Thermal-responsive Photonic Crystals based on Physically Cross-linked Inverse Opal Nanocomposite Hydrogels
2021-04-16ZHENGHangLIJinSONGWeizhengHEGuangyaoWANGYifengCHENYanjun
ZHENG Hang, LI Jin, SONG Weizheng, HE Guangyao, WANG Yifeng, CHEN Yanjun
(School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China )
Abstract: A thermal-responsive photonic crystal material was fabricated by forming an inverse opal nanocomposite hydrogel of poly(N-isopropylacrylamide) (IONHPNIPAm) within the interstitial space of a polystyrene photonic crystal template. In IONHPNIPAm, PNIPAm were physically cross-linked with two kinds of nanoparticles (carbon dots and laponite clays). The integration of carbon dots and laponite clays for physical crosslinking endowed IONHPNIPAm sufficient strength and self-healing property. IONHPNIPAm films can be completely peeled from the substrates to be utilized as an independent photonic crystal material. The structural color and optical diffraction of the IONHPNIPAm exhibits a rapid reversible change in response to external thermal stimuli due to its physical cross-linking feature. Moreover, the IONHPNIPAm shows clear fluorescence due to the introduction of carbon dots, which enables a convenient way for chemical detection (such as the detection of silver ions). This stimuli-responsive photonic crystal materials based on physically cross-linked inverse opal nanocomposite hydrogels with fast response and good mechanical stability are promising for applications in the fields of smart optical detectors, thermal-responsive sensors and chemical detectors.
Key words: photonic crystals; inverse opal; physical cross-linking; thermal-responsive; nanocomposite hydrogel
1 Introduction
Photonic crystals (PCs) have increasingly attracted the attention of researchers owing to their unique structural color properties[1,2]and important application values in the fields of optical detectors, sensors[3-5],textile[6,7]and inkless printing[8]. According to the distinguished Bragg’s law, the structural color of photonic crystals depends on the periodic lattice constant and the refractive index of component materials. Nowadays,polymer photonic crystals are the most widely studied,which are formed by regular arrangement of polymer colloidal particles with good monodispersity. Polymer photonic crystals are particularly attractive because of the low cost of large area deposition, the diversity of polymers, and the ease of three-dimensional fabrication using self-assembly method. Polymer photonic crystals can be directly used in photonic devices[9-11]. More interestingly, polymer photonic crystals can be used as a photonic crystal template to fabricate more photonic crystal materials.
Responsive photonic crystals can adjust their structural colors in respond to external stilmuli, so they have wide application prospects in the fields of intelligent display[12], anti-counterfeiting identification[13], and sensing detection[14]. Polymer hydrogels with elastic polymer network can undergo conformational transformations with changes in the external environment,so preparing inverse opal hydrogels (IOH) through combining photonic crystals and polymer hydrogels is an effective method to realize responsive photonic crystals[15-26]. Zhaoet al[23]polymerized hydrogel monomers within the interstitial space of silica colloidal crystal template to fabricate IOH whose structural color has pH, temperature and light responses. Quanet al[24]reported poly(vinylidene fluoride-co-hexafluoropropylene) IOH which showed a thermo-sensitive shape-memory property and a reversible optical property. Polymer-infiltrated SiO2inverse opal photonic crystals were prepared for colorimetrically selective detection of xylene vapors[25]. However, it is worth noticing that most of reported IOHs were fabricated based on chemically cross-linked polymer hydrogels.Compared with chemically cross-linked systems, the volume change of physically cross-linked polymer hydrogels are more sensitive to the external environment due to their reversible network structures[26]. Moreover,nanocomposite hydrogel with physical cross-linking networks has excellent mechanical properties[27]and self-healing property[28]. So, it can be expected that physically cross-linked nanocomposite hydrogels will endow responsive photonic crystals with some interesting properties, such as a faster response speed and some, a stronger mechanical strength and self-healing.
