Immunomodulatory effects of polysaccharides from edible fungus: a review
2021-05-24ZhenghuaYinZhenhuaLiangChangqinLiJinmeiWangChangyangMaWenyiKang
Zhenghua Yin, Zhenhua Liang, Changqin Li, Jinmei Wang, Changyang Ma, Wenyi Kang,*
a National R & D Center for Edible Fungus Processing Technology, Henan University, Kaifeng, Henan 475004, China
b Joint International Research Laboratory of Food & Medicine Resource Function, Kaifeng, Henan 475004, China
c Functional Food Engineering Technology Research Center, Kaifeng, Henan 475004, China
Keywords:Edible fungus Polysaccharides Immunomodulatory effect Mechanisms-immunomodulatory relationship
ABSTRACT The polysaccharides from edible fungus showed many kinds of biological activities, including anti-tumor, immunomodulatory, anti-inflammatory, anti-diabetes, improving functional constipation activities.In particular, the immunomodulatory effects have been paid more and more attention by scholars, but there was no systematic introduction of their immunomodulatory mechanism.So, this review introduced the immunomodulatory effects and mechanism of edible fungus polysaccharides in recent years, and then the relationships between structure and immunomodulatory effect were also discussed.
1.Introduction
Immune response, as an important physiological process, can identify and destroy foreign harmful substances or organisms to protect disease, in which macrophages play significant roles in phagocytosis, cytotoxicity and intracellular killing activities [1].Once activated, macrophages can resist pathogens either directly through phagocytosis or indirectly by producing related factor, including nitric oxide (NO), interleukins (IL), tumor necrosis factor-α (TNF-α) and reactive oxygen species (ROS).The mechanism of action is related to mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) via pattern recognition receptors (PRRs) [2].In addition, it can play the role of immune regulation through oxidative stress and lymphocytes.At present, the edible fungus have attracted increasing attention due to their nutritional and medicinal values and their bioactive components, especially their immunomodulatory activity.
Edible fungus, also known as mushrooms, are found around 15 000 species all over the world, of which 650 species have been reported to have medicinal value [3].Mushrooms have attracted more and more attention because of nutritional and medicinal values and rich bioactive components [4].Mushrooms contained many bioactive components, including polysaccharides, active proteins, glycosides, alkaloids, volatile oils, phenols, ketones and organic acids, which showed immune regulation, anti-tumor, anti-hypoglycemia activities [5].Polysaccharides, as a major bioactive substance of mushroom, showed anti-tumor, anti-inflammatory, immunomodulatory, antidiabetes, improving functional constipation activities [6-10], the immunomodulatory activities have been attracting increasing attention.
In this review, the mechanisms of immunomodulatory effects of polysaccharides from edible fungus were summarized.In addition, the relationships between structure and immunomodulatory activities were also discussed.
2.Immunomodulatory mechanisms of polysaccharides from edible fungus
2.1 Effects of polysaccharides on cytokines and other related molecules
Macrophages play important roles against microbial infections in the host defense system, which produce various inflammatory mediators, cytokines, and phagocytic activities to attack invaders, such as bacteria or viruses [11].Macrophages are in the static state under normal circumstances.But activated macrophages enhance the production of mediators, like NO, ROS, TNF-α, IL-1β and IL-6 [12].However, excessive activation of macrophages leads to the production of pro-inflammatory factors, which can cause damage to living organisms [13].Among them, NO is an important intracellular signal molecule that mediates a series of host defense functions of activated macrophages.At the same time, it is also a cytotoxic agent to regulate immune response [14].The effects of pro-inflammatory factors TNF-α and IL-1β on macrophages can enhance the immune response and induce the expression of other immunomodulatory factors.IL-6 has effects on phagocytosis, antigen presentation and inflammation regulation, and provides the third signal to T cells to regulates cellular and humoral immunity [15].IL-10, as a major anti-inflammatory cytokine, shows immunosuppressive effect [16].
