Phylogenetic analysis and protective effects of thymol and its chromatographic fractions from a novel wild mushroom in combating oxidative stress
2021-05-24SuulkshmiSugpriyDhnskrnAirmiKnnnPlnippnDivyVnugopl
M.Suulkshmi, Sugpriy Dhnskrn*, S.Airmi, M.Knnn, R.Plnippn, Divy Vnugopl
a Department of Microbiology, K.R.College of Arts and Science, Kovilpatti, Tamil Nadu, India
b Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, Wadi Al Dawaser, Saudi Arabia
c Department of Microbiology, Kamaraj College, Thoothukudi, Tamil Nadu, India
d Research Department of Zoology, V.H.N.Senthikumara Nadar College (Autonomus) Virudhunagar, Tamil Nadu, India
e Research Department of Computer Science, V.H.N.Senthikumara Nadar College (Autonomus) Virudhunagar, Tamil Nadu, India
Keywords:Antioxidant Anti-proliferation Dietary mushrooms HeLa cells Novel Mushrooms Thymol
ABSTRACT Mushrooms are good sources of phytochemicals that have antioxidant and anti-proliferative effects.This study identifi ed a unique isoform of 18S rRNA gene (864 bp) from a novel wild mushroom (SMK-1) (GenBank accession number: SUB3267363).Thin layer chromatographic (TLC) profiling of the methanolic extract of the dried mushroom fruiting bodies of SMK-1 revealed the presence of phenolic and fl avonoid fractions with retention factor (Rf) values of 0.955 and 0.927 respectively.The GC/MS chromatograms of the SMK-1 methanolic extract identifi ed the main bioactive compound was phenol, 5-methyl-2-(1-methylethyl) (74.00%) (thymol).The radical scavenging activity for the fl avonoid fraction was greater than the phenolic fractions (Rf-phenolics fractions > Rf-fl avonoid fractions) with the antioxidant activity more than that of standard ascorbic acid.Also, the phenolic and flavonoid fractions of SMK-1 expressed cytotoxic effects in HeLa cells with IC50 values ranging from 5 μg/mL to 80 μg/mL in a dose-dependent manner.This present research highlights the presence of high thymol concentration in a novel wild mushroom that has antioxidant and anti-poliferative potential with therapeutic benefi ts.The application of thymol natural products from novel mushroom SMK-1 as nutrition supplements could inhibit oxidative stress triggered by numerous pathologies that may pave the way to develop a new therapeutic natural drug.
1.Introduction
In recent years, consumption of wild mushrooms has risen globally, increasing their earning potential and economic contribution by around two billion dollars [1].Mushrooms are non-timber forest goods that are significant for both their pharmacological and nutritional values.They are sources of several pharmacologically active molecules that can improve to strengthen the immune function and defend against carcinogenic substances [2].A recent study has found that mushrooms contain bioactive compounds that have innumerable therapeutic benefits such as immune-modulation, antitumor and chronic bronchitis improvement [3].
Several types of mushrooms are nutritious and edible, providing vitamins, carbohydrates, protein, amino acids, minerals and bioactive substances [4].Gloeophyllum sepiarium(Rusty gilled polypore) is an inedible wood-decay fungus but possesses medicinal properties and grows on coniferous trees in small, dark brown/green brackets [5].A study reported that largest edible basidiomycete mushroom,Agaricus bisporus, belonging to the genusAgaricaceaehas a delicious taste with much more nutritional benefits and has been used in the food industry for its very strong aroma or fl avoring taste [6].A balanced diet is the supporting treatment for the prevention of illness and especially against oxidative stress.Mushroom extract is an excellent source of different nutraceuticals for dietary supplements that can promote human health by the synergistic effects of all the bioactive compounds present [7,8].Numerous molecules synthesized by mushrooms are known to be bioactive, and these bioactive compounds are present in cultured mycelium and in fruiting bodies.The bioactive compounds include fats, proteins, minerals, polysaccharides, alkaloids, glycosides, terpenoids, volatile oils, tocopherols, flavonoids, phenolics, lectins, carotenoids, ascorbic acid, folates, enzymes, and organic acids [9-12].These bioactive compounds comprise of key functional roles such as anticancer, antioxidant, immunomodulation, antiallergic, cardiovascular protector, anticholesterolemic, antidiabetic, antibacterial, antiparasitic, antiviral, antifungal, and hepatoprotective effects; they also protect against tumor development and inflammatory processes [9-12].
The Western Ghats in south India extending over an area of 140 000 km2contains wild macro-fungi varieties [13].The selected wild novel mushroom, SMK-1 is short lived, found abundantly soon after the monsoons and the polyporus species has been collected from the Kalakkad Mundanthurai Tiger Reserve & Range, Tamil Nadu, India.The present study aimed to identify the phylogenetic relationship of this novel mushroom SMK-1 and to analyse its phytochemical content and biomedical potency.