In this paper, we synthesized a physically crosslinked poly(N-isopropylacrylamide) inverse opal nanocomposite hydrogel (IONHPNIPAm) by using polystyrene photonic crystal template. Instead of chemical crosslinking agents, nanoparticles, such as carbon dots (C-dots) and laponite clays, were introduced into poly(N-isopropylacrylamide) hydrogels to provide physical crosslinking points. Our method not only can prepare IONHPNIPAmthin films on substrates, but also can fabricate IONHPNIPAmmaterials independent on substrates. We tried to discuss the size effect of nanoparticles on the preparation of IONHPNIPAm. Due to the thermo-sensitive property of PNIPAm hydrogels, the structural color and optical diffraction of IONHPNIPAmcan rapidly vary in respond to temperature changes. In addition, C-dots endow IONHPNIPAmwith fluorescence and direct determination of silver ions. Therefore, the IONHPNIPAmshows thermal-responsive character, fluorescence property and chemical detection function,which benefits the applications of analytical detection and sensors.
2 Experimental
2.1 Materials
Styrene (St) was purchased from Sigma Chemistry Co., Ltd. and was distilled under reduced pressure before use. Acrylic acid (AA) and N-isopropylacrylamide (NIPAm) were obtained from Aladdin Chemistry Co., Ltd. and were used as received. N, N, N’,N’-Tetramethyl-ethylenediamine (TEMED), potassium persulfate (KPS),β-Cyclodextrin (purity=96%) and hydrochloric acid (HCl) were obtained from Sinopharm Chemical Reagent Co., Ltd. and were used as received.Laponite clays (92.32wt% of Mg5.34Li0.66Si8O20(OH)4Na0.66, 7.68wt% Na4P2O7) were provided by Aoyuan New Material Technology Co., Ltd. and used after drying at 125 ℃ for 2 h. C-dots were prepared by usingβ-cyclodextrin as carbon source via the low temperature hydrothermal method based on the previous report[29]. Pure water was produced by deionization and filtration using a Millipore apparatus (resistivity=18.2 MΩ·cm).
2.2 Preparation of monodispersed polystyrene (PS) colloid
The polystyrene (PS) colloids were synthesized by soap-free emulsion polymerization. First, Styrene(St) was washed by 10wt% NaOH solution and distilled under reduced pressure before use. Then, 150 mL of deionized water and 13 mL of St monomer were added to the three-necked flask and mixed by stirring(300 r/min) for half an hour with nitrogen protection.Next, 0.2 g of acrylic acid (AA) and 0.3 g of potassium persulfate (KPS) were added to the mixture. Then, the temperature of the reaction system was slowly heated in a water bath to 75 ℃. After 8 hours’ reaction, PS emulsion was obtained. Then, PS emulsion was centrifuged and washed to obtain monodispersed PS colloid.
2.3 Preparation of PS photonic crystal templates
PS photonic crystal templates were prepared by the vertical deposition self-assembly method[30,31]. PS colloid obtained in the previous step were configured into a PS aqueous dispersion which having a mass fraction of 2%. The PS colloid dispersion were place in a beaker, and then treated glass slides were vertically inserted into the PS colloid dispersion. Then the beaker was kept in an oven at 65 ℃ for a few days. After the water was evaporated completely, the temperature of the oven was increased to 98 ℃ and kept for 3 hours.PS photonic crystal template with a structure color was obtained.
According to the Bragg’s law[32,33](1), theλmaxvalue of the diffraction peak of the PS photonic crystals is determined by size of PS colloid through the Eq.(2):

where,λmaxis the peak wavelength of the Bragg diffraction spectrum of the photonic crystals;dhklis the interplanar spacing of the photonic crystals;neffis the effective refractive index of the photonic crystals (for PS photonic crystals with a face-centered cubic (fcc)structure,neff=1.47); θ is the incident angle of the light(θ=90°); andDis the diameter of the PS colloidal microspheres.
2.4 Preparation of the physically cross-linked IONHPNIPAm
Physically cross-linked IONHPNIPAmfilm was manufactured by a capillary-attraction-induced method as follows. First, 0.04 g C-dots and 0.2 g XLS were dispersed in 10 mL deionized water by ultrasonic dispersion for about 1 h. 1.13 g NIPAm was added into 10 mL aqueous suspension of C-dots and XLS. Under nitrogen atmosphere, the mixture was kept for 1 h at low temperature (T<10 ℃) under continuous and vigorous stirring. Then, 0.5 mL KPS (20 mg/mL) and 5 μL of TEMED were added under stirring and the pre-solution was degassed for 10 minutes. PS photonic crystal template was then refilled by the precursor and allowed to permeate for 10min until the template became transparent. The polymerization of the monomer NIPAm was conducted for 24 h at 25 ℃ to form physically crosslinked PNIPAm hydrogel with PS photonic crystal template. After that, the hydrogel was rinsed in xylene to perfectly remove the PS colloids template for 48 hours,and a colored photonic crystal thin film with inverse opal structure was obtained. The IONHPNIPAmfilm was carefully transferred to deionized water to reach the equilibrium swelling. Table 1 shows the preparation formula of the physically cross-linked IONHPNIPAm.