Edible fungus polysaccharides play immunomodulatory roles by regulating cytokines and other factors in RAW264.7 macrophage cells.For example, the polysaccharides fromTremella fuciformisstimulated the production of NO, IL-6, IL-1β and TNF-αby increasing mRNA expressions of inducible nitric oxide synthase (iNOS),IL-6,IL-1βandTNF-α[17].Two polysaccharides FVPB1 and FVP2 isolated fromFlammulina velutipesincreased the concentrations of NO, TNF-α, IL-6 or IFN-γ [18,19].Polysaccharide PIP2-1 that extracted fromPaxillus involutussignificantly increased the releases of TNF-α and IL-6 [20].Polysaccharide LEPs fromLentinula edodesinduced the secretions of NO, TNF-α and IL-6 in RAW264.7 cells [21].Polysaccharide IP3a isolated fromInonotus obliquuspromoted the secretions of IL-2, IL-6, IL-12 and TNF-α [22].Polysaccharide GP11 that isolated fromGrifola frondosapromoted the productions of NO, TNF-α and IL-1β by stimulating mRNA expression ofiNOSandTNF-α[23].Polysaccharide GFP isolated fromG.frondosafruiting body increased the levels of TNF-α, IL-6 and IFN-γ, and also increased the levels of MIP-1β, MIP-1α and MIP-2 [24].In addition, Se-rich polysaccharide (Se-GP11) that isolated from Se-enrichedG.frondosaincreased the relatively TNF-α and IL-2 levels, and promoted NO production [25].Two polysaccharides (CEPSN-1 and CEPSN-2) isolated fromAuricularia auricula-judaepromoted releases of NO, TNF-α, IL-6 and IL-10 [26].
Aβ-D-glucan H6PC20 obtained fromHercium erinaceusenhanced the expression of IL-6, TNF-α and IL-1β that secreted by THP-1 macrophages [27].In addition, polysaccharide HEP-S increased the secretion of IL-2, IL-4 and IFN-γ in spleen lymphocytes [28].Ganoderma lucidumpolysaccharides GL-PS increased the concentration of serum IL-2, TNF-α and IFN-γ in rats [29].Table 1 summarizes the effects of polysaccharides on cytokines and other related molecules.
2.2 Effects of polysaccharides on MAPK and NF-κB signal pathways
The NF-κB transcription factors control the activities of genes related to apoptosis, cell senescence, immunity and inflammation.As dimmers, NF-κB is present in the cytoplasm and isolated by NF-κB inhibitor protein α (IκB-α).Under stimulation, IκB-α protein undergoes a series of phosphorylated, ubiquitinated, and degraded, thus releasing NF-κB dimers to activate the transcription of their target genes [30,31].MAPKs, including extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38, play key roles in the regulating cell growth, differentiation and cell responses to cytokines and stresses [32].MAPKs also play roles for NF-κB transcriptional activation.
Edible fungus polysaccharides regulated cytokines and other factors via NF-κB and MAPKs signal pathways.Polysaccharide FVPB2 fromF.velutipessignificantly increased production of IL-10 through the ERK1/2 and NF-κB signaling pathways [33].Polysaccharide EPA-1 fromPleurotus eryngiisignificantly induced the releases of NO, IL-6, TNF-α and IL-1 by NF-κB/MAPK signaling pathways [34].HEP enhanced innate immune responses via macrophage phagocytosis and NK cell activity in mice and promoted adaptive immune responses via enhancing cell-mediated and humoral immunity, upregulated secretory immunoglobulin A (SIgA) secretion in the lamina propria, which was related to MAPK and protein kinase B (AKT) signaling pathways [35].PSG-1 enhanced the levels of IL-2, IFN-γand IL-12 via Ca2+/CaN and MAPK pathways [36].A glucogalactomanan was obtained fromAgaricus bisporus, and could activate ERK/MAPK and IκB/NF-κB pathways via increasing ERK1/2 and IκB-αphosphorylation levels, which increased the production of NO and promoted the expression of IL-6, IL-1β and TNF-α, iNOS and cyclooxygenase-2 (COX-2)[37].Cordyceps militarispolysaccharide CMP-III could increase macrophage phagocytosis and secretion of factors via MAPKs/NF-κB signaling pathways [38].Table 2 summarizes the effects of polysaccharides on MAPK and NF-κB signal pathways.
2.3 Effects of polysaccharides on oxidative stress
Oxidative stress is related to abnormal immune response [39].Based on the oxidation-inflammation theory, because oxidants are inflammatory factors, excessive production of oxidants cause inflammation response.Oxidative stress lead to decrease of immune cells function [40].
