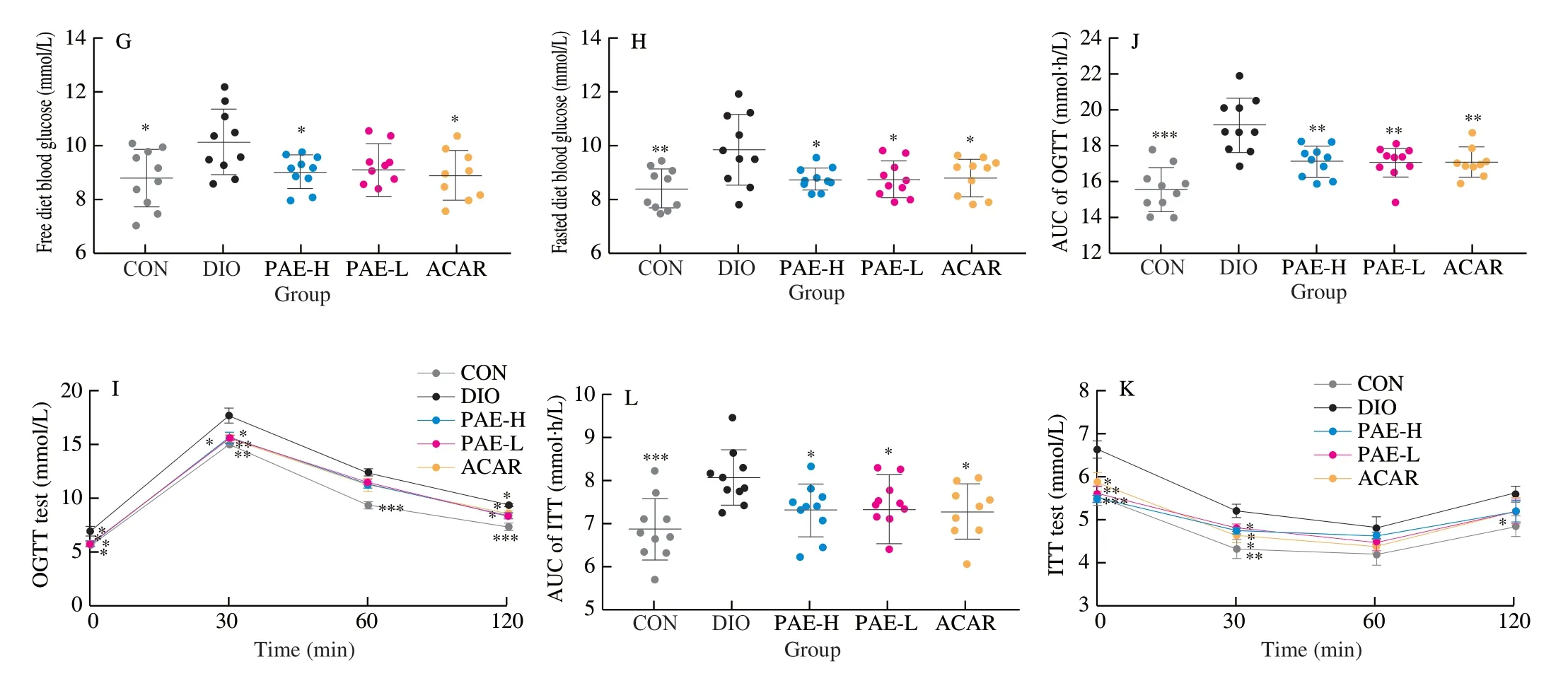
Fig.2 (Continued)
3.3 PAE reduced hyperlipidemia and hepatic steatosis
Comparing with the normal chow diet mice, DIO mice showed that the plasma TG, TC and LDL-C levels significant increased by 1.33, 1.59, and 2.76 times, respectively, indicating the occurrence of hyperlipidemia by long-term intake of high fat diet.Oral administration of PAE at 20 mg/kg greatly inhibited the HFDinduced increase of plasma TG, TC and LDL-C levels (Fig.3A, 3B).Specifically, the levels of the plasma TG and TC were reversed to the normal state as detected in the control group.Treatment with PAE at 10 mg/kg reduced the levels of plasma LDL-C, but there was no significant difference.
