Anti-colon cancer activity of water-soluble polysaccharides extracted from Gloeostereum incarnatum via Wnt/β-catenin signaling pathway
2021-05-24JiweiHeAnhuiYngXuyuZhoYngLiuShuynLiuDiWng
Jiwei He, Anhui Yng, Xuyu Zho, Yng Liu, Shuyn Liu,*, Di Wng,*
a Engineering Research Center of Chinese Ministry of Education for Edible and Medicinal Fungi, Jilin Agricultural University, Changchun 130118, China
b School of Life Sciences, Jilin University, Changchun 130012, China
Keywords:Gloeostereum incarnatum Water-soluble polysaccharides Colon cancer Wnt/β-catenin
ABSTRACT The incidence rate of colon cancer ranks the third among malignant tumors worldwide.Gloeostereum incarnatum (GI), a fungus with homology of medicine and food, has multiple pharmacological activities.It was investigated in this study that the anti-colon cancer effect of polysaccharides purifi ed from GI (GIPS) in ApcMinC/Gpt mice (an in situ colon cancer mouse model).Eight-week administration with GIPS at doses of 30 or 90 mg/kg strongly inhibited tumor growth including the reduction on numbers and the suppression of the size without infl uencing the animals’ body weight and organ functions.According to the proteomics performing by antibody array, among 308 detected cytokines, GIPS significantly regulated 89 of them.Compared with vehicle-treated mice, GIPS effectively reduced the levels of interleukin (IL)-1β, IL-4, IL-6, IL-17, IL-22, tumor necrosis factor (TNF)-α, matrix metalloproteinase (MMP)-2, and enhanced the levels of IL-15 and IL-18 in serum and/or colon tissues, which suggested its anti-inflammation of GIPS.GIPS suppressed nuclear aggregation of β-catenin, affected the expression of WNT1 and related proteins, thereby regulated the activation of the Wnt signaling.Altogether, GIPS can inhibit the growth of colon cancer, at least partially, via inhibiting the Wnt/β-catenin signaling pathway.
1.Introduction
As the highly frequent malignant tumor of the digestive tract, colon cancer often occurs at the junction of the rectum and the sigmoid, the incidence rate of which ranks the third among malignant tumors worldwide [1].Colon cancer shows familial and genetic feature, and it has been reported that its pathogenesis is associated with high fat and low fiber diet [2].Due to the poor prognosis, colon cancer always transfers to liver, kidney or other organs, and finally leads to death [3].
According to the previous conclusion, by combining with Frizzled and low-density lipoprotein receptile-related proteins 5/6 (LRP5/6), Wnt proteins dissociate from the destruction complex, thus inhibit the phosphorylation of β-catenin and prevent its degradation by proteasome.Free β-catenin enters the nucleus and binds to the transfer factor T-cell factor (TCF)/lymphoid enhancement factor (LEF), thereby initiating the expression of cancer-related target genes [4].Nuclear accumulation of β-catenin can be observed in 80% of patients with colon cancer, which may serve as a therapeutic target [5].
Till now, the common treatments for colon cancer in clinics are still surgery, chemoradiotherapy and immunotherapy, which are expensive and easy to relapse [6].Accordingly, 5-fl uorouracial, capecitabine, oxaliplatin (OXA), and irinotecan (IRI) are commonly used drugs for colon cancer therapy [7].However, a series of disadvantages including poor tolerance, non-specifi city and adverse effects such as nausea, vomiting, diarrhea, neutropenia were noted [8].Developing new alternative agent for colon cancer therapy is desiderated.
Natural products have become attractive research hotspot due to their huge resources and low toxicity.As one of the most important consistent of natural plants, polysaccharides show various effects including anti-tumor and immunity regulation [9].In our group, it was confirmed that polysaccharides obtained fromTermitomyces albuminosusshow anti-diabetic nephropathy activities via the regulation of nuclear factor-κB (NF-κB) [10], polysaccharides fromAntrodia cinnamomeaimprove cyclophosphamide monohydrate (CTX)-induced immunosuppression in mice through oxidative stress [11], and polysaccharidesfromSarcodon imbricatusenhancing the hematopoietic function of bone marrow [12].According to the previous studies, polysaccharides obtained fromScutellaria baicalensisGeorgi [13],Spinacia oleracea Linn[14]andAnoectochilus formosanus[15]have been confirmed to show anticancer effects, especially on colon cancer.Gloeostereum incarnatum(GI), a kind of precious fungus with homology of medicine and food, has been reported to possess various pharmacological efficacies including diarrhea protection, gastrointestinal tract regulation and anti-dysentery [16].Polysaccharides purified from GI (GIPS) have been successfully confirmed to show immune-enhancing, antiinflammatory, and anti-bacterial activities [17].In our previous research, we found that the GIPS possessed the immunomodulatory activities in CTX-induced immunosuppression mice [18].Till now, no research systemically reports the anti-colon cancer effects of polysaccharides obtained from GIin situcolon cancer mouse models.
It was investigated in this study that the anti-colon cancer effect of GIPS inApcMinC/Gptmice, which carries a missense mutation of the adenomatous polyposis coli (APC) gene and spontaneously develops hundreds of polyps, mimicking the development of colon cancer in mice [19].Our study confirmed that GIPS showed an inhibitory effect on the occurrence and development of colon cancer via regulating the Wnt signaling pathway.