Some polysaccharides enhanced immune function by protecting the body from oxidative stress.In the spleen and thymus immunosuppressed mice,G.atrumpolysaccharide PSG-1 improved immune dysfunction by regulating the production of ROS and apoptosis [41].C.militarispolysaccharides (CMP) improved the immune function in mice, which was related to protection against oxidative stress [42].
The nuclear factor-erythroid 2-related factor 2 (Nrf2) signaling pathway participated in regulating inflammation and immune response.Nrf2 regulated antioxidant and eliminated excess ROS [43].Wang et al.[44]proved thatSarcodon imbricatuspolysaccharide SIPS acted on Nrf2-mediated oxidative stress to effectively reverse cyclophosphamide (CTX)-induced immunosuppression.The relevant mechanism is shown in Fig.1.
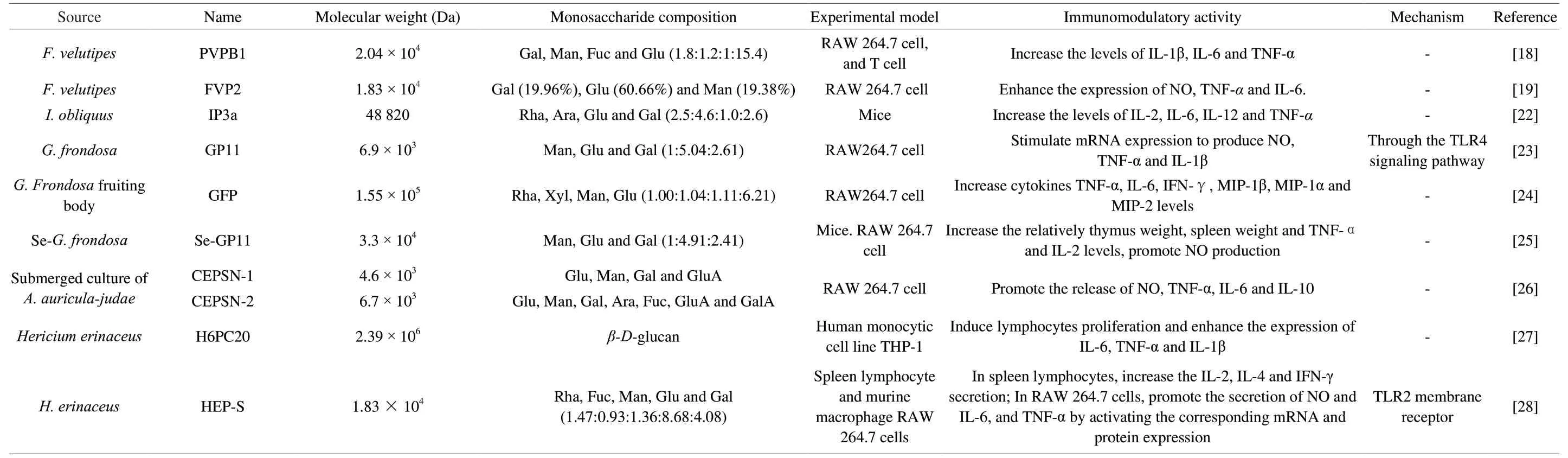
Table 1 The effects of polysaccharides on cytokines and other related molecules.

Table 2 The effects of polysaccharides on MAPK and NF-κB signal pathways.
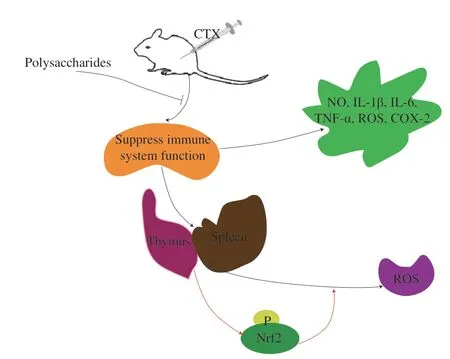
Fig.1 The relevant mechanism of polysaccharides from edible fungus on oxidative stress.
