Formation Mechanism and Existing Form of Sb in Heat Resistance Mg-Gd-Y-Sb Alloy
2021-04-16HUANGYuanchunLUOXiaoyuLIUYuXIAOZhengbing
HUANG Yuanchun, LUO Xiaoyu*, LIU Yu, XIAO Zhengbing
(1. Light Alloy Research Institute, Central South University, Changsha 410083, China; 2. State Key Laboratory of High Performance and Complex Manufacturing, Central South University, Changsha 410083, China; 3. School of Material Science and Technology, Central South University, Changsha 410083, China)
Abstract: The existing form and reaction mechanism of Sb in heat resistane Mg-Gd-Y-Sb rare earth magnesium alloy were investigated by inductive coupled plasma emission spectroscopy(ICP), scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), and X-ray diffraction (XRD). It is found that Sb tends to form high melting point intermetallics with rare earth elements of Gd and Y. The existing form of Sb is determined to be GdSb and SbY, respectively, which has high melting point (GdSb: 2 142 ℃/SbY: 1 782℃). Meanwhile, the first principle calculation and electronegativity difference calculation were performed to further understand the reaction mechanism. Therefore, the forming heat and binding energy were calculated.The experimental results show that the binding tendency of Sb element to Gd and Y is much stronger than that of it with other elements in this alloy, which results in the formation of high melting point of Gd-Sb and Y-Sb intermetallics, and finally leads to the high temperature resistant further improvement of the Mg-Gd-Y magnesium alloy.
Key words: Mg-Gd-Y-Sb alloys; rare earth elements; heat resistance property; intermetallics; first-principles calculation
1 Introduction
High-strength and high-toughness rare earth magnesium alloy[1]is currently recognized as one of the most promising new structural engineering materials. It has important requirements in the aerospace, defence[2],and is also considered to be an important material for the future automotive lightweight. However,insufficient heat resistance has always been an important factor limiting the application of many series of magnesium alloys in these industries.
Among the rare earth magnesium alloys, the Mg-Gd-Y series alloy is a relatively mature commercial rare earth magnesium. The addition of Gd and Y elements[3]has a certain effect on improving the heat resistance of magnesium alloys. A rare earth magnesium alloy of Mg-7Gd-5Y The best heat resistance is 250 ℃/140 MPa[4]. Another research has shown that the heat resistance of Mg-6Gd-3Y-0.5Zr rare earth magnesium alloy is 250 ℃/247 MPa and 300 ℃/146 MPa, Mg-10Gd-3Y-0.5Zr alloy, heat resistance property can be reached to 250 ℃/310 MPa and 300 ℃/182 MPa[5],which is better than common heat-resistant rare earth magnesium alloys WE43 and WE54[7]. Liet alstudied an Mg-Gd-Y-Sm-Zr alloy of GW1221 (with Sm added), whose heat resistance is about 250 ℃/282.61 MPa and 300 ℃/275.18 MPa[8]. The tendency of these alloys is that UTS decreases sharply with increasing temperature. The high-temperature strengthening phases of these rare earth magnesium alloys are mainly Mg-RE phase and LPSO phase[8,9], but when over 250℃, these strengthening phases will turn to soften,leading to failure at higher temperatures.
To further improve the heat resistance of rare earth magnesium alloys. Method of adding trace elements into alloy has been widely investigated.Studies have shown that after the addition of trace Sb in AZ and AS series magnesium alloys, the Mg3Sb2phase with good thermal stability is formed[10], and it has a morphology of the second phase. The shaping effect of these alloys further improves the heat resistance of these alloys. Liet alstudied the application of Sb in heat-resistant magnesium alloys[11], and analyzed the reaction mechanism and reaction products of Sb and Mg, While the reaction mechanism of Sb and Gd,Y is still unknown.