2.Materials and methods
2.1 The preparation of GIPS
GI purchased from Shandong Province, China, were smashed, dried, and extracted by hot water at 80 °C for 3 h twice.The supernatant was collected, concentrated, and removed proteins using trichloroacetic acid (TCA) method.After neutralization using 1.0 mol/L NaOH, polysaccharides were precipitated using 80% anhydrous ethanol placing at 4 °C for 12 h.The collected crude polysaccharides were dialyzed for a week at 4 °C, and then purified by Diethylethanolamine-52 (DEAE-52) (3.5 × 40 cm) (CX0092) (Solarbio, Beijing, China).The purified polysaccharides were freezedried and entitled GIPS.The detail information of GIPS can be found in our previous publication [18].
2.2 Animal experiments and protocols
Animal research according to the requirements of the Experimental Animal Ethics Committee of Jilin University has been approved with the NO.SY201905005.Thirty-six maleApcMinC/Gptmice (4-6 weeks old, body weight 20-25 g) were purchased from GemPharmatech Co., Ltd., Nanjing, China (SCXK(SU)2018-0008).Mice were bred adaptively in a specific pathogen-free (SPF) animal laboratory, at (23 ± 1) °C, 50%-60% humidity, and under light/dark conditions for 12 h, with unlimited access to adequate food and water cycles.
After one-week adaptive feeding, mice were randomly divided into 3 groups including vehicle-treated mice (CTRL) (n= 12) orally received with 10 mL/kg of normal saline, GIPS-treated mice orally received with 30 mg/kg (n= 12) or 90 mg/kg (n= 12) of GIPS every other day for 8 weeks.The doses of GIPS were chosen based on the previous studies [20,21].From the 1st to 2nd week, the mice were fed with normal diet, and from the 3rd to 8th week, the mice were fed with 60% high-fat diet (60% fat, 20% protein, 20% carbohydrate) (D12492, Research Diets, America) combining with normal diet for tumorigenesis.The body weight of mice were recorded once a week throughout the period of experiment.Within 8-week experimental period, the survival rate of mice in the CTRL group was 83.3%, and the GIPS-treated groups were 91.7% (30 mg/kg) and 91.7% (90 mg/kg).After 8-week administration, blood was sampled from the orbital venous plexus, and then all mice were euthanized via the inhalation of ether.Tissues including colon, liver, spleen and kidney were collected.
Nearly 8 cm of the colon was removed from each mouse and photographed under fluorescent lights to observe the numbers and size of colon tumors.
2.3 Histological analysis
According to our previous study [22], tissues including liver, spleen, kidney and colon tumor were fixed in 10% formaldehyde solution, dehydrated with different concentrations of ethanol and embedded in paraffin.After being cut and dewaxed, the slides were stained with hematoxylin and eosin (H&E), and photographed with an optical microscope (400 ×, Olympus, Japan).
2.4 Antibody array assay
The L-series Mouse Antibody Array Kit (AAM-BLG-1-4) purchased from RayBiotech (Tebu-Bio, The Netherlands) was used to analyze the changes of 308 cytokines which related to immunity and tumor-related proteins.Examples of colon tissues were collected from vehicle-treated mice and 30 mg/kg GIPS-treated mice.The Cell & Tissue Protein Extraction Reagent (KC-415, KangChen, China) was used to extract the proteins from colon, and the bicinchoninic acid (BCA) protein assay kit (KC-430, KangChen, China) was used to analyze the concentration of proteins.The antibody array membranes were balanced for 30 min at room temperature, unpacked and dried at room temperature for 1 h, and then 5% bovine serum albumin (BSA) sealant was added.After 1-h blocking, the test samples containing 100 μg of proteins were added and incubated at 4 °C overnight.After removing the samples, the membranes were washed with buffer solution, and then the biotin-labeled antibodies were added for 2-h incubation at room temperature.The membranes were washed and then reacted with fluorescent agent-streptavidin-Horseradish peroxidase (HRP) at room temperature.Membranes were thoroughly washed and scanned by scanner (GenePix 4300A, Axon, US).
2.5 Enzyme-linked immunosorbent assay (ELISA)
The washed colon tissues were homogenized with normal saline contained 1% protease inhibitor cocktail (P8340) (Sigma-Aldrich, St.Louis, MO, USA) and 2% phenylmethanesulfonyl fluoride (PMSF) (P7626) (Sigma-Aldrich, St.Louis, MO, USA).The levels of cytokines in both serum and colon lysis, including interleukin (IL)-1β (KT2040-A), IL-4 (KT2165-A), IL-6 (KT2163-A), IL-15 (FY2172-A), IL-17 (KT2170-A), IL-18 (KT2169-A), IL-22 (KT9441-A), tumor necrosis factor-α (TNF-α) (KT2132-A) and matrix metalloproteinase (MMP)-2 (KT2149-A) (Jiangsu Kete Bio-Technology Co., Ltd., Jiangsu, China) were analyzed using the commercial ELISA kits according to the manufacturer’s instructions.
2.6 Western blot analysis
The washed colon tissues were lysed using radio immunoprecipitation assay (RIPA) (R0010) (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) buffer, containing 1% protease inhibitor cocktail and 2% PMSF.The concentrations of protein samples were analyzed using BCA kit.40 μg of protein lysates were fractionated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to 0.45 μm polyvinylidene difluoride (PVDF) membranes (Merck Millipore, Billerica, MA).