2.4 Effects of polysaccharides on PRRs
In most cases, macromolecular polysaccharides cannot enter cells directly because of their large molecular mass, but polysaccharides can recognize PRRs to enter cells [45].PRRs, includingβ-glucan receptor, dectin-1 receptor, mannose receptor (MR), complement receptor type 3 (CR-3) and toll-like receptors (TLRs), activate macrophages by binding of polysaccharides [46].TLRs are one kind of important PRRs in macrophages.Among them, TLR2 and TLR4 are involved in the recognition of a wide range of pathogen associated molecular patterns [47].
Edible fungus polysaccharides could activate macrophages via PRRs that activating NF-κB, MAPKs and other signal pathways, and further to regulate cytokines and other factors.For example, HEP-S activated the corresponding mRNA and protein expression to release NO and IL-6, and TNF-α, which involved TLR2 membrane receptor [38].Amillariella melleapolysaccharide AAMP-A70 increased the secretions of NO, ROS, TNF-α, IL-6 and IL-1β by activating macrophages via NF-κB/MAPK signaling pathways and TLR2 receptor [48].CMPB90-1 activated TLR2, MAPK/NF-κB pathways, which promoted lymphocyte to secrete NO, TNF-α and IL-2, upregulated T-cell subsets, enhanced phagocytosis of macrophages and induced M1 [49].GP11 acted on TLR-4 pathway to stimulate the releases and mRNA expression ofNOand inflammatory factors [23].Agaricus brasiliensispolysaccharides stimulated TLR4/myeloid differentiation 88 (MyD88) and TLR4/TIR-domain-containing adapter-inducing interferon-β (TRIF) pathways to induce the secretions of inflammatory factors and COX-2 [50].Polysaccharide DIP fromDictyophora