Long-term intake of HFD usually causes accumulation of hepatic lipid, inducing fatty liver, especially non-alcoholic fatty liver disease [38].In this study, the hepatic lipid dysbiosis and live injuries were observed in the model group.Daily treatment with PAE at 20 mg/kg for 7 weeks significantly reduced the levels of TG, TC, and LDL-C in the livers of DIO mice (Fig.3C, 3D).Treatment with PAE at 20 mg/kg notably reduced the levels of plasma ALT and AST by 30.65% and 16.41% and increased the level of plasma SOD while had no effect on MDA, as compared with that of the model group (Fig.3E-3H).SOD can affect intestinal redox state and intestinal flora distribution by reducing oxygen free radicals, thereby improving liver function and hyperlipidemia [25].Furthermore, histopathological observation of H&E staining was performed to confirm the improvements on liver damages by PAE.Compared with the accumulation of fat droplets in the liver of the DIO mice, DIO mice treated with PAE showed less cytoplasmic vacuolation, legible cell boundaries, a clear nucleolus, and a visible nucleus (Fig.3I).All above results indicated that oral administration of PAE ameliorated lipid metabolism, hepatic steatosis and liver injury in DIO mice.
3.4 PAE alleviated inflammation and maintained intestinal integrity
It has been demonstrated that long-term intake of HFD can cause a gut microbiota dysbiosis featured with the increase in conditioned pathogens and the damages on the integrity and permeability of the intestinal wall.These alterations promote the infiltration of the gut bacteria-derived LPS into the bloodstream, and thus induce chronic low-grade inflammation.Chronic inflammation has been proven to be a critical factor involved in the development of obesity and obesityrelated dysfunctions [39,40].Goblet cells in the intestine tract play an important role in maintaining the intact mucous layers and separating the gut bacteria.The loss and defects of goblet cells is closely related to inflammatory bowel disease [41].In this study, we determined the number of goblet cells in the gut tract by histological analysis of the small intestine tissues stained with alcian blue periodic acid schiff.As a result, oral treatment with PAE and acarbose effectively prevented the reduction of goblet cells in the gut tract and largely maintained the integrity of intestinal wall (Fig.4A, 4B).Next, we analyzed the mRNA expression levels of tight junction proteins (ZO-1andOccludin) and LPS-related response in the PAE-treated (20 mg/kg) mice that showed significant improvement on gut wall of DIO mice.In comparison with that of DIO mice, the expression levels ofZO-1andOccludinwere enhanced (Fig.4C, 4D); and the levels of endotoxin LPS as well as inflammatory mediators TNF-α and IL-6 were decreased (Fig.4E-4G).However, the level of IL-1β remained unchanged after PAE treatment (Fig.4H).Based on these results, we concluded that oral treatment with PAE protected the intestinal wall of mice from damaging due to high fat diet consumption.
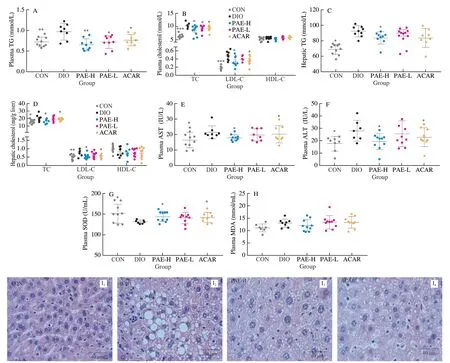
Fig.3 PAE regulated lipid metabolism, hepatic steatosis.(A) Plasma triglyceride; (B) Plasma total cholesterol, LDL-C, and HDL-C; (C) Hepatic triglyceride; (D) Hepatic total cholesterol, LDL-C, and HDL-C; (E) Plasma AST; (F) Plasma ALT; (G) Plasma SOD; (H) Plasma MDA; (I) Representative images of H&E staining of the liver (n = 3 mice per group).Scale bars, 40 μm. Data are presented as the mean ± standard error of the mean (SEM).n = 7-10 mice per group.Statistical analysis was done using one-way ANOVA followed by the Tukey post hoc test.*P < 0.05; **P < 0.01; ***P < 0.001 vs DIO.