The membranes were blocked for 6 h at 4 °C with 5% BSA in trisbuffered saline (TBS).Primary antibodies were exposed to membranes overnight at 4 °C including β-catenin (dilution at 1:1 000) (bs-1165R), Kremen-2 (dilution at 1:2 000) (bs-16813R), MMP-2 (dilution at 1:1 000) (bs-0412R), MMP-9 (dilution at 1:1 000) (bs-4593R) (Beijing Bioss Biotechnology Co., Ltd., Beijing, China), LRP5/6 (dilution at 1:2 000) (ab51910) (Abcam, Cambridge, MA, USA), WNT1 (dilution at 1:2 000) (A2475), Dickkopf-related protein 1 (DKK1) (dilution at 1:1 000) (A2562), total glycogen synthetase kinase (GSK)-3-beta (T-GSK-3β) (dilution at 1:1 000) (A2081), phosphor (P)-GSK-3β (dilution at 1:1 000) (AP1088), Frizzled-7 (dilution at 1:1 000) (A4213) (ABclonal Biotechnology Co., Ltd, Wuhan, China) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (dilution at 1:2000; E-AB-20032) (Elabscience Biotechnology Co., Ltd, Wuhan, China).After being washed, the membranes were incubated with secondary antibodies at 4 °C for 4 h including goat anti-rabbit IgG (E-AB-1003) and goat anti-mouse IgG (E-AB-1001) (horseradish peroxidase-conjugated) (Elabscience Biotechnology Co., Ltd, Wuhan, China).The bands of proteins were visualized using Electro Chemi Luminescence (ECL) kit (Merck Millipore, Billerica, MA, USA) and gel imaging system (BioSpectrum 600, Bioss Inc., Shanghai, China), and quantified with Image J software (National Institutes of Health, Bethesda, MD).
2.7 Statistical analysis
All data are expressed as the mean ± SEM.SPSS 16.0 software (IBM Corporation, Armonk, NY) was used for one-way analysis of variance (ANOVA), followed by multiple post-hoc multiple comparisons (Dunn’s test).TheP-value less than 0.05 is considered as statistically significant.
3.Results
3.1 The protection of GIPS against colon cancer
UsingApcMinC/Gptmice, the protection of GIPS against colon cancer was successfully confirmed, suggesting its inhibition on tumor growth including the reduction on numbers (Fig.1A, 1B) and the suppression tumor size (Fig.1A) compared with those of vehicletreated mice.Due to the specific location of the tumors, their volumes were failed to be measured.GIPS failed to influence the bodyweight of mice with colon cancer (Fig.1C).
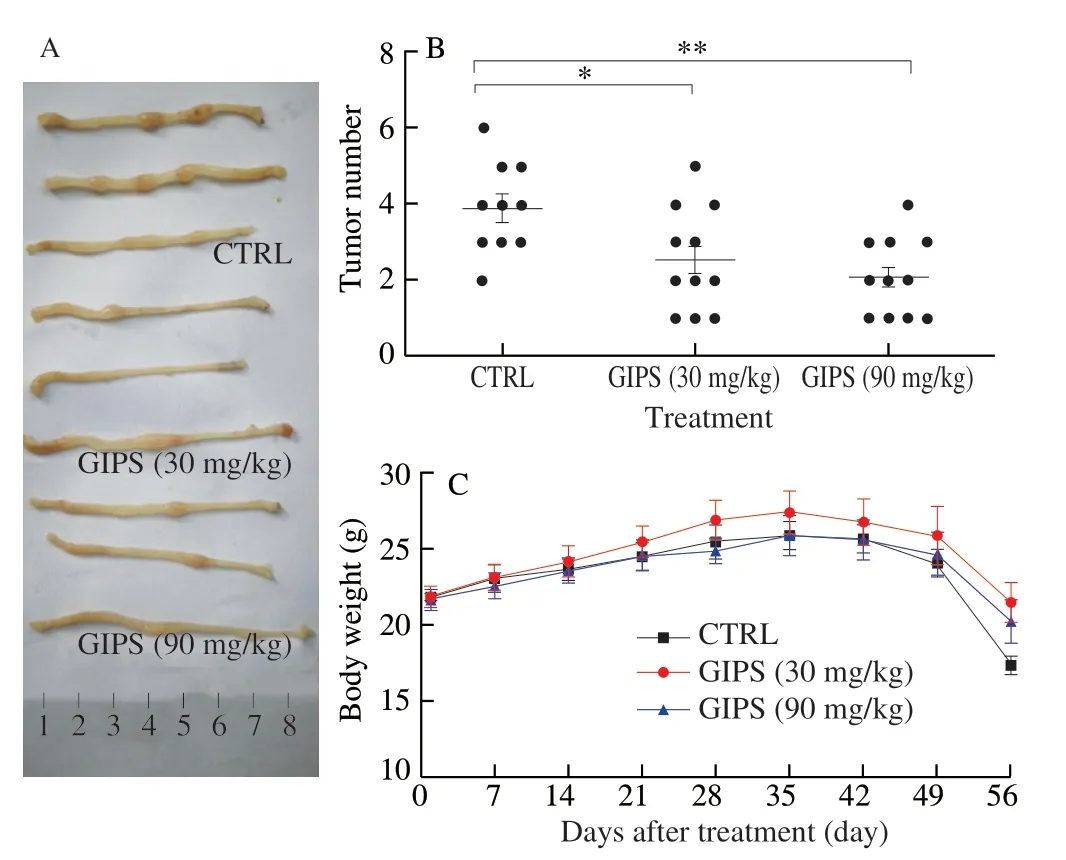
Fig.1 GIPS suppressed the tumor growth in ApcMinC/Gpt mice with colon cancer after 8-week administration.(A) Examples of tumors tissues form vehicle and GIPS-treated groups.(B) The numbers of colon tumors in mice.(C) GIPS failed to influence the body weights of ApcMinC/Gpt mice.The data were analyzed using a one-way ANOVA and expressed as means ± SEM (n = 10 for CTRL group, n = 11 for GIPS-treated group).* P < 0.05 and ** P < 0.01 vs.CTRL group.