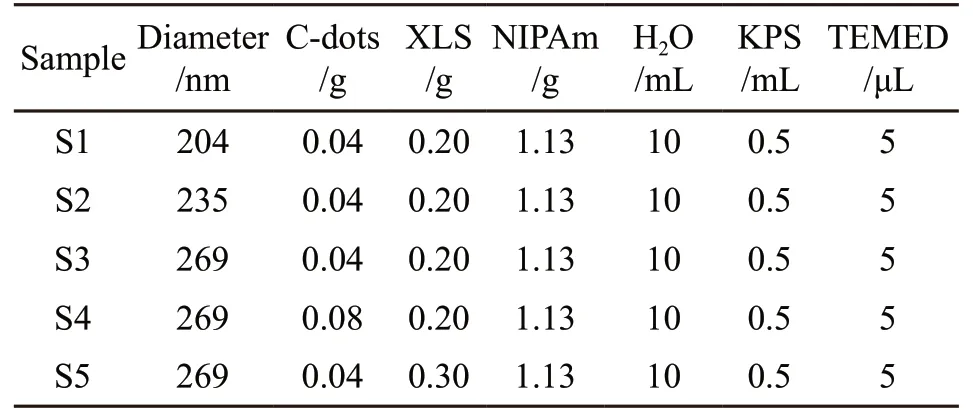
Table 1 Preparation formula of the physically cross-linked IONHPNIPAm
2.5 Characterization
The PS photonic crystal and IONHPNIPAmwere coated with gold by a sputter coater (EM SCD005,LEICA, Germany), and the microstructures of the PS photonic crystal template and IONHPNIPAmwere analyzed by field emission scanning electron microscopy(FE-SEM, JEOL JSM7500F) at voltages of 5.0 kV and 2.0 kV, respectively. The fluorescence of IONHPNIPAmwere observed under 420 nm UV light at 25 ℃ (970CRT,Shanghai). The reflection spectra of IONHPNIPAmwere recorded by a fiber optic spectrometer (NOVA,Ideaoptics, Shanghai) which was mounted on a microscope (ECLIPSE 50iPOL, Nikon, Japan) in vertical direction. For the thermosensitive test, the IONHPNIPAmwas soaked in the deionized water from 25 ℃ to 46℃ for 1 h to reach a stabilization. The diameter and size distribution of PS colloids were determined using a Malvern Zetasizer Nano-S dynamic light scattering(DLS) device (wavelength: 633 nm). The images of PS photonic crystal template and IONHPNIPAmwere captured using the digital camera. The swelling measurement was carried out by monitoring the diameter of the cylindrical gel in different temperature deionized water.
3 Results and discussion
3.1 Characterization of PS photonic crystal templates
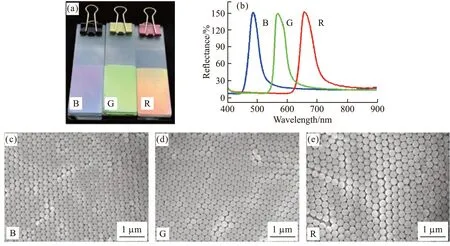
Fig.1 Optical photograph(a), the reflection spectrum(b) and SEM image(c), (d) and (e) of three kinds of PS photonic crystal templates
Three kinds of monodispersed PS colloids were first prepared and their average hydrated particle sizes are 212 nm, 244 nm, and 280 nm, respectively, which were measured by dynamic light scattering (DLS). PS colloids are monodispersed because their DLS PDI are 0.004, 0.04, and 0.03, respectively. Then, three PS photonic crystal templates were obtained by the vertical deposition of PS colloids on glass substrates. As shown in Figure 1a, three PS photonic crystal templates show bright blue (B), green (G), and red (R) structural colors,respectively. The position of the reflectance peak wavelength of three PS photonic crystal templates respectively lies at 490 nm, 566 nm, and 656 nm in Figure 1b, which are in agreement with the calculated peak position of 488 nm, 563 nm, and 645 nm according to the modified version of Bragg’s law (Eq.(2)).