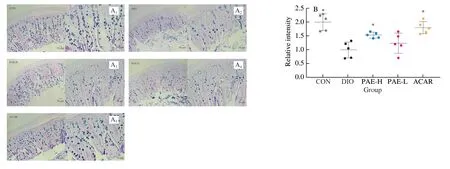
Fig.4 PAE alleviated inflammation and maintained intestinal integrity.(A) Representative images and distribution of goblet cells in small intestine through ABPAS.Scale bars, 50 μm and 20 μm.(B) Relative densities of goblet cells in the mice small intestine (n = 5 mice per group); the relative mRNA expression level of ZO-1 (C) and Occludin (D) in ileum; (E) Plasma LPS; (F) Plasma TNF-α; (G) Plasma IL-6; (H) Plasma IL-1β; Data are presented as the mean ± standard error of the means (SEM).n = 5-8 mice per group.Statistical analysis was done using one-way ANOVA followed by the Tukey post hoc test.*P < 0.05; **P < 0.01; ***P < 0.001 vs DIO.
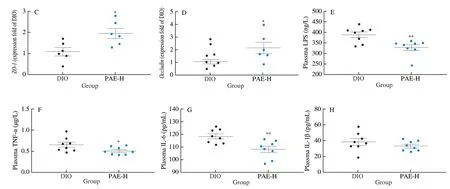
Fig.4 (Continued)
3.5 Effected of PAE on gut microbiota
The amelioration of the integrity and permeability of gut wall in DIO mice is inevitably correlated with the changes in the composition of gut microbiota.Next, sequencing the V3-V4 regions of the cecal bacterial 16S rRNA gene was performed to investigate the gut microbiome of DIO mice administrated with PAE at the dose of 20 mg/kg.In total, 575 OTUs were detected from IonS5TMXL sequence reads.Theβdiversity was calculated by the unweighted UniFrac distances-based PCoA, which revealed a significant microbial structural difference between PAE-H group and the DIO group (Fig.5A).Further analysis with linear discriminant analysis (LDA) effect size (LEfSe) revealed a significant decrease in the relative abundance of Ruminococcaceae(Fig.5B-5E) in the PAE-treated mice.At genus level, treatment with PAE at 20 mg/kg significantly reduced the relative abundance ofIntestinimonas,Anaerotruncus,Butyricicoccus,Papillibacter,Harryflintia, andAngelakisellain the family of Ruminococcaceae, in comparison with that in the vehicle-treated DIO mice.(Fig.5F).The abundance of the family ofRuminococcaceae was increased in obese patients whose diet was rich in saturated fats and simple sugars as well as in patients with irritable bowel syndrome [42,43].The relative abundance ofAnaerotruncuswas found to be casually correlated with obesity and its associated metabolic disorders in early studies [44,45].