2.Materials and methods
2.1 Collection and extraction of isolated novel mushroom (SMK-1)
The isolated novel, wild mushroom SMK-1 as shown in Fig.1, were collected from logs of wood, palm logs and humus soil at different locations.The collections were done early in the morning during the period of January to December 2018 in the Kalakkad Mundanthurai Tiger Reserve, Tamil Nadu, India.The mushroom SMK-1 was cleaned and washed with sterile distilled water, dried in shade and finely blended to powder and stored in sterile plastic bags at 4 °C until further use (extraction).About 25 g of the blended SMK-1 powder was subjected to successive extraction in a Soxhlet apparatus with 95% methanol as the extraction solvent [14].After extraction, the methanol solvent was evaporated by concentrating under vacuum with rotary evaporator (Senco Rotary Evaporator, Model RE 801) at 40 °C under reduced pressure and stored at 2-8 °C separately until use.The dried methanolic extracts were reconstituted to 50 mg/mL in 2% dimethyl sulfoxide (DMSO) solution.The solvent free methanol extract was thereafter evaluated.

Fig.1 Isolated novel mushroom SMK-1.(A) Fresh basidiomata in the field.(B) Habitat.
2.2 Molecular identification
2.2.1 DNA extraction
Mycelia of novel mushroom SMK-1 was separated from spawn and incubated on complete yeast media (CYM) agar plates at 25 °C for 10 days.The agar plates with the grown mycelia were cut into small pieces, transferred into 100 mL CYM liquid medium and cultured at 25 °C for 14 days.Genomic DNA was extracted from freeze-dried mycelia of SMK-1 by using the method of Tang et al.[15].The DNA quality was confirmed by 1.0% (m/V) agarose gel electrophoresis, and concentrations were determined with a BioPhotometer 6131 (Eppendorf, Germany), then diluted to 50 ng/μL for PCR amplification.
2.2.2 PCR amplification and sequence analysis
PCR amplifications for rDNA of region of novel SMKSD-1 was performed with universal PCR primers viz.ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) (MWG Biotech Pvt.Ltd: Bangalore, India) specific to the nuclear ribosomal internal transcribed spacer (nrITS) region [16].PCR amplification was performed as per the protocol of Khatua et al.[17]in a Biorad gradient thermal cycler (BioRad, USA).PCR cycling was started with denaturation for 4 min at 94 °C, followed by 30 cycles consisting of denaturation for 30 s at 94 °C, annealing for 30 s at 55 °C, extension for 1 min at 72 °C, and a final elongation step of 5 min at 72 °C.The PCR product was further detected using gel electrophoresis and extracted using the Genei®Gel Extraction Kit (Bangalore, India).Cycle sequencing was performed with the help of ABI3730xl DNA Analyzer (Applied Biosystems, Carlsbad, California, USA) at the R&D Centre, Department of Microbiology, Bharathiyar University, Coimbatore, India, based on the Sanger dideoxy sequencing technique using identical primers used for amplification of nrITS region.The newly generated sequences were deposited in GenBank.Moreover, the ITS nucleotide sequences of SMK-1 was set as outgroup in the construction of the combined phylogenetical tree [18].
2.2.3 GenBank search
Sequence search was performed using the BLAST standard nucleotide-nucleotide basic local alignment search tool [National Center for Biotechnology Information (NCBI), Library of Medicine, Bethesda, MD, USA (http://www.ncbi.nlm.nih.gov/BLAST/)].All GenBank, EMBL, DDBJ, PDB sequences were searched with the expectation frequency minimized at 0.0001.Sequences were filtered for low complexity.Sequences were deposited in GenBank (http://www.ncbi.nlm.nih.gov/).
2.3 Phytochemical screening
The concentrated methanolic extract of SMK-1 was screened for phytochemicals in accordance with Kumar et al.[19]and the minerals were determined according to Gençcelep et al.[20].
2.4 GC/MS analysis and identification of chemical constituents
Qualitative and quantitative GC/MS analyses were carried with a Hewlett-Packard 5890 Series II Plus gas chromatograph interfaced to an HP 5989B mass spectrometer.Separation was done on a HP5-MS capillary column (25 m × 0.25 mm) coated with 0.50 μm 5% phenyl 95% methylpolysiloxane.Temperature programming was set at 70-250 °C, at a rate of 3 °C/min.The carrier gas used was helium at a constant flow rate of 1.9 mL/min.Injector and interface temperature was adjusted to 250 and 280 °C, respectively.Electron ionization (EI) mass spectra were recorded at 70 eV ionization voltage (source temperature 250 °C).Bioactive compounds extracted from methanolic extracts of SMK-1 were identified by mass spectral comparison based on GC retention time on HP-5MS column and matching of the spectral data was compared with linear retention indices with computer software data of standards (Replib and Mainlab data of GC-MS systems).
2.5 Analysis and chromatographic separation by thin layer chromatography (TLC)
The methanolic extract of novel mushroom SMK-1 was separated with TLC for the qualitative determination of phytoconstituents (preliminary chemical analysis) such as phenolic compounds and flavonoids.Methanolic extract of SMK-1 was spotted on TLC plates (solid absorbent - silica gel 60 F254 plates [Merck, Darmstadt, Germany]GF plates).A variety of solvent systems have been tested with solvents of increasing polarity, but acceptable resolution has been achieved in the mobile phase of ethyl acetate:methanol (7:3,V/V) for the determination of flavonoids; toluene-acetoneformic acid (5:5:1,V/V) for the determination of phenolic compounds.The plates were dried and the resolution of flavonoids and phenolic compounds spots were visualized and confirmed on chromatogram using modified Folin Ciocalteau method according to Minnusi et al.[21]for phenolic content; and exposed to ammonia vapor for flavonoids.Each spot’s distance from its application was monitored and measured, and the retention factor (Rf) value was determined.The TLC spot validates a positive reaction for the presence of phenolic compounds and flavonoids and these fractions were collected separately for further studies.