According to the H&E staining, significant inflammatory cell infiltration was observed in the colon tissues of vehicle-treated mice, which were strongly suppressed after GIPS administration (Fig.2A).Furthermore, GIPS suppressed the tumor growth indicating by the smaller tumor area of colon tissues compared with those of vehicle-treated mice (Fig.2A).GIPS failed to influence the structure of liver, spleen and kidney of mice with colon cancer after 8-week administration (Fig.2B).
3.2 The anti-inflammation of GIPS during its anti-colon cancer
Using the antibody array, 308 cytokines were detected (Table 1).Compared with CTRL group, 30 mg/kg GIPS significantly regulated the levels of 89 factors (over 1.5-fold changes) (accounting for 28.9% of total detected cytokines) (Fig.3A, 3B, Table 1).The notably changed cytokines were further analyzed by KEGG pathway, which suggested that GIPS regulated inflammatory related pathways, especially Wnt signaling, during its anti-colon cancer inApcMinC/Gptmice (Fig.3A, 3B).
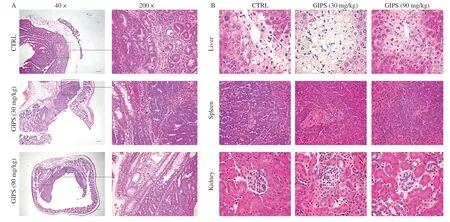
Fig.2 The histopathological examination of colon and organs were analyzed by H&E staining.(A) H&E staining of colon tissue (40 ×, Scale bar: 200 μm) (200 ×, Scale bar: 50 μm) (n = 6).Arrows indicate the inflammatory cell infiltration.(B) H&E staining of liver, spleen, and kidney tissues (400 ×, Scale bar: 20 μm) (n = 6).
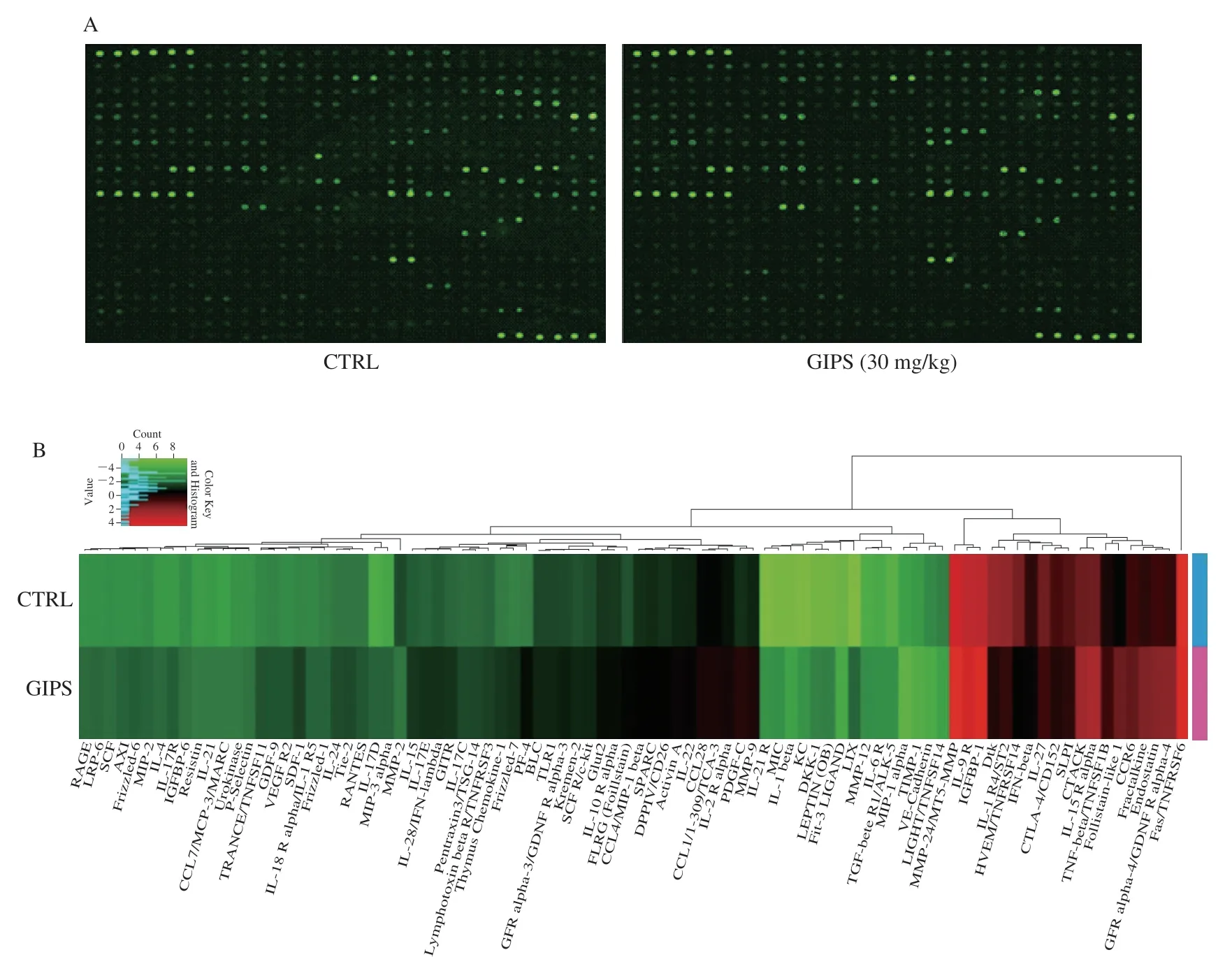
Fig.3 The effects of GIPS on the 308 cytokines in colon tissues of ApcMinC/Gpt mice detected by the RayBio® L-Series Mouse Antibody Array Kit (n = 3).(A) The fluorescent graphical representation of cytokine expressions.(B) Compared with CTRL mice, 30 mg/kg GIPS significantly regulated the levels of 89 kinds of cytokines (over 1.5-fold changes), as shown by heatmap.