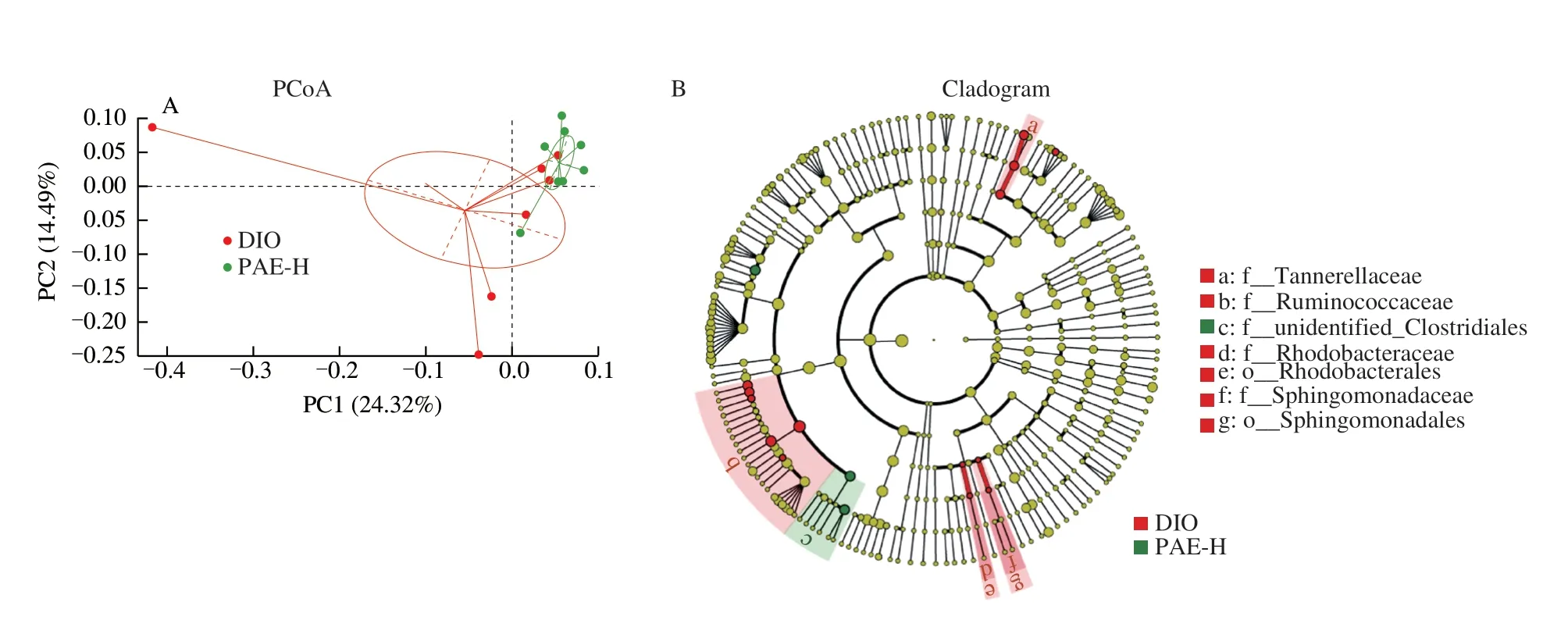
Fig.5 Effected of PAE on gut microbiota composition.(A) Principal component analysis based on OUT abundance; (B, C) Taxonomic cladogram generated from LEfSe analysis of 16S rRNA gene sequences.Each circle’s size is proportional to the taxon’s abundance.Red color represents enriched taxa in the DIO group; Green color represents enriched taxa in the PAE-H treated group; (D, E) Comparison of the taxonomic abundance among the indicated groups; (F) The relative abundance of the bacterial genus detected in Ruminococcaceae fecal samples; (G) The bacterial genera in Ruminococcaceae were correlated with clinical indices in the DIO and PAE groups.The color is scaled with the correlation coefficients, positive correlation is red, and negative correlation is blue.Data are presented as the mean ± standard error of the mean (SEM).n = 8 mice per group.Statistical analysis was done using one-way ANOVA followed by the Tukey post hoc test.*P
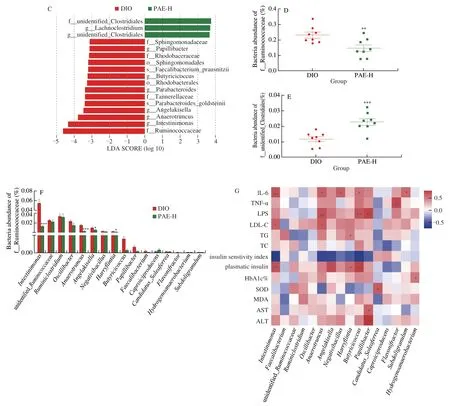
Fig.5 (Continued)
To further investigate the relationship between the gut bacteria in the family of Ruminococcaceae and therapeutic effects of PAE, a Spearman’s correlation analysis was performed.As shown in Fig.5G,AnaerotruncusandIntestinimonasshowed a significantly positive correlation with the level of LDL-C.The relative abundance ofAnaerotruncus,Butyricicoccus, andPapillibacterwere positively correlated with the production of LPS.Intestinimonas,Anaerotruncus,Negativibacillus,Butyricicoccus, andSubdoligranulumhave positively relationship with the level of IL-6.Anaerotruncus,Angelakisella,Butyricicoccus, andIntestinimonasexhibit significantly negative correlation with insulin sensitivity index.The abundance ofPapillibacterwas also significantly positively correlated to AST and ALT.Although not significant, a negative relationship between the level of SOD andAnaerotruncusandHarryflintiawas identified.Therefore, we deduced that the beneficial efficacies of PAE on HFDinduced obesity and its related complications were closely associated with the modulation of gut microbiota.