In the study of further improving the heat resistance of Mg-Gd-Y rare earth magnesium alloys,no reports and related studies have been found on the addition of trace amounts of Sb to Mg-Gd-Y rare earth magnesium alloys. Therefore, we carried out related research on the addition of trace Sb. After adding trace Sb, we found that its heat resistance was greatly improved compared to the same series of magnesium alloys. After aging, its heat resistance property can be improved to 250 ℃/362.91 MPa and 300 ℃/338.26 MPa. Therefore, we deeply studied the existing form and reaction mechanism of adding micro-Sb in the system and the reason for the improvement of its heat resistance.
2 Experimental
Melting and casting are performed in a 200 kg semi-continuous casting system with protective atmosphere protection. The raw materials used for melting are pure magnesium, pure zinc, Mg-30%Gd master alloy, Mg-30% Y master alloy, Mg-30%Zr master alloy, and Sb2O3powder. The melting temperature is about 750 ℃. Then adding #6 refining agent (compositions: 26% MgCl2, 22% KCl, 29% BaCl2,14% CaF2, 4% CaCl2, 4% NaCl, and 1% MgO)[12].The next step was refining and removing the slag followed by casting as the final step. The whole process was under the protection of the protective gas which mixed with SF6+CO2in proper proportion. Following homogenization, the ingot was hot extruded to 46 mm×66 mm square bar and then aged at 200 ℃ for 45 h.
The composition of the ingot sample was characterized by inductive coupled plasma emission spectroscopy(ICP). microstructure observations of the alloys were carried out on Phenom ProX scanning electron microscope, scanning electron microscopy(SEM) and energy dispersive spectroscopy (EDS).X-ray diffraction(XRD)analysis used ADVANCE 8D series X-ray diffractometer. The sample was scanned in the range from 5° to 105° and the target material of the X-ray diffractometer is a copper target[13]. The result of XRD in Fig.4 is obtained from the ingot sample.
The reaction behavior and reaction mechanism were calculated by these ways which conclude electronegativity calculation and first-principle calculation. Electronegativity is the scale of the ability which elemental atoms to attract electrons to form compounds[14,15]. Therefore, the electronegativity difference can explain the possibility of two elements to compound. There is a rule that the greater the difference of electronegativity, the easier the binding to compound and the more obvious the electron pair migration.
The most important calculation and analysis are based on the first-principles method. The first-principles method is a quantum mechanics method with density functional theory[14]. It adopts the CASTEP quantum mechanics calculation package in Material Studio 7.0.The specific calculation method is as follows:
The total energy plane wave pseudopotential method is used to replace the ion potential with the pseudopotential. The wave functions of the electrons are expanded by the plane wave basis set. The exchange and correlation potentials of the electronelectron interaction are corrected by the generalizedgradient-approximation (GGA)[15].
The maximum cut-off energy truncated value(ultra fine) calculated by plane wave is 400 eV for GdSb, 380 eV for MgY, 380 eV for Mg24Y5, 400 eV for Mg3Gd phase, 380 eV for Mg3Sb2phase, and 290 eV for SbY phase. In reciprocal space, the selected density of K-point in the first Brillouin region (fine) for GdSb is 4×4×4, and the selected density of MgY is 8×8×8,2×2×2 for Mg24Y5and 4×4×4 for Mg3Gd, Mg3Sb2is 6×6×4, and SbY is 4×4×4.
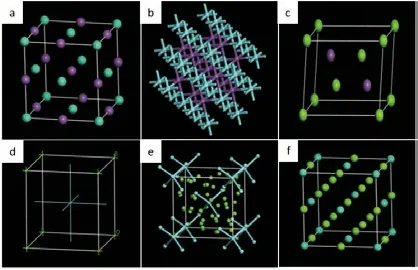
Fig.1 The cell structures of calculated: (a) GdSb; (b) SbY; (c)Mg3Sb2; (d) MgY; (e) Mg24Y5; (f) Mg3Gd
At the same time, the Broyden-Fletcher-Goldfarb-Shanno (BFGS) method is used to optimization the lattice constants[16]and the specific positions occupied by the atoms in the cell. The energy convergence value of SCF is set to 5×10-6eV/atom[17], the interaction force between atoms is no more than than 0.01 eV/A,the maximum spacing is not more than 5×10-4A°, the maximum stress is not more less than than 0.02 GPa,and the maximum force is no greater not more than 0.01 eV/A. Next, the calculation of heat and binding energy is made.