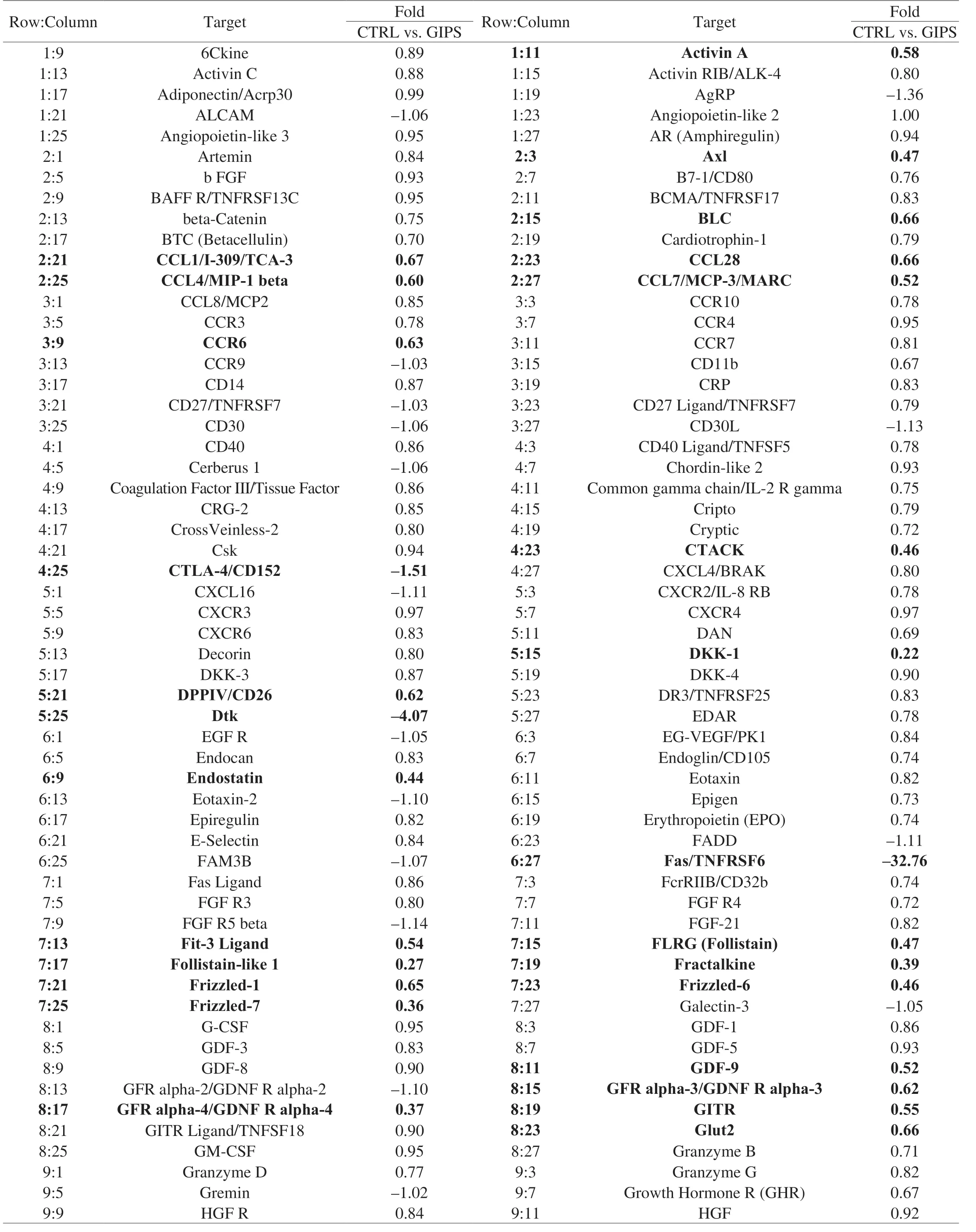
Table 1 All of the detailed parameters of target cytokines decreased or increased among experimental groups in colon tissues of ApcMinC/Gpt mice.