SEM images of three PS photonic crystal templates are shown in Figs.1(c), 1(d), and 1(e). The average diameters of PS colloids in photonic crystal templates are 204 nm, 235 nm, and 269 nm from SEM results. All the three kinds of PS colloids are organized into a face-centered cubic (fcc) structure with smooth edges of the microspheres. Some spaces between the PS colloids are observed, which provides opportunity for the infiltration of hydrogel precursor. Moreover, the space becomes narrow with the decrease of the average diameter of PS colloids in photonic crystal templates.
3.2 Fabrication of the physically crosslinked IONHPNIPAm
The fabrication process of physically cross-linked PNIPAm inverse opal hydrogel is showed in Fig.2(a).Firstly, PS photonic crystal template with obvious structure color was prepared by the vertical deposition of monodispersed PS colloids on glass substrates.Then, hydrogel precursor including NIPAm monomers,C-dots and XLS particles (their structure were showed in Fig.2(b) and Fig.2(c), respectively) was filled into the space of the PS photonic crystal template. Next,PS/PNIPAm composite hydrogel was formed by in situ polymerization of NIPAm monomers. At the same time, physical networks were formed inside the hydrogel through hydrogen bonds between PNIPAm chains and active groups on the surface of XLS particles and C-dots, as shown in Fig.2(d). PS/PNIPAm composite hydrogel shows light color, similar as PS photonic crystal template. After PS/PNIPAm composite hydrogel was immersed in xylenes for 48 h, PS colliods were selectively removed. Finally, the physically cross-linked inverse opal hydrogel (coded as IONHPNIPAm) was obtained and it has brilliant structural color.
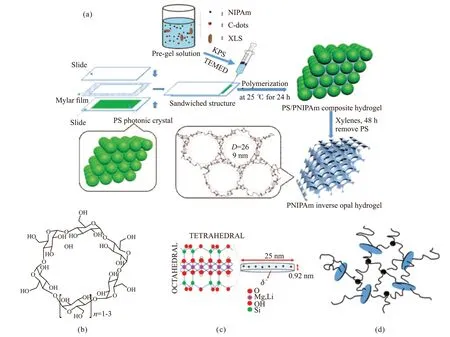
Fig.2 The fabrication of the physically cross-linked IONHPNIPAm (a); The structure of C-dots(b) and XLS particles(c); The physical network of PNIPAm, C-dots and XLS inside the hydrogel(d)
Three kinds of physically cross-linked IONHPNIPAm(S1, S2, and S3) were prepared by using different PS photonic crystal templates and the fixed contents of C-dots and XLS particles. As shown in the photos of S1, S2, and S3 (Figs.3(a)-3(c)), S3 exhibits a complete reddish blue color, while S2 has an incomplete blue color and S1 scarcely has structural color. It is found from Fig.1 that the spaces of the PS photonic crystal templates become wide from S1 to S3. It is obvious that bigger spaces benefit the infiltration of hydrogel precursor. Therefore, S3 has more crosslinking structures than S1 and S2, and completely kept the fcc structure after PS colloids were removed. S3, S4, and S5 (Figs.3(c)-3(e)) were prepared by using the same photonic crystal template. S4 still has complete structural color as S3,even if the amount of C-dots increased from 0.04g to 0.08 g. However, S5 exhibits incomplete structural color when the amount of XLS nanoparticles change from 0.2 g to 0.3 g. These results indicate that C-dots and XLS nanoparticles have different effects on the formation of physically cross-linked IONHPNIPAm. C-dots are spherical shape with the average particle size of 4 nm,while XLS nanoparticles are sheet shaped with 20 nm diameter and 0.8 nm thickness. Thus, the enter of XLS nanoparticles is more greatly limited by the space of PS photonic crystal templates, comparing to spherical C-dots with small sizes. Moreover, a high content of XLS nanoparticles increases the viscosity of hydrogel precursor. For these reasons, S5 has less crosslinking structures of and just keep part of the fcc structures.Therefore, it is worth noticing that the matching of the sizes of the nanoparticle crosslinking agents and the spaces of the photonic crystal templates is particularly important for a physically cross-linked inverse opal hydrogel system.