2.6 In vitro antioxidant activity
Different concentrations from 5 μg/mL to 100 μg/mL of phenolic and flavonoid fraction of methanolic extract of SMK-1 were assessed for their antioxidant activityin vitro.The antioxidant property was measured by using DPPH radical scavenging assay [22].Nitric oxide (NO-) scavenging assay [23]and ferric reducing antioxidant potential (Fe3+-Fe2+) [24]were also evaluated.Using a nonlinear regression algorithm, the concentration of fractions needed to quench free radicals (IC50) was evaluated.Ascorbic acid was used as a reference compound for all antioxidant assays performedin vitro.All the tests were carried out in triplicates.
2.7 In vitro anti-proliferative property
2.7.1 Cell line and culture
HeLa cell line (human cervical cell line) was obtained from National Center for Cell Science (NCCS), Pune, India, and maintained further in Centre for Bioscience and Nanoscience Research laboratory, Coimbatore, India.The cells were cultured in DMEM medium (Gibco Co., Germany) supplemented with 10% fetal calf serum (FCS), with glucose, sodium carbonate, penicillin (100 IU/mL), and streptomycin (100 μg/mL), at 37 °C in a 5% CO2incubator.
2.7.2 Cell treatment
HeLa cells (2 × 105cells/100 μL/well) were plated in 96 well plates and incubated for 48 h.After 48 h of incubation, the cells were treated with phenolic and flavonoid fraction of SMK-1 at different concentration ranges (25-150 μg/mL), and incubated for 24 h at 37 °C in a 5% CO2incubator.Control cells were treated with 2% dimethyl sulfoxide (DMSO) alone.All the experiments were performed in triplicate.
2.7.3 Cytotoxicity assays
After treatment, the medium (100 μL) were removed and the cells were assessed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide]assay according to Prasad et al.[25].IC50inhibition of cell viability was calculated.The blanks for the experiments were cells treated with 2% DMSO.Cytotoxic effects were observed under inverted light microscope at × 45 magnification using Olympus Laboratory Microscope.Cytotoxic efficacy of phenolic and flavonoid fractions was expressed as the percentage of cell viability, according to Dai and Mumper [26],
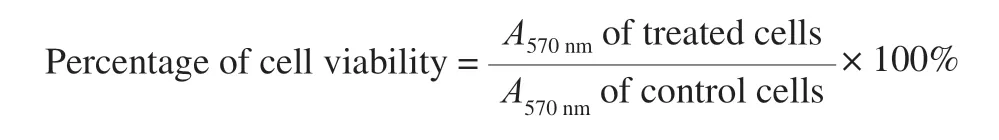
2.8 Statistical analysis
The data for the effect of all extracts were analyzed by ANOVA using SPSS version 16.0 Software.Statistical inferences were made at a significance level ofP< 0.05.
3.Results
3.1 Macroscopic examination
The unknown Fungi named as SMK-1, GenBank accession number is SUB3267363.
Common, collected from dead trees, saprobic and terricolous.Basidiocarps of SMK-1 vary in size (6-12 cm wide and 10-24 cm across).The distinctive characteristics of the fruiting bodies are milky white colour, with irregular shape, large sized basidiocarp with fleshy stipe and broadly adnate to decurrently gills.The pores of the basidiocarps are milky white in colour.Stipe was 5 cm long when young, and later it becomes short and firmly attached at the base of decayed trees which grows whole year as shown in Fig.1.
3.2 Molecular characterization and classification
3.2.1 18S rRNA analysis of SMK-1
Based on 18S rRNA molecular studies, the novel SMK-1 was identified as a new fungal species.The rRNA region of SMK-1 was amplified, and then submitted to GenBank (GenBank accession number SUB3267363).The region was 864 bp long with unique sequences shown in Fig.2A.Following DNA sequence alignment with the filamentous fungi the 18S rRNA region, the sequence from 170 bp to 300 bp in the 5’ end region was G-rich with (GGGGC)nrepeat, and (TTTGGG)nrepeat found in the sequence from 430 bp to 530 bp.
3.2.2 Phylogenetic analyses based on ITS sequence
The newly identified species sequencing products ranged from 864 nucleotides.The phylogenetic relationships of novel SMK-1 were derived from the MCMC analysis based on nuclear ribosomal DNA (NrITS) sequences obtained by this study and from GenBank.The ITS sequences have been paired with ends to construct 864 pairs.The newly created nrITS and other GenBank sequences used for the phylogenetic analysis will be shown in Fig.2B.The phylogenetic tree constructed by MCMC methods based on the ITS alignment showed the same clustering patterns (Fig.2B).Four major clades were labeled; cluster I consisted of 3 strains reported as unknown fungus; cluster II contained 3 strains, which was also reported as unknown; cluster III included 1 strain reported as unknown; cluster IV merely consisted of the strains 1 (SMK-1) isolated as novel.