Crystal structure and model: the GdSb and SbY belong to the cubic system[18,19], and its space point group is Fm-3m, space group number is 225; Mg3Sb2belongs to the triclinic system with a spatial point group of P-3m1 and the space group number of 12.MgY phase belongs to cubic system, its space point group is Pm-3m, space group number is 221; Mg24Y5belongs to cubic system, its space point group is I-43m, space group number is 217; Mg3Gd belongs to cubic system[20,21], and its space point group is Fm-3m and space group number is 225. The cell structures of each phase are shown in Fig.1.
3 Results and discussion
3.1 Hot tensile tests
High-temperature tensile tests were carried on an INSTRON high-temperature tensile mechanical testing machine at 250 ℃ and 300 ℃, respectively, at a tensile speed of 0.2 mm/min. There are three parallel samples,and the test results are shown in Fig.2.
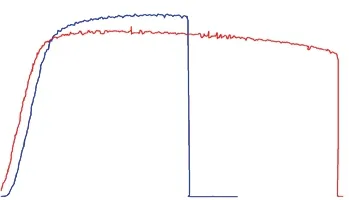
Fig.2 Hot tensile curves at different temperatures

Table 1 The comparative of high temperature property
At 250 ℃, the tensile strength of the alloy can reach 362.91 MPa, while at 300 ℃, the tensile strength is still 338.26 MPa. From Table 1, we compared with the GW103 (Mg-10Gd-3Y) alloy without Sb, the heatresistant strength of the alloy at the same temperature is much higher. At 250 ℃, it is increased by 17.07%,and at 300 ℃, it is increased by 85.86%. Especially at higher temperature, the improvement is more obvious,which can reflect the improvement effect of heat resistance after adding Sb.
3.2 Reaction production
According to the inductive coupled Plasma emission spectroscopy(ICP) result in Table 2, it can be seen that the actual composition of the ingot approach to the standard.
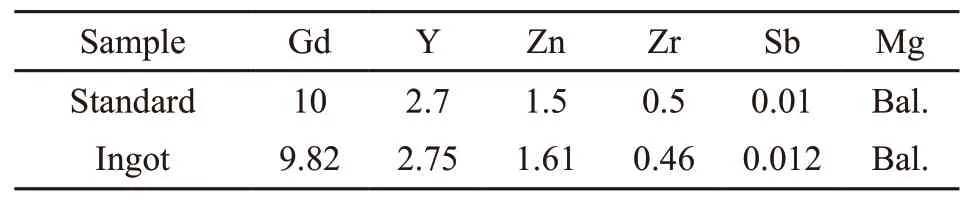
Table 2 Theoretical value and element analysis of ingot ICP
X-ray diffraction (XRD) was used to recognize the existing form of Sb in Mg-Gd-Y alloy for further accurate analysis.
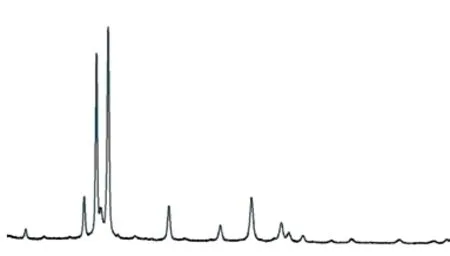
Fig.3 XRD diffraction pattern of sample
From the Fig.3, the XRD results show that the phases of GdSb and SbY intermetallic compounds have been accurately found. That means Sb and rare earth elements truly have metallurgical reactions that form two-element intermetallic compounds GdSb and SbY.