3.6 Effected of PAE on secondary bile acids
Secondary bile acids synthesized in the gut have important physiological activities in regulating the glucose and lipid metabolism.Higher levels of deoxycholic acid (DCA) and lithocholic acid (LCA) induced by HFD contribute to obesity-associated insulin resistance, disruption on intestinal epithelial integrity due to its hydrophobic nature, and damages on DNA through producing reactive oxygen species [46-48].Ruminococcaceae, especially the genus ofIntestinimonasandOscillibacter, are well known for secreting 7α-dehydroxylase [49-51]that catalyzes the key step in the transformation of primary bile acids into secondary bile acids.In cholesterol gallstone patients, it has been found that the increased 7α-dehydroxylating bacteria in gut is correlated with the increase of DCA [52].In this study, the decrease of gut bacteria in the family of Ruminococcaceae by PAE may result in reduction of DCA and LCA.As expected, the level of the total bile acids, CA, DCA and LCA in the feces of PAE-treated DIO mice were much lower than that of vehicle-treated DIO mice (Fig.6A, 6B), which causatively benefited for the maintenance of intestinal integrity and the reduction of inflammation.All these evidences confirmed a gut microbiota related mechanism for the anti-metabolic syndrome effects of the polyphenolic alkaloids from the edible mushroomS.leucopus.
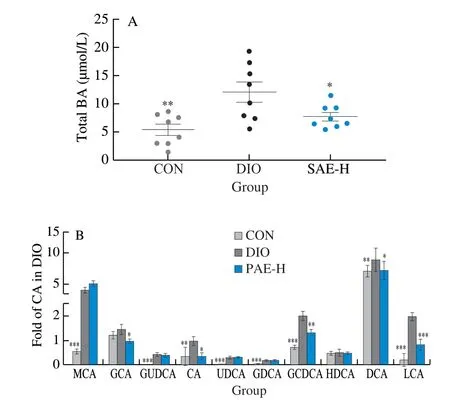
Fig.6 Effected of PAE on bile acid metabolism.(A) The total BA concentration in fecal sample; (B) Levels of BAs in fecal sample.MCA, muricholic acid; GCA, glycocholic acid; GUDCA, glycoursodeoxycholic acid; CA, cholic acid; UDCA, ursodeoxycholic acid; GDCA, glycodeoxycholic acid; GCDCA, glycochenodeoxycholic acid; HDCA, hyodeoxycholic acid; DCA, deoxycholic acid; LCA, lithocholic acid.Data are presented as the mean ± standard error of the mean (SEM). n = 8 mice per group.Statistical analysis was done using one-way ANOVA followed by the Tukey post hoc test.*P < 0.05; **P < 0.01; ***P < 0.001 vs DIO.
4.Conclusion
In summary, we prepared a PAE from the fruiting body of a wild edible mushroomS.leucopusand investigated its therapeutic effects on metabolic disorders.Oral treatment with PAE significantly improved glucose and lipid metabolism, reduced the inflammation and hepatic steatosis, and altered the gut microbiota in the DIO mice.The significantly decreasedIntestinimonasandOscillibacterin the family of Ruminococcaceae that be responsible for the production of the secondary bile acids (DCA and LCA) in gut were found to be causally associated with the improvement of obesity-related disorders.The current study lays a foundation for the application of PAE fromS.leucopusas functional foods or prebiotics.
Declaration of Competing Interest
The authors declare no competing financial interest.
Acknowledgments
This study was supported by the Project of Science and Technology Department of Tibet (XZ201901-GB-19).
杂志排行
食品科学与人类健康(英文)的其它文章
- Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia
- Phylogenetic analysis and protective effects of thymol and its chromatographic fractions from a novel wild mushroom in combating oxidative stress
- Immunomodulatory effects of polysaccharides from edible fungus: a review
- Advances in research on chemical constituents and pharmacological effects of Paecilomyces hepiali
- Healthy function and high valued utilization of edible fungi
- Antrodia Cinnamomea ameliorates neointimal formation by inhibiting infl ammatory cell infi ltration through downregulation of adhesion molecule expression in vitro and in vivo