indusiatainduced NO, IL-1β, IL-6 and TNF-α production by increasing mRNA expression levels of related factors via TLR4/NF-κB signaling pathways [51].G.frondosapolysaccharide (GFPS) activated macrophages via TLR4-MyD88-inhibitor kappa B kinase β (IKKβ)-NF-κB p65 signaling pathways to induce the release of NO, and increase the levels of IL-10, IL-1β, granulocytecolony stimulating factor (G-CSF), monocyte chemotactic protein (MCP)-3 and interferon inducible protein-10 (IP-10) in RAW264.7 cells [52].Polysaccharide CRP isolated fromCollybia radicataactivated murine macrophages through TLR4, and induced release of NO, iNOS and inflammatory factors [53].Glucogalactan TLH-3 fromTricholoma lobayenseincreased secretions of NO, IL-6 and TNF-α by activating the IκB-α-NF-κB pathways via TLR-4 [54,55].Polysaccharide F2isolated fromSchizophyllum communeactivated NF-κB/MAPK pathways via acting on CR-3 and TLR-4, furthermore, a large amount of NO and other cytokines were produced by increasing the expression levels of related mRNA [56].The relevant mechanism is shown in Fig.2.Table 3 reports a summary of effects of polysaccharides on PRRs.
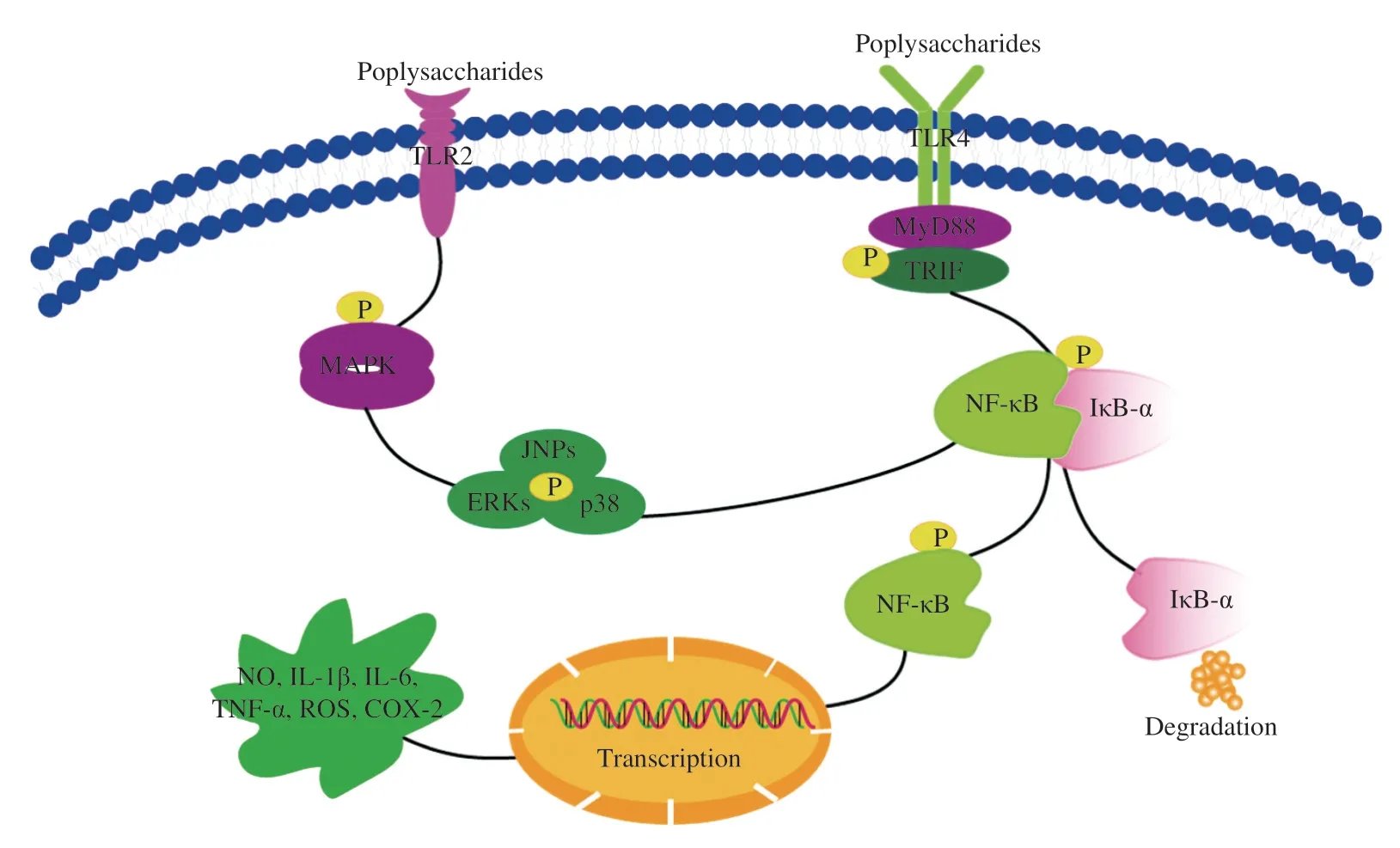
Fig.2 The NF-κB and MAPK signal pathways via TLR-2 and TLR-4.
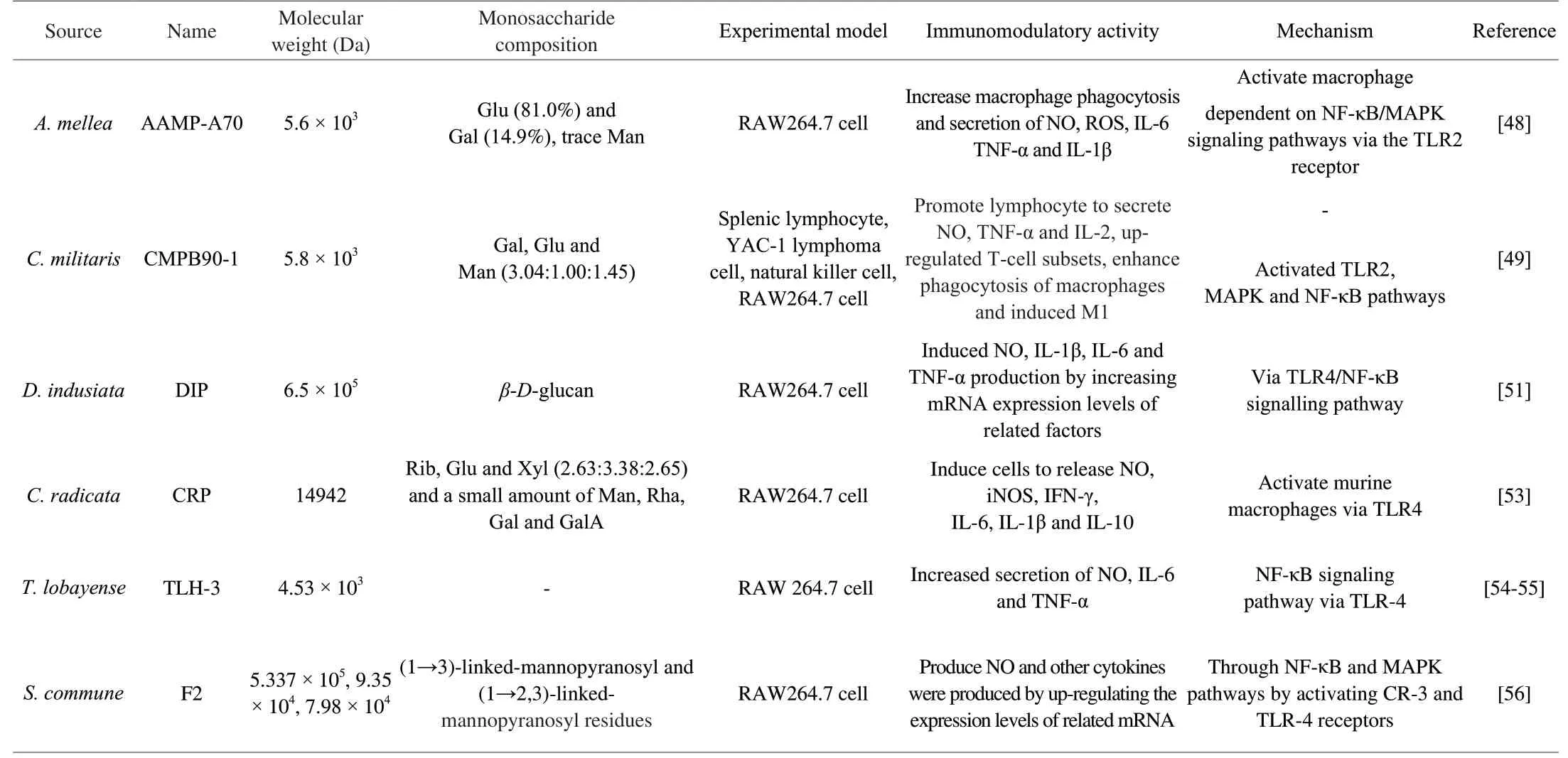
Table 3 The effects of polysaccharides on PRRs.
2.5 Effects of polysaccharides on intestinal microflora
Intestinal microflora has important effect on immune system development and immune function regulation [57].Disorder of intestinal microflora affects human health [58].Xu et al.[59]demonstrated that polysaccharide L2 derived fromL.edodesaltered intestinal microbiota spatial structure in mice.Polysaccharide PEP isolated fromP.eryngiicould alter fecal microbiota composition and regulate the immune response of the host [60].FVP2 also impacted on gut microbiota in rats [19].