3.3 Nutritional and mineral studies
The novel SMK-1 shows presence of crude fibres (14.7%), ash content (9.3%), and moisture content (88.6%) in 100 g of dried samples.The presence of mineral elements were abundantly seen in SMK-1 that include iron (258 mg/100 g), copper (8 mg/100g), manganese (3 mg/ 100 g), magnesium (247 mg/ 100 g), and calcium (13 mg/ 100 g) as shown in Table 1.
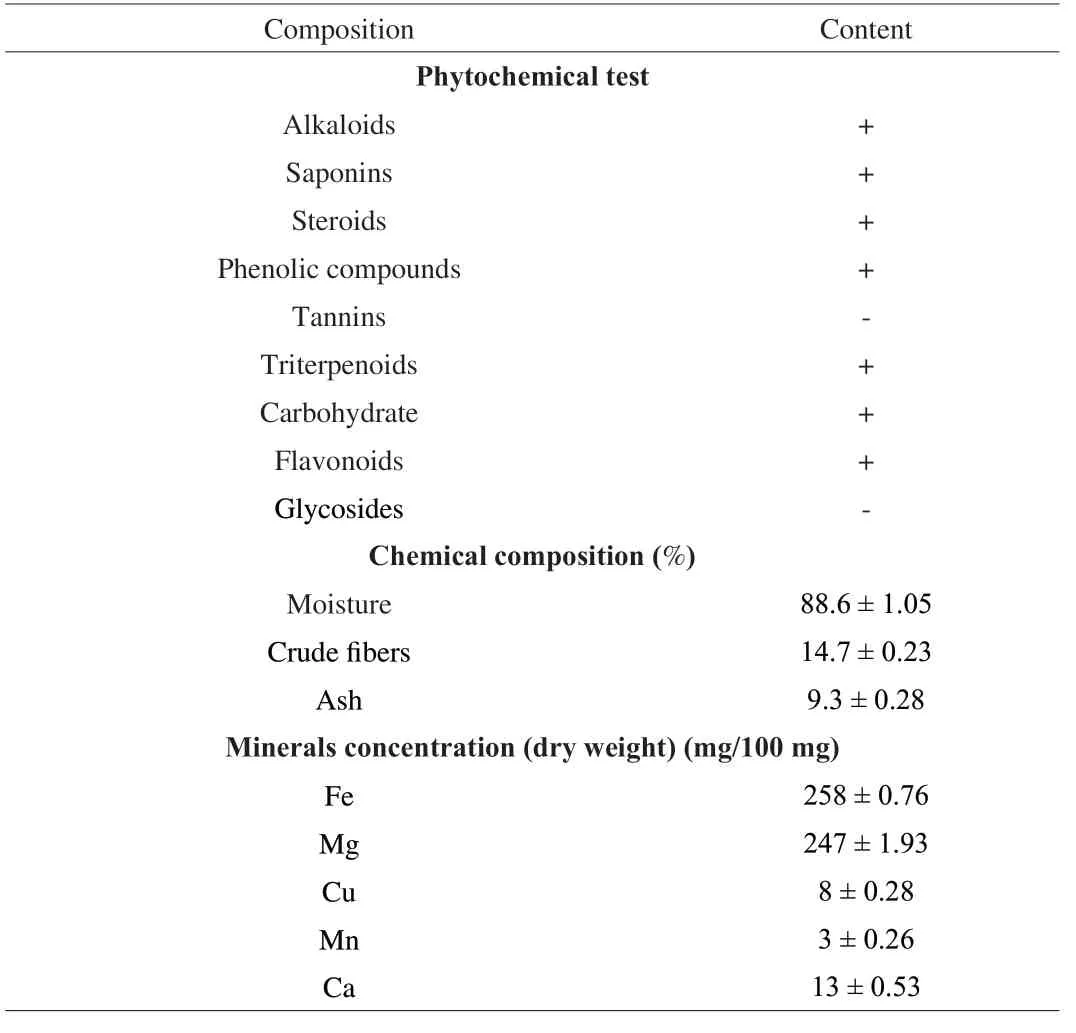
Table 1 Qualitative estimation of the phytochemicals, chemical composition and mineral concentration present in the novel mushroom SMK-1.
3.4 Qualitative phytochemical screening
Phytochemical screening of methanol extracts obtained from novel SMK-1 revealed the presence of alkaloids, flavonoids, cardiac glycosides, saponins, terpenes, steroids, tannins and phenolics as discussed in Table 1.