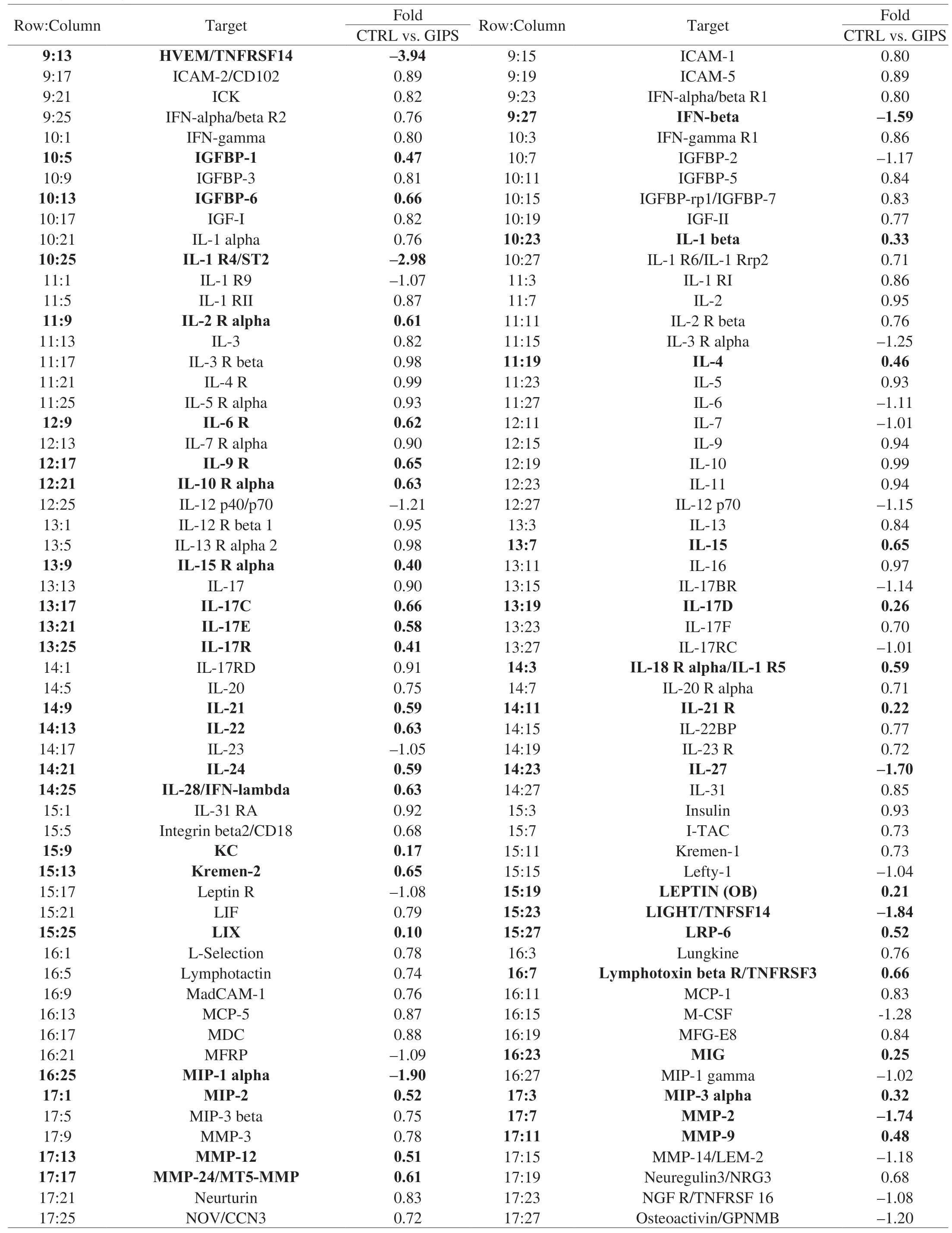
Table 1 (Continued)
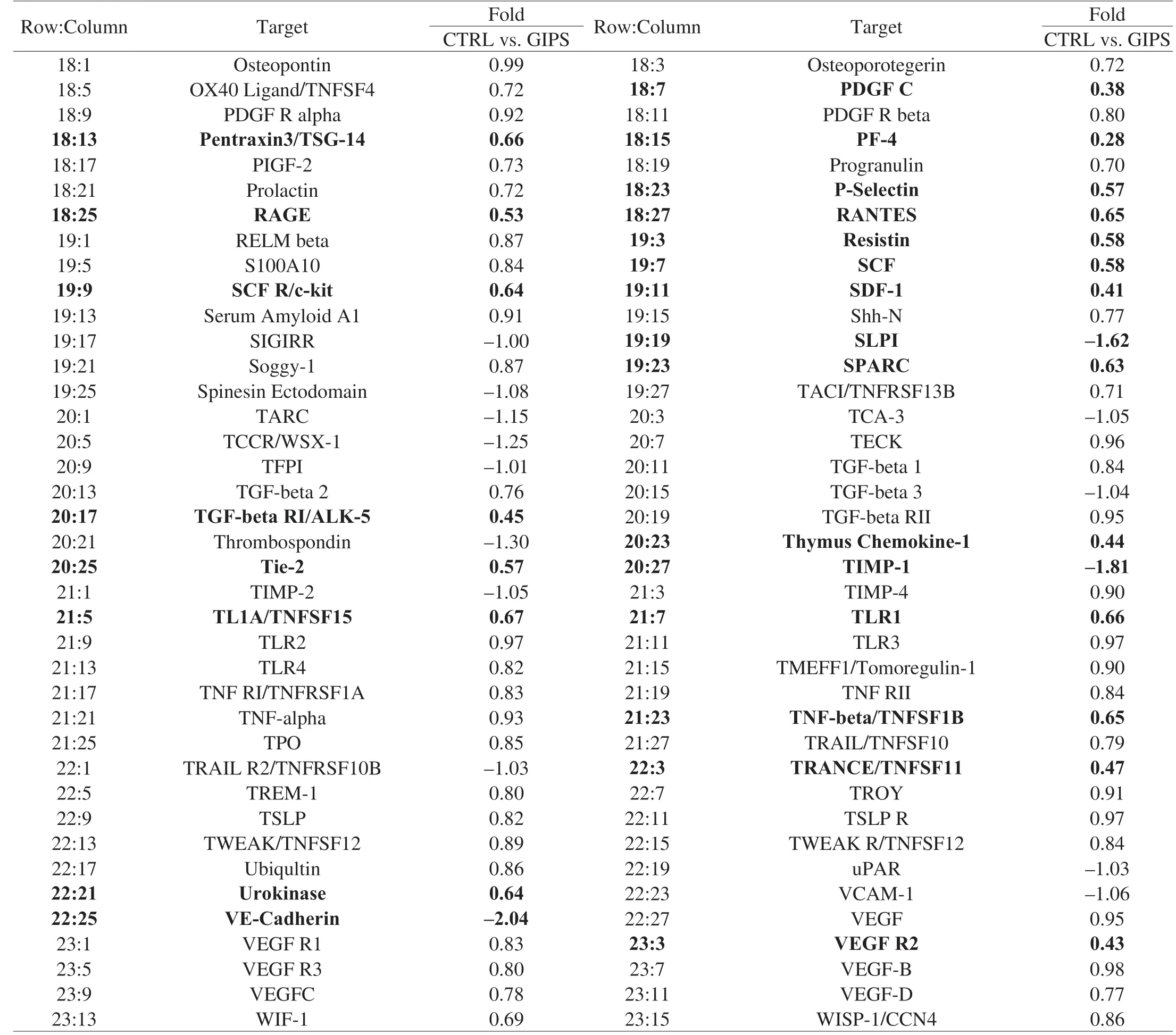
Table 1 (Continued)
Functionally, in colon cancer and other diseases, interleukins, a class of cytokines in the tumor microenvironment, are involved in maintaining the inflammatory process [23].TNF-α plays a role as a proinflammatory cytokine in the pathogenesis of tumor, and MMPs are shown to be important in the progression, invasion and metastasis of tumor [24,25].In colon tissues, compared with CTRL, GIPS significantly suppressed the levels of IL-1β (P< 0.001), IL-6 (P< 0.05), IL-17 (P< 0.05), MMP-2 (P< 0.05) and TNF-α (P< 0.05), and enhanced the levels of IL-15 (P< 0.05) and IL-18 (P< 0.05), In serum, compared with CTRL, GIPS significantly suppressed the levels of IL-1β (P< 0.01), IL-4 (P< 0.05), IL-6 (P< 0.001), IL-22(P< 0.05) and MMP-2 (P< 0.05), and enhanced the levels of IL-15(P< 0.01) and IL-18 (P< 0.001) (Fig.4).
3.3 GIPS regulated the Wnt/β-catenin signaling to show anticolon cancer effects
Wnt/β-catenin signaling pathway plays a significant role by promoting cancer cell proliferation and resistance to chemotherapy in colon cancer [26].After 8-week administration, GIPS significantly reduced the expression levels of β-catenin (P< 0.001), Frizzled-7 (P< 0.01), WNT1 (P< 0.001), LRP5/6 (P< 0.001), MMP-2 (P< 0.001) and MMP-9 (P< 0.001), enhanced the expression levels of DKK1 (P< 0.001) and Kremen-2 (P< 0.001), and suppressed the phosphorylation of GSK-3β (P< 0.001) in colon tissues ofApcMinC/Gptmice (Fig.5).
4.Discussion
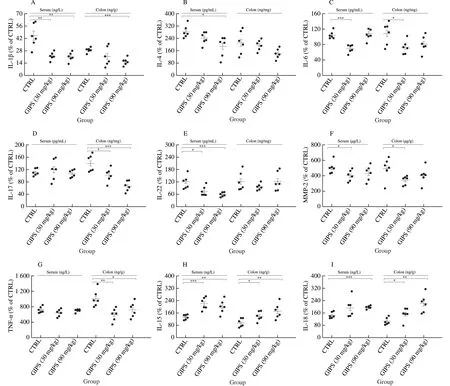
Fig.4 The effect of GIPS on inflammatory factor in serum and colon.GIPS significantly reduced the levels of (A) IL-1β, (B) IL-4, (C) IL-6, (D) IL-17, (E) IL-22, (F) MMP-2, (G) TNF-α, and enhanced the levels of (H) IL-15 and (I) IL-18.The data were analyzed using a one-way ANOVA and expressed as means ± SEM (n = 6).* P < 0.05, ** P < 0.01 and *** P < 0.001 vs.CTRL group.