3.3 Structure of the physically cross-linked IONHPNIPAm
Fig.4(a) shows the SEM image of the surface of the polystyrene photonic crystal template filled with the PNIPAm hydrogel. The well-ordered face-centered cubic (fcc) structure and uniform PS colloidal spheres are observed. Meanwhile, at the spaces between the PS colloidal spheres, PNIPAm are observed, meaning PNIPAm hydrogel were formed around the PS colloidal spheres.
After completely etching the PS photonic crystal template, IONHPNIPAmfilm was obtained. As shown in Fig.4(b), the three dimensions highly ordered macropores form in the IONHPNIPAmfilm. The macropores with long-range order ensure the free diffusion of water in the IONHPNIPAmfilm. The average size of the macropores is 192 nm, smaller than the diameter of the PS colloids in Fig.1(e). The reason for this phenomenon is that the IONHPNIPAmfilm is swollen at the room temperature in the deionized water.
The inset photos in Fig.4(c) shows that the IONHPNIPAmfilm exhibits a clear reddish blue color at a swelling equilibrium state. Fig.4(c) shows that the IONHPNIPAmfilm still displays a sharp single reflection peak and the position of the reflectance peak wavelength lies at 485 nm, which was calculated according to the modified version of Bragg’s law[34]. Bragg’s law provides an explanation for the structural color of IONHPNIPAm:


Fig.3 Optical photographs of sample S1, S2, S3, S4, and S5 in Table 1 of the physically cross-linked IONHPNIPAm

Fig.4 SEM images of the surface of PS/PNIPAm composite hydrogel (a) and the IONHPNIPAm film (b); Reflectance spectrum and optical photograph (c) of IONHPNIPAm film
where,dis the diameter of the PS colloidal microspheres,mis the order of the Bragg diffraction,neffis the refractive index of the IONHPNIPAm,θis the incident angle of the light, andD/D0is the degree of equilibrium swelling of the IONHPNIPAm.
3.4 Mechanical property and self-healing property of the physically cross-linked IONHPNIPAm
The physically cross-linked IONHPNIPAmfilm prepared by our method is easily removed from the glass substrate and exhibits a good mechanical tensile property. After stretched several times, the IONHPNIPAmfilm is not broken and keeps its brilliant blue color, as showed in Fig.5. Therefore, IONHPNIPAmis not just a thin film on substrates and can be used independently without substrates.

Fig.5 The IONHPNIPAm could be properly stretched

Fig.6 Optical images of the self-healing process of two rectangle segments
To impart the structural color hydrogels with application diversity, healable test was set up and implemented. Two rectangle-shaped IONHPNIPAmfilms were put together to let them contact to each surface in room temperature. After 48 hours, the two IONHPNIPAmfilms formed an integrated rectangle hydrogel film, as shown in Fig.6. The internal structure of the hydrogel is restored, because C-dots and XLS can reform hydrogen bonds with the polymer chain on the interface of the two IONHPNIPAmfilms. Therefore, the physically crosslinked property imparts self-healing property to the IONHPNIPAm.
3.5 Thermal-responsive properties of the physically cross-linked IONHPNIPAm
Poly(N-isopropylacrylamide) (PNIPAm) is a wellknown temperature-responsive polymer[35]and has a low critical solution temperature around 34 ℃. We explored the response of IONHPNIPAmfilms to temperature. As shown in the photos of Fig.7(a), the IONHPNIPAmshows reddish blue color at 25 ℃. After the temperature increases to 46 ℃, the structural color of the IONHPNIPAmchanges to green-dish blue color. With the increase of temperature, the reflectance peak wavelength of the reflectance spectrum of IONHPNIPAmsustains blue-shift in Fig.7(b). The solid line in Fig.7(a) shows the relationship of the temperature and the reflectance peak wavelength. It can be found that the reflectance peak wavelength obviously decreases from 25 ℃ to 40 ℃. When the temperature is higher than 40 ℃, the reflectance peak wavelength scarcely changes with temperature.With the increase of temperature, PNIPAm chains transfer from a hydrophilic state to a hydrophobic state, so the volume of IONHPNIPAmshrinks (as shown in Fig.7(a),dotted line), which results in a reduction in the size of the voids of the IONHPNIPAm. When the temperature is higher than 34 ℃, the IONHPNIPAmreaches a hydrophobical state and its volume will not shrink further. Therefore, the reflectance peak wavelength of the reflectance spectrum has little change.