On the other hand, there are mainly as-cast second phases of Mg3Gd and intermetallic compounds of Mg-Y series in as-cast sample, which is Mg24Y5[22,23]phases. However, the second phase phase Mg3Sb2in Mg-Sb alloys was not be found.
The results of energy dispersive spectrometer(EDS) analysis the magnesium matrix and the fishbone shape Mg (Gd, Y) as-cast second phase[24]in the Fig.4. While the distribution of Sb is uneven, the spot 1 is almost completely undetectable Sb. In contrast,according to spot 2, Sb can be seen exist in the as-cast fish-bone second phase where rich in Gd and Y rare earth elements. Sb has a stronger affinity with Gd and Y elements from the experiment result observation.
The EDS mapping analysis is used to further detective the distribution of Sb, Gd, Y elements in the entire observation region of the ingot and the correlation among three elements. Using the 15 kV accelerating voltage, the electron beam type was set to streaming, and setting the resolution to 512 pixels and the step time to 50 ms. The precision mapping analysis is obtained as follows. The distribution of the four elements of Mg, Gd, Y, and Sb are analyzed. Its result is shown on Fig.5.
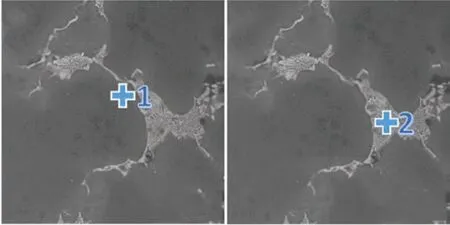
Fig.4 EDS analysis images and results of ingot sample: (a) The position 1 in ingot sample; (b) The position 2 in ingot sample; (c) Position 1 EDS analysis; (d) Position 2 EDS analysis
Blue is Mg in the magnesium alloy matrix, while the Gd and Y elements rich in the second phase. At the same time, it can be clearly seen that although micro-Sb was added, it is mainly distributed in the bonelike second phase with Gd and Y, the distribution concentration in the matrix is very small less. this indicate that Sb in Mg-Gd-Y alloy is GdSb and SbY binary intermetallic compounds. It’s distribution mainly can be found in the bone-like second phase, but very rarely distribute in the magnesium alloy matrix.
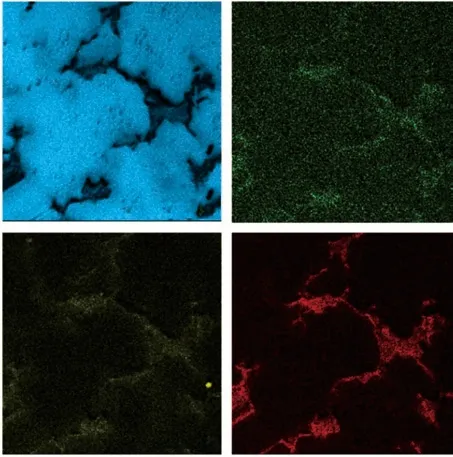
Fig.5 EDS mapping analysis results: (a)Mg; (b) Sb; (c) Y; (d) Gd
By comparing these results, we can further explain the existing form of Sb in Mg-Gd-Y alloy is GdSb and SbY binary intermetallic compounds. It’s distribution mainly be found in the bone-like second phase, but very less distribute in the magnesium alloy matrix. That means due to the addition of Sb,Gd, and Y elements in Mg-Gd-Y alloys reacting with Sb, resulting in a number of Sb, Gd, and Y elements primarily formation reaction products such as GdSb and SbY, whose high melting point can reached to 2 141 ℃ and 1 782 ℃, respectively. These high melting point products distribution in bone-like second phase.
The whole reaction processing is when adding Sb2O3to the melt, reducibility magnesium melt will first restore Sb2O3to produce pure Sb and MgO at high temperature.