In China, the incidence of colon cancer is the highest among people in their 40s, and it shows the youth trend [27].G.incarnatum, one of the precious traditional medicines,has attracted researchers’ attention due to its intestinal protection.Based on the antiinflammatory and ulcerative colitis protective effects of GI reported in our group previously, we successfully demonstrated the anti-colon cancer effects of GIPSin situcolon cancer mouse model in this study.Beginning from the 7th week, the body weight of mice were dropped significantly which may due to the rapid growth of the colonic tumors leading to the damage on digestive and absorptive capacity of the intestine.The similar symptoms were also observed in patients with colorectal cancer [28].
Recently, numerous polysaccharides have been reported to have immunoregulatory activities, especially those of fungal origin [9,29].For instance, polysaccharides fromGanoderma lucidumplay an anti-tumor role by increasing the activity of natural killer cells [30], and polysaccharides obtained fromPhellinus igniariusexerts anticancer effects via stimulating T lymphocytes and dendritic cells (DCs) [31].The relationship between the structural characteristics and the pharmacological efficacy of polysaccharides has rarely been elaborated.Due to the limitation of this study, we failed to investigate the accurate structure of GIPS, which contains serval types of polysaccharides.The natural and crude character of GIPS not only supports the low toxicity with various pharmacological efficacy, but also helps to explain its non-dose dependent manner during our experiments, which may show anti-colon cancer via multiple targets.In fact, the non-dose dependent manner can be noted in the research related to Traditional Chinese Medicine [32].