Fig.7 The peak wavelength of IONHPNIPAm from the Bragg diffraction spectrum and relative swelling ratio of IONHPNIPAm in different temperatures deionized water(a), and Reflectance spectra of IONHPNIPAm at different temperatures(b)
The recoverability of the IONHPNIPAmfor temperature was investigated by changing the ambient temperature between 25 ℃ and 46 ℃ for 6 times. Fig.8 gives reciprocating shifts of reflectance peak wavelength for the IONHPNIPAmfilm over 6 cycles, indicating the IONHPNIPAmhas good recoverability for temperature. Moreover, the film remaining intact after multiple cycles shows that they have good physical stability.This temperature-dependent structure color of the IONHPNIPAmindicates that the IONHPNIPAmis promising for fabrication of thermo-sensitive devices or sensors.

Fig.8 Reversible changes of reflectance peak wavelength for the IONHPNIPAm when the temperature was changed between 25℃ and 46 ℃
3.6 Fluorescence properties of the physically cross-linked IONHPNIPAm
Because of the addition of C-dots with strong fluorescence, the IONHPNIPAmis endowed fluorescent property. The IONHPNIPAmemit strong blue fluorescence under excitation of UV light (365 nm) and the largest fluorescence emission peak is at 515 nm, as shown in Fig.9.

Fig.9 Fluorescence spectra (a) of the IONHPNIPAm under excitation wavelength of 420 nm and optical photograph (b) of the IONHPNIPAm under UV light (365 nm) at 25 ℃
Moreover, C-dots have strong reducing property, so they can quickly reduce Ag+in sunlight[36]. IONHPNIPAmfilm was immersed in five AgNO3aqueous solutions with different Ag+concentrations (30, 35, 40, 45, and 50 μg/mL, respectively) for 30 min. Then, the fluorescence intensity of IONHPNIPAmfilm was detected. C-dots in IONHPNIPAmfilm excite electrons and reduced the Ag+to Ag nanoparticles, so the fluorescence intensity of IONHPNIPAmwill change. Fig.10(a) clearly shows that the fluorescence intensity of the IONHPNIPAmfilms gradually decreases with an increase of Ag+concentration.Further establishing the relationship between the Ag+concentration of AgNO3aqueous solutions and the fluorescence intensity of the IONHPNIPAm, it is found that the Ag+concentration is linearly correlated with the fluorescence intensity (Fig.10(b)). The linear correlation coefficient is 0.995, which can meet the requirement of quantitative analysis. Hence, we can make use of this fluorescent characteristic of the IONHPNIPAmto detect silver ions in water environment.
4 Conclusions

Fig.10 Photoluminescence response of C-dots-based IONHPNIPAm upon addition of increasing concentrations of Ag+ (a) and calibration plot of the quenching of C-dots-based IONHPNIPAm versus Ag+ ions(b)
In summary, the thermal-responsive IONHPNIPAmfilms have been synthesized by combining physically crosslinked nanocomposite hydrogels and PS photonic crystals for the first time. This method is simple, fast and reliable to IONHPNIPAmfilms with a fast respond speed, good mechanical stability and self-healing property. The fabricated IONHPNIPAmfilms have brilliant structure color, thermo-responsive property and fluorescence property. The observed phenomena or results reveal that the Bragg diffraction wavelength of the IONHPNIPAmfilms will have blue-shifts when the temperature rises from 25 ℃ to 46 ℃. Meanwhile the IONHPNIAPmfilms have excellent cycle stability when the temperature changes between 25 ℃ and 46 ℃.Moreover, the IONHPNIPAmfilms can emit strong blue fluorescence under excitation of UV light (365 nm)and quickly reduce Ag+in sunlight. Therefore, they can be applied in the detection of Ag ions. Compared with chemically cross-linked IOH, the physically crosslinked IONHPNIPAmopen new possibilities towards the development of smart optical, thermo-responsive and fluorescent sensing devices.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Experimental Study on Viscosity Characteristics of Expanding Polymer Grout
- Evalution of Thermal Oxidative Degradation of Trimethylolpropane Trioleate by TG/DTA/DSC
- Transformation Characteristics and Microstructure of Rail under Low Stress during Continuous Cooling
- Formation Mechanism and Existing Form of Sb in Heat Resistance Mg-Gd-Y-Sb Alloy
- Rapid Dendrite Growth in Solidification of Highly Undercooled Alloys
- Research of Ultrafine Cemented Carbides for PCB Microdrills