2.6 Effects of polysaccharides on immune system
Edible fungus polysaccharides had the effect on immune system.For example, Yu et al.[61]found that PSG-1 activated spleen lymphocytes via the Ca2+/CaN/nuclear factor of activated T cells (NFAT)/IL-2 and protein kinase C (PKC)/NFAT/IL-2 signaling pathways.Lentinan, tremellan, and pachymaran polysaccharides increased index of thymus in CXT-induced immunosuppression mice, increased the levels of serum antibody immunoglobulin (Ig) G and IgM [62].GFP-A fromG.frondosaincreased thymus index and spleen index, and lactate dehydrogenase (LDH) and acid phosphatase (ACP) levels in spleen, the mRNA levels of inflammatory factors in spleen.In addition, it also increased CD4+and CD8+splenic T lymphocytes expression [63].For CTX-treated mice, GFP-22 enhanced immune organ index, splenocyte proliferation and cytokines levels in splenocytes, further improved immunosuppression [64].
CMPB90-1 significantly enhanced the proliferation of lymphocytes,increased the activation potential of T cell and B cell, and enhanced humoral and cellular immunity, also enhanced the killing activity of splenocytes NK cells.In the presence of CMPB90-1, the percentage of CD4+and the ratio of CD4+/CD8+T lymphocytes increased [49].GLP-1 isolated fromG.lucidumprotected spleen and thymus and promoted hematopoiesis and improving the level of IgA in serum [65].Table 4 reports a summary of effects of polysaccharides on immune system.