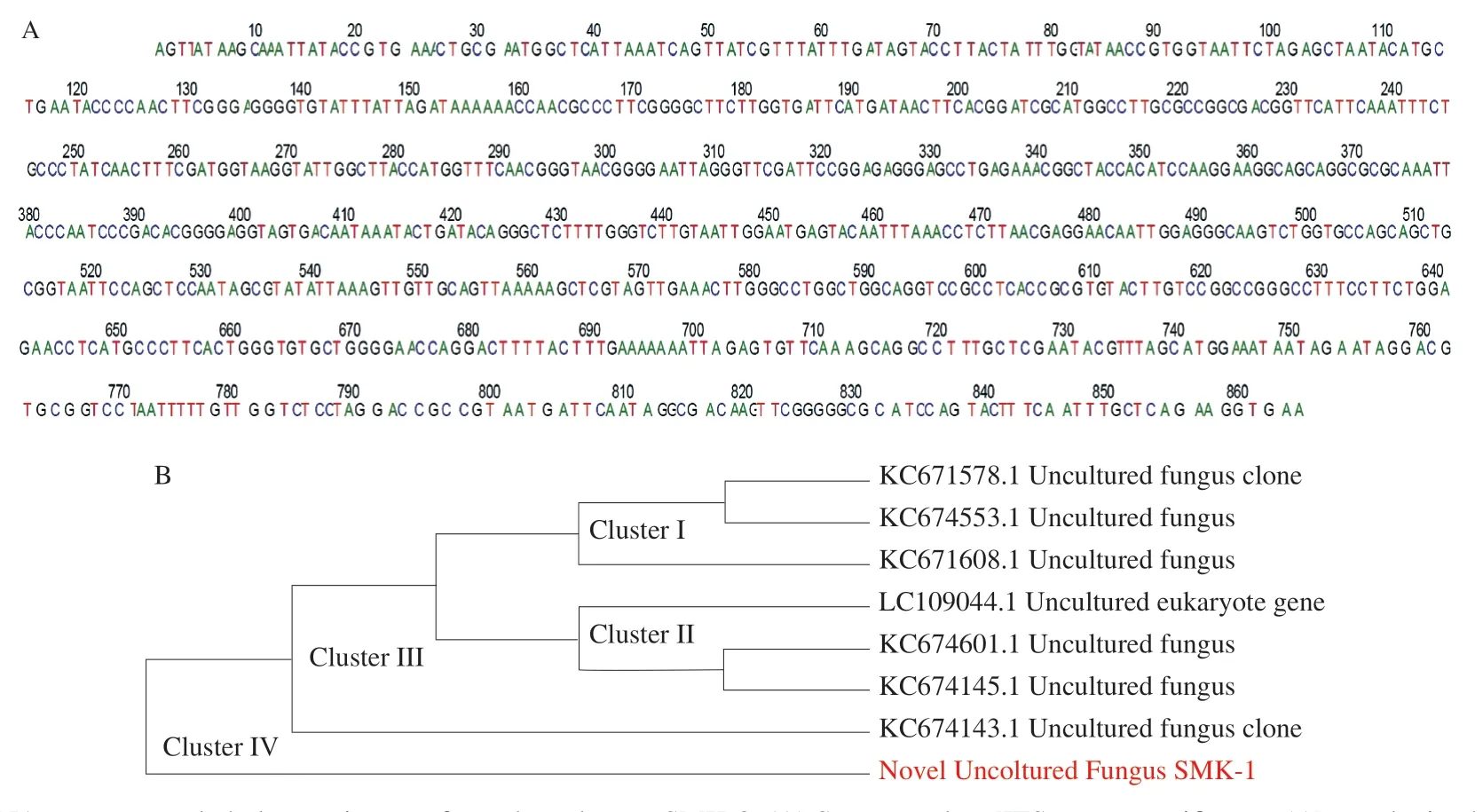
Fig.2 18S rDNA sequence and phylogenetic tree of novel mushroom SMK-3.(A) Sequence data [ITS taxon-specific gene(s)]are obtained directly from a fungal fruiting body and a BLAST search is conducted in International Nucleotide Sequence Database (INSD), such as GenBank or other specialized databases.Identification is based on overall sequence similarity (e.g., 97%-100% sequence similarity) with other reference sequences in INSD, thereby providing an identification of an unknown sequence of 864 bp.(B) Consensus phylogram (50% majority rule) resulting from a Bayesian analysis of the nrITS sequence alignment of SMK-1 species, showing mean branch lengths, obtained from 10 generations of an MCMC analysis.
3.5 Bioactive compounds present in the extracts based on GC/MS analysis
Table 2 shows the bioactive compounds present in the methanol extracts obtained from novel SMK-1.Their recognition and characterization in an HP-5MS column was based on their elution order.The molecular formula, elution time, and the amount of these bioactive compounds were also presented.The highest bioactive compound was phenol, 5-methyl-2-(1-methylethyl) (74.00%) known as thymol, followed by 1-butene, 3-chloro-2-methyl (8.93%), 9,12-octadecadienoic acid (Z,Z) (7.62%) and hexadecanoic acid (6.36%).All the other compounds were presented in trace amounts as mentioned in Table 2.The GC chromatograms of the MES-1 extract presented in Fig.3 shows the time of retention in the column and the peaks detected that correspond to the bioactive compounds present in the extract.
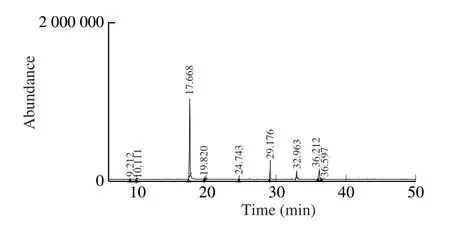
Fig.3 GC-MS of methanolic extract of novel mushroom SMK-1.