The reaction equation is as follows:
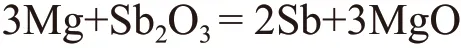
The reduced Sb will be uniformly distributed in the melt as a simple substance free state, and preferentially react with Gd and Y elements to form high melting point intermetallic compounds GdSb and SbY. The magnesium oxide will be removed from the melt with the subsequent refining and slag raking process, and the reacted product MgO will not damage to the properties of the ingot.
The all of Sb has already be reacted, thus Mg3Sb2not be produced and can not be found. For investigating on this phenomenon and metallurgical reaction mechanism. The way of electronegativity analysis and first principles was used to further exploration.
As to the mechanism of improving the high temperature performance of Sb. From SEM and EDS energy spectrum scanning, it can be observed that the fine phases GdSb and SbY with high melting point are dispersed in the second phase of the alloy.In the process of thermal deformation, these phases will play a role in hindering the grain boundary movement, pinning the grain boundary and impeding the coordinated deformation mechanism of multigrains, thus hindering the sliding and deformation of the alloy at high temperature, and achieving the effect of improving the heat resistance strength of the alloy.
3.3 Reaction mechanism
Based on the series of experiments and studies,the reaction product after adding antimony components to Mg-Gd-Y magnesium alloys are verified. The reaction mechanism and products of the metallurgical reaction are explored by means of electronegativity calculation and further first-principles calculation.
3.3.1 Electronegativity calculation
The calculated results are given in Table 3. It shows that the order of electronegativity difference from large to small is Sb-Gd > Sb-Y > Sb-Mg > Mg-Gd > Mg-Y, while the electronegativity difference of Zn and Zr with Mg, Gd, Y, and Sb is far less than above sequence, so they will not be preferentially reformed.Based on the electronegativity principle[23], Sb-Gd and Sb-Y are most easily generated in this system.
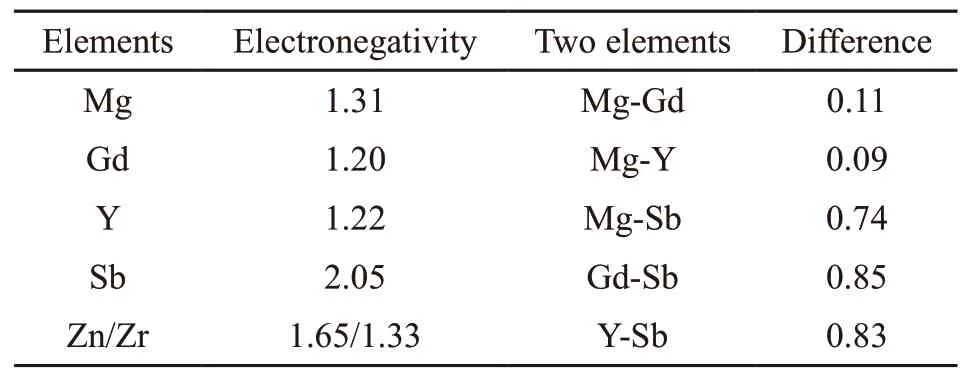
Table 3 The Electronegativity difference between different elements
From the view of electronegativity, the main reason is that Sb has a stronger affinity with Gd and Y rare earth elements. The difference of electronegativity among Sb, Gd, and Y is not only far greater than that between Sb and Mg matrix, but also greater than that among Gd, Y, and Mg matrix. The sequence from large to small is Sb-Gd > Sb-Y > Sb-Mg > Mg-Gd > Mg-Y,which can explain to a certain extent that Sb-Gd and Sb-Y compounds will be preferentially formed. At this time, the addition of less proportion of Sb has been fully reacted, while Gd and Y have a surplus, so there is no major Mg-Sb strengthening phase such as Mg3Sb2in the ingot.
3.3.2 First-principles calculation
The calculated results of forming heat and binding energy are given in Table 4.