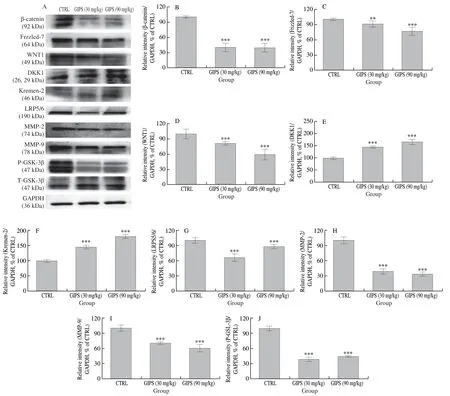
Fig.5 Wnt/β-catenin signaling is involved in GIPS-mediated anti-colon cancer in ApcMinC/Gpt mice.GIPS reduced the expression levels of β-catenin, Frizzled-7, WNT1, LRP5/6, MMP-2, MMP-9 and P-GSK-3β, and enhanced the expression levels of DKK1 and Kremen-2 in the colon tissue lysis.The quantitative expression of each protein was normalized using GAPDH.The data expressed as means ± SEM (n = 4) and analyzed using a one-way ANOVA.** P < 0.01 and *** P < 0.001 vs.CTRL group.
Natural polysaccharides have anti-tumor effects by regulating human’s own immune system, which show unparalleled safety compared with chemical drugs.GIPS can effectively reduce the number and suppress the size of tumors in the colon ofApcMinC/Gptmice.According to the proteomics, there were 89 relevant cytokines notably regulated by GIPS, among which, Wnt signaling pathwayrelated factors and interleukins were found.Linkages between inflammation and cancer, including colon cancer, are widely accepted.Cancer induces an inflammatory microenvironment, which promotes the cellular proliferation and invasion, and alters the immune responses [33,34].Interleukins show multiple immunomodulatory functions, including cell proliferation, maturation, migration and adhesion [23], among which IL-6 is considered as an important tumor promoter in various human cancers by inhibiting the regulatory T cell differentiation and inducing effector T cells [35].Additionally, pro-inflammatory and tumor-promoting activities of IL-6 have been considered to have an important link between inflammation and cancer development [36,37].In colon cancer, IL-6 is highly expressed, affecting the tumor microenvironment and promoting colon cancer metastasis [38,39].Furthermore, IL-1β is produced by macrophages in the tumor microenvironment, initiates the inflammatory cascade, and thereby induces the expression of IL-6, which forms a loop feedback effect [33,40].IL-22 activates signal transducers and activators of transcription protein 3 (STAT3), consequently causes the proliferation of colon cancer cell [41].As reported, colorectal cancer stem cells evolved mechanisms to escape death-inducing stimuli by producing IL-4 [42].The combination of IL-4 and IL-6 significantly increased the resistance of cancer stem cell to chemotherapy agents [43].Pro-inflammatory cytokine IL-17 can promote angiogenesis by up-regulating the expression of vascular endothelial growth factor (VEGF), thus enhancing tumor metastasis and invasion [44,45].IL-17 promotes the production of IL-6, and its cooperation with IL-6 helps to recruit neutrophils to inflammatory tissues [46].Accordingly, IL-6 plays a central role during the anti-colon cancer property of GIPS.
Abnormal activation of the Wnt/β-catenin signaling pathway can be found in multiple tumorigenesis, including colon cancer, which can affect cancer stem cells, leading to malignant tumors and poor prognosis [47,48].Concomitantly, the expression of Frizzled-7 and a large number of inflammatory changes occur.As the channel transmits Wnt signaling to nucleus [49], β-catenin is stabilized and accumulated in the nucleus under the presence of Wnt, which can be regulated via the activation of GSK-3β [50,51].Frizzled-7 is a G protein-coupled receptor protein that forms heterodimers with LRP5/6 to act as Wnt receptors, conducting Wnt signaling on cell membranes [4].Frizzled-7 and LRP5/6 are highly expressed in colon cancer.TheDKK1gene is an upstream inhibitor of the Wnt signaling, binding with LRP5/6 and Kremen-2 competitively to destroy the Wnt receptor [52,53].As the downstream genes of the Wnt signaling,MMP-2andMMP-9degrade the extracellular matrix and secrete VEGF, and their expressions and activities are frequently elevated in malignant cancer [39].Meanwhile, MMP-9 can enhance the expression of proinflammatory cytokines such as TNF-α, IL-1β and IL-6 [54].Natural polysaccharides, such as fucoidan (polysaccharides derived from brown algae), and polysaccharides fromGrifola frondosa, exert antitumor effects by down-regulating the Wnt/β-catenin pathway[25,55].GIPS can reduce the expression level of β-catenin and that of Wnt receptors on the cell membrane, thus inhibiting the Wnt/β-catenin pathway and regulating the expressions of its downstream proteins.
5.Conclusions
In conclusion, it is demonstrated in this study that GIPS, polysaccharides purified fromGloeostereum incarnatum, can inhibit the growth of colon cancer, which may be achieved by inhibiting the Wnt/β-catenin signaling pathway.
Conflict of Interest
The authors have declared that there is no conflict of interest.
Acknowledgements
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (Grant No.2018YFE0107800), the “13th Five-year” Science and Technology Projects from Education Department in Jilin Province of P.R.China (Grant No.JJKH20190108KJ) and Industrial Technology Research and Development Projects from Development and Reform Commission of Jilin Province (Grant No.2019C050-8).
杂志排行
食品科学与人类健康(英文)的其它文章
- Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia
- Amelioration of metabolic disorders by a mushroom-derived polyphenols correlates with the reduction of Ruminococcaceae in gut of DIO mice
- Immunomodulatory effects of polysaccharides from edible fungus: a review
- Advances in research on chemical constituents and pharmacological effects of Paecilomyces hepiali
- Healthy function and high valued utilization of edible fungi
- Antrodia Cinnamomea ameliorates neointimal formation by inhibiting infl ammatory cell infi ltration through downregulation of adhesion molecule expression in vitro and in vivo