3.6 Preliminary thin layer chromatography
Preliminary TLC separation and identification of phenolic compounds and flavonoids of MES-1 was performed using the chromatographic system with silica gel GF as stationary phase and the corresponding mobile phase mentioned above.Gallic acid and quercetin were used as standards for phenolic compounds and flavonoids respectively.Visualizing agents used to clearly observe the color of the sample’s spot were dominantly appeared for above mentioned constituents.Spots of standards were easy to detect and compared with samples spots.Rfvalues of gallic acid and quercetin was 0.57 and 0.66 respectively for corresponding mobile phase mentioned earlier.The TLC analysis in various solvent systems for each specimen revealed the presence of spots.Each spot is presumably due to a pure natural product or phytochemical.Each also has a specificRfvalues.TheRfvalues ranged from 0.955 to 0.856 for SMK-1 as shown in Fig.4.
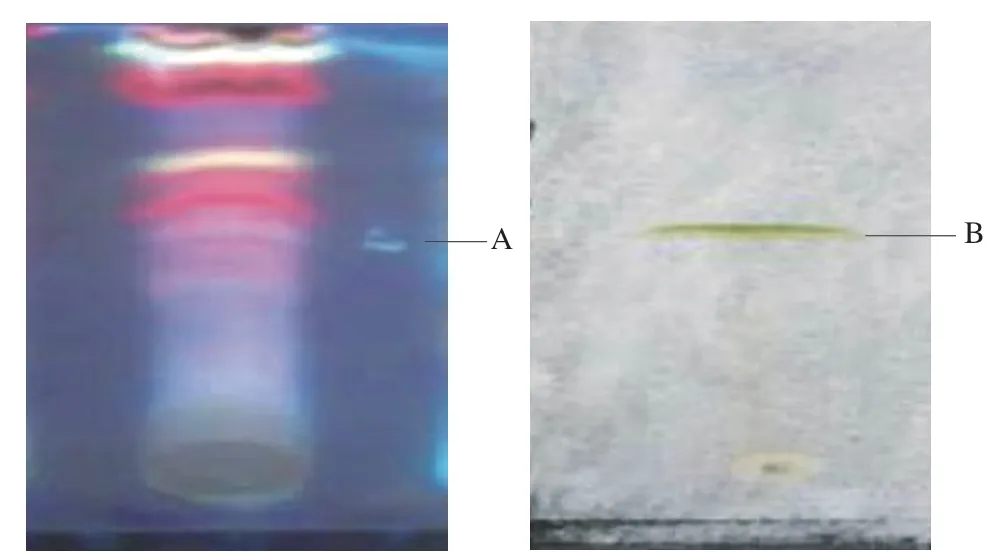
Fig.4 TLC of methanolic extract of novel mushroom SMK-1.(A) The phenolic fractions under UV light, (B) the flavonoid fraction under visible light.
3.7 Antioxidant profile of TLC fractions
3.7.1 DPPH scavenging activity
Table 3 shows the dose-response effects of phenolic and flavonoid fractions on the DPPH radical scavenging activity in comparison to ascorbic acid.The IC50of phenolic and flavonoid fractions were 18.57 and 15.69 μg/mL, respectively.Comparatively, the flavonoid fractions showed greater involvement in scavenging DPPH radicals than the phenolic fractions.
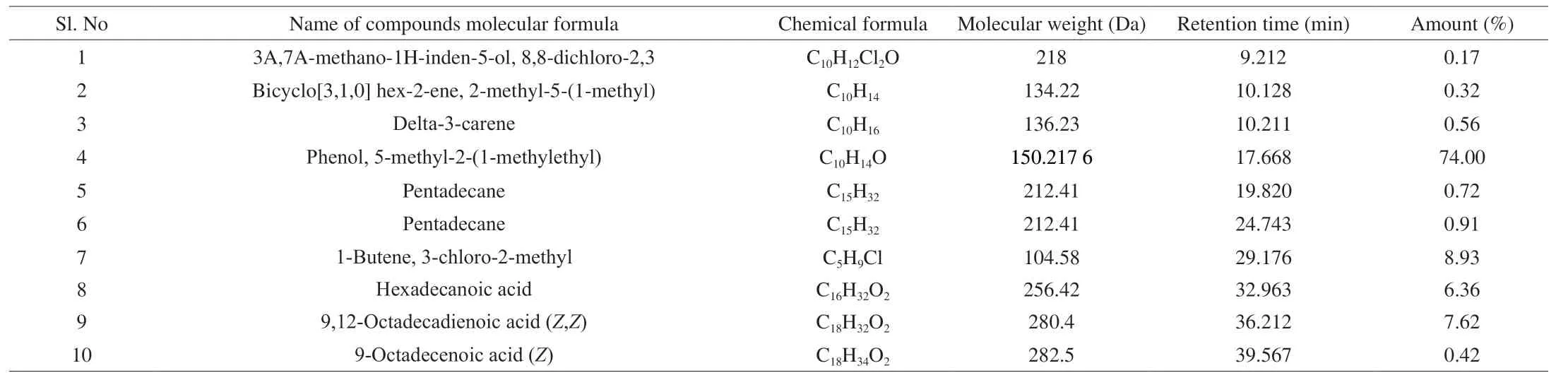
Table 2 GC-MS analysis of methanolic extract of novel mushroom SMK-1.