3.Structure-immunomodulatory activity relationship
The immunomodulatory activities of polysaccharides areaffected by their molecular weight, monosaccharide composition,α/β-configuration, conformation, glycosidic linkage, branching degree, length of side chains, and so on.
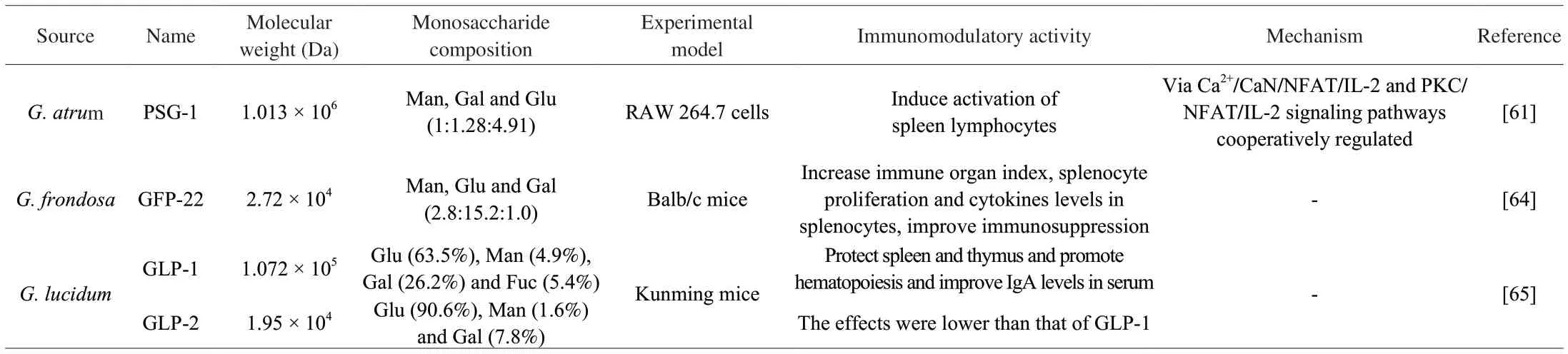
Table 4 A summary of effects of polysaccharides on immune system.
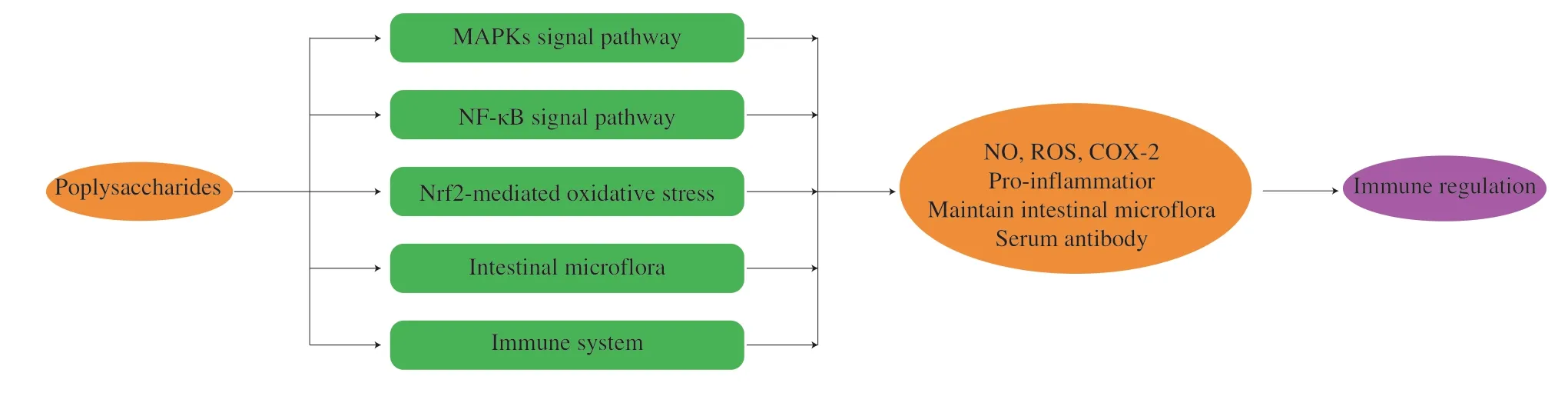
Fig.3 The immunomodulatory of polysaccharides from edible fungus.