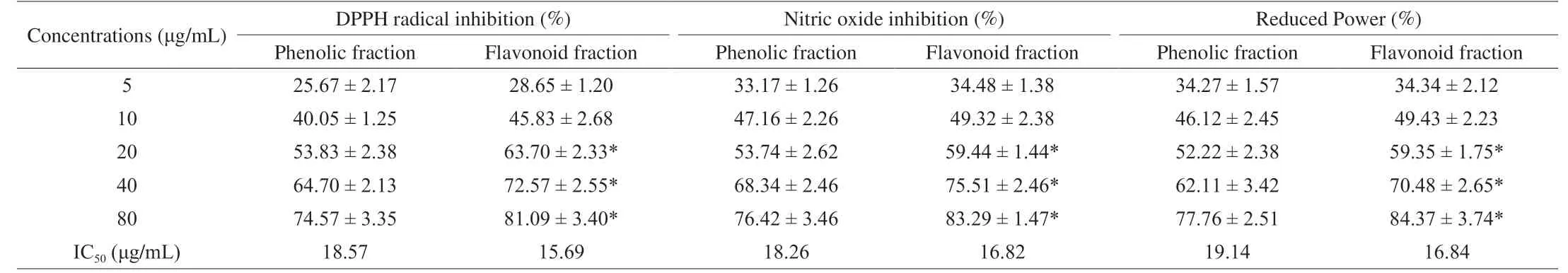
Table 3 Free radicals scavenging property of isolated phenolic and flavonoid fraction of SMK-1.
3.7.2 Nitric oxide scavenging activity
Nitric oxide radical scavenging activity of the phenolic and flavonoid fractions possesses dose-response effects, compared with ascorbic acid as mentioned in Table 3.It was observed that flavonoid fraction of the SMK-1 had higher activity than that of the phenolic one.The IC50of phenolic and flavonoid fractions were 18.29 and 16.82 μg/mL, respectively.Comparatively, the flavonoid fractions displayed higher activity than phenolic fractions in scavenging nitric oxide radicals.
3.7.3 Reducing power
Fe3+was reduced to Fe2+in the presence of both phenolic and flavonoid fractions and the reference compound ascorbic acid to assay the reductive capability.The highest reductive activity was shown at a minimum dose by the flavonoid fraction (20 μg/mL) as compared to phenolic fractions and its IC50value were 18.26 and 16.82 μg/mL respectively as shown in Table 3.
3.8 In vitro anti-proliferative activity of TLC fractions
Cytotoxic effect (MTT assay) of the phenolic and flavonoid fractions on HeLa cells are shown in Fig.5.Cytotoxicity of the fractions was measured at various concentrations (5-80 μg/mL).Cell viability decreased in a dose-dependent manner with the minimum viability (23.4%) for flavonoid fraction at 80 μg/mL, while for phenolic fractions it was 25.4% at the same concentration.The IC50of the flavonoid fraction was 38.6 μg/mL while for phenolic fractions it was 36.1 μg/mL.The cytotoxic ability of phenolics and flavonoids fractions reveals novel SMK-1 as promising anti-proliferative agents.
4.Discussion
Mushrooms are commercially valuable since they are a source of food, medication and bioactive substances used in therapeutic formulations for the pharmaceutical industry [27].The classification and identification of newly isolated novel mushroom belonging to Basidiomycota division based on conventional taxonomy are difficult [28].In recent years, DNA sequencing has been used for solving taxonomic problems in unknown fungi and in the related genera [29].ITS fragments of nuclear ribosomal DNA have acted as a valuable molecular marker for identifying organisms with related morphological characteristics [29].Fungal DNA research relies on the ribosomal DNA sequence for results and classification.Due to the various genetic changes of the rDNA domains, the conservative degree of every rDNA region was uneven.Since the ITS region is variable, especially for classification and species identification [30], the 18S rDNA regions are heavily conserved (Fig.2A) and are often included in alignment and family phylogeny research (Fig.2B) [31].A recent study on intergenic spacer (IGS) has been effectively useful to understand the genetic diversity of numerous edible mushrooms, includeRhodocollybia laulaha[32],Ferula sinkiangensis[24], andTuber borchii[33].
TLC analyses revealed that the methanolic extract of SMK-1 consists of two different fractions of phenolic compounds and flavonoids.The antioxidant and anti-proliferative potential of the phenolic compound and flavonoid fractions were evaluated.Sample bands showed blue color under UV light (Fig.4A) and visible yellow-green (brown) spot indicating the presence of flavonoids in the methanolic extract of SMK-1 (Fig.4B).The bright yellowgreen (brown) color spot shows the presence of flavone glycoside biflavonols [34].In the present study, a total of one flavonoid spot and one phenolic band were isolated from methanolic extract of SMK-1 (Fig.4).The results showed theRfvalues of phenolic and flavonoid fractions were 0.856 and 0.955 respectively and maybe related to flavonols in particular to quercetin 3-O-rutinoside, with aRfvalue of 0.9 [34,35].
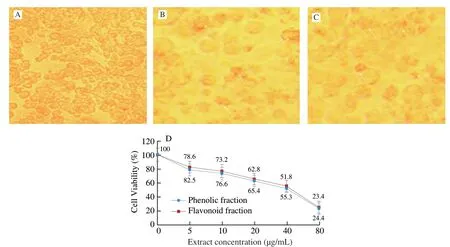
Fig.5 Anticancer effect of isolated phenolic and flavonoid fraction of SMK-1.(A) Control cells HeLa cells alone; (B) phenolic fraction treated HeLa cells; and (C) flavonoid fraction treated HeLa cells.(D) Cytotoxic effect (MTT assay) of isolated phenolic and flavonoid fraction of SMK-1.
Qualitative phytochemical research revealed the presence of alkaloids, saponin, steroids and sterols, phenols, tannins, proteins, carbohydrate, terpenoids, glycosides and flavonoids in methanolic extract of SMK-1.Such phytochemicals are considered to be biologically active and used as a protective mechanism for animals, human and plants toward many microorganisms, insects and predation [36].The GC/MS result revealed that high concentration 74.00% of phenol, 5-methyl-2-(1-methylethyl), which is a thymol present in novel mushroom SMK-1 revealed cell death may be intrinsic apoptotic pathway due to interaction of thymol with DNA resulting in cell cycle arrest (Fig.3).Polyphenolic components are potential free radical scavengers.
The antioxidant activity of phenolic compounds is related to the number and position of phenolic hydroxyl groups and the nature of substitutions on the aromatic rings that directly determines the capacity for donating electrons, hydrogen atoms, scavenging free radicals, and/or chelating metal cations [37-40].The hydrogen atoms of the adjacent hydroxyl groups (O-diphenol), located in various positions of the rings A, B and C, the double bonds of the benzene ring, and the double bond of the oxo functional group (C=O) of flavonoids provide high antioxidant activity [37-40].The position of the OH group in the phenolic ring is the major significant element.The hydroxyl groups present in phenolic compound of thymol (SMK-1) are strong hydrogen donors, with reactive oxygen/nitrogen species on other hand, the hydrogen-donating antioxidants will react with the termination reaction to breaking the loop of new radical’s production.For example, the presence of the OH group in ortho position in thymol has stronger biomedical potency that may enhance the pharmocological efficacy of novel mushroom SMK-1.
We found that the phenolic and flavonoid fractions exhibited an antioxidant effect due to its strong radical scavenging activity (Table 2).Earlier studies also found a significant association between the total phenolic and flavonoid content and its antioxidant activity [40,41].The anti-proliferative effect was also examined in HeLa cervical cancer cells.According to the National Cancer Institute (NCI) guidelines, the cytotoxic level for plant crude extract was IC50< 20 μg/mL, and our results show a better cytotoxic effect with the IC50values for flavonoids much stronger than the phenolic fractions on treated HeLa cervical cells (Fig.5).Similar to our study, Hip et al.[42]found phenolic contents in several wild mushrooms.Phenolic derivatives have important biomedical and pharmacological properties [43,44]and many also exhibit remarkable ability to modify the conjugation of sulfates.The bioactivity of polyphenols can be attributed to its ability to chelate metals, inhibit lipoxygenase and scavenge free radicals [45,46].Since these mushrooms analyzed here contain polyphenolic components, indicates its promise for future pharmaceutical formulations.
5.Conclusions
Phylogenetic analysis of the ITS region validates the uniqueness of the mushroom SMK-1.This study also revealed the phenolic and flavonoid fractions of SMK-1 methanolic extract has an active therapeutic agent.Interestingly, GC-MS of SMK-1 methanolic extract shows the presence of thymol as the main constituent that has significant effects for quenching of free radical to combat against oxidative stress.Moreover, the chromatographic fractions showed strong cytotoxic effects against HeLa cells contributing to the anti-proliferative machinery.Further studies on isolation of the bioactive phytochemical component and its correlation of molecular targets may pave the way for providing a better therapeutic intervention of cancer.
Acknowledgment
The author is very thankful to the Deanship of Scientific Research, Prince Sattam bin Abdulaziz University for supporting this work.The author expresses hearty thanks to Our Team for their timely assistant and personally grateful to Mr.Muthamil Selvan for his understanding, full-hearted support and encouragement.This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Interest
The authors declare no conflict of interest.
Appendix A.Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2021.04.007.
杂志排行
食品科学与人类健康(英文)的其它文章
- Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia
- Amelioration of metabolic disorders by a mushroom-derived polyphenols correlates with the reduction of Ruminococcaceae in gut of DIO mice
- Immunomodulatory effects of polysaccharides from edible fungus: a review
- Advances in research on chemical constituents and pharmacological effects of Paecilomyces hepiali
- Healthy function and high valued utilization of edible fungi
- Antrodia Cinnamomea ameliorates neointimal formation by inhibiting infl ammatory cell infi ltration through downregulation of adhesion molecule expression in vitro and in vivo