The chain conformation of polysaccharides affected the immunomodulatory activity.Polysaccharides have different chain conformations in solution, including random crimping, double or triple strands, rod-like and spherical [66].Among them, the activity of polysaccharides is higher at the triple helix state [67,68].In addition, Li et al.[65]found that polysaccharide GLP-1 was aD-galactoglucan and had flexible random linear conformation.Homogeneousβ-D-glucan GLP-2 was a spherical conformation, but GLP-1 had better protective effect on the spleen and thymus and promoted hematopoiesis and improved the level of IgA in serum.
At the same time, conformation changed with the variation of molecular weight.Generally, molecular weight has been recognized as a critical parameter of immunomodulatory activity [69,70].As can be seen in Table 2, polysaccharides with immunomodulatory activity were isolated from edible fungus, the molecular weight of most polysaccharides is more than 104Da, and some are even higher than 107Da, but there are also some polysaccharides whose molecular weights are less than 104Da.
In addition, polysaccharides and polysaccharide-protein complexes showed different immunomodulatory activities.For example, Liu et al.[71,72]found that a polysaccharide-protein complex (PRW) obtained from sclerotia ofPolyporus rhinoceroswas more potent immunomodulatory activities on human monocytes THP-1 and bone marrow dendritic cells than that of an alkali-soluble polysaccharide (mPRSon), high MW and DB mushroom sclerotial polysaccharide rich in hetero-glycan and protein have stronger immunopotent than that of pure homoglycan.
The types and positions of glycosidic linkages had effect on immunomodulatory activities of polysaccharides.Previous studies demonstrated someα-glucans was composed of high (1→6)-α-branched (1→4)-α-glucan, which showed immunomodulatory activity [73].For example, Zhang et al.[26]provedA.auricula-judaeexopolysaccharides with (1→4)-α-D-glucan as backbone had potential immunomodulatory effect.In addition,β-(1→3), (1→6) glycosidic linkages could enhance immunomodulatory effects.Li et al.[74]found (1→6)-β-D-glucans fromL.edodesactivited macrophage to increase the release of NO and ROS.
4.Conclusions
The immunomodulatory activities of edible fungus polysaccharides have attracted wide attention.The polysaccharides could activate the corresponding signal pathway by recognizing the corresponding membrane receptors, so as to stimulate the releases of related factors, which played a role in immune regulation.In addition, the polysaccharides could also regulate immune activity by acting on intestinal microorganisms and immune organs (Fig.3).
Based on the analysis of structure-activity relationship, structure of polysaccharides affected immunomodulatory activities.At present, because of the complexity of structure and components of polysaccharides, the complete structure was difficult to determine.Therefore, the structure-activity relationship could not be completely determined.
Most of the reported on the studies of immunomodulatory activities have been performedin vivoorin vitro, which could not fully reflect the actual role of polysaccharides in human body.Therefore, the mechanism and application of polysaccharides in body still needs to be further studied.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgement
This research was financially supported by Major Public Welfare Projects in Henan Province (201300110200), National Key R&D Program of China (2018YFD0400200), Key scientific and technological key projects of Henan science and Technology Department (192102110214 and 202102110283), Henan Province Industry-University-Research Cooperation Project (182107000033), the special fund project of Zhengzhou basic and applied basic research (ZZSZX202003).
杂志排行
食品科学与人类健康(英文)的其它文章
- Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia
- Phylogenetic analysis and protective effects of thymol and its chromatographic fractions from a novel wild mushroom in combating oxidative stress
- Advances in research on chemical constituents and pharmacological effects of Paecilomyces hepiali
- Healthy function and high valued utilization of edible fungi
- Antrodia Cinnamomea ameliorates neointimal formation by inhibiting infl ammatory cell infi ltration through downregulation of adhesion molecule expression in vitro and in vivo
- Auricularia polytricha noodles prevent hyperlipemia and modulate gut microbiota in high-fat diet fed mice