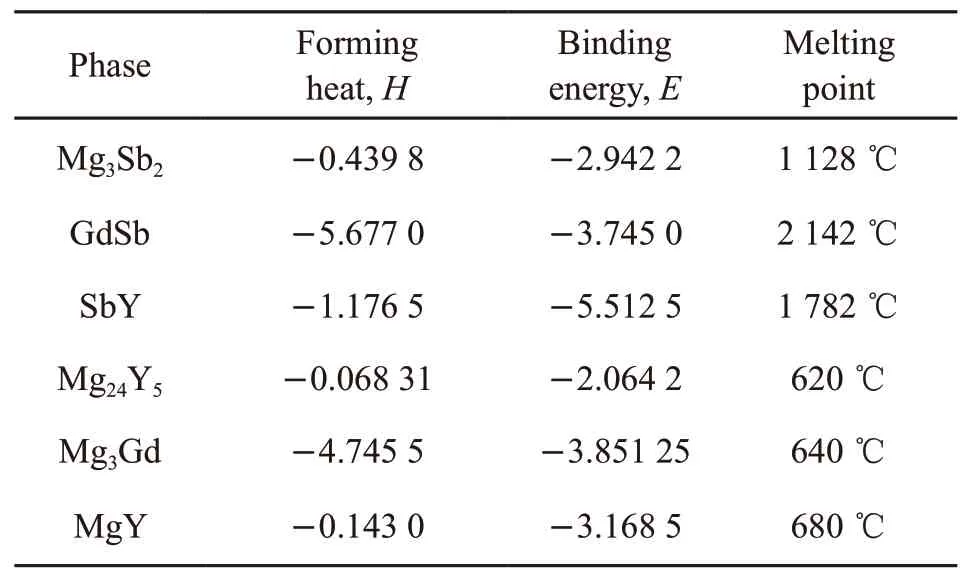
Table 4 Calculation results of forming heat and binding energy of reaction phase
According to the calculation results, the forming heat and binding energy contrast diagrams are made. In order to convenience to comparison, the binding energy data is taken absolute value, and the result is shown in Fig.6.
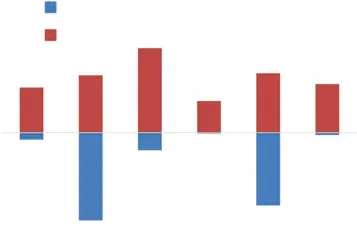
Fig.6 Comparison of calculated forming heat and binding energy of each phase
The forming heatHcan reflect the alloying ability of alloy components, and can be used to indicate the degree of difficulty in the formation of compounds[25,26].The binding energyEcan be used to indicate the stability of compounds after formation. The value of binding energy is defined as the work required for the decomposition of a compound into a single atom after the formation of a compound. For a binary compound AxBy, the forming heatHand binding energyEare calculated by the formulae (1) and (2), which are shown as follows:
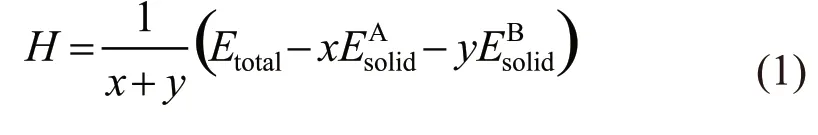
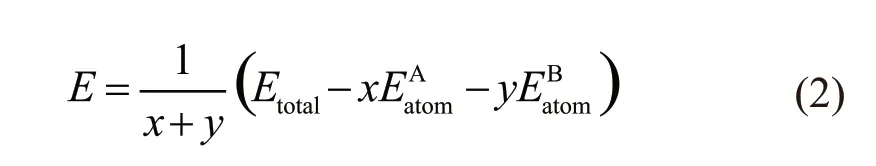
In the formulae (1) and (2), the subscript ‘total’is the total ground state energy of the compound after structural optimization, and the subscript ‘atom’ is the energy of a single atomXin the solid-state.
For example,H< 0 indicates that the formation of the formed phase is an exothermic spontaneous reaction process, and the greater the absolute value ofH, the easier the reaction can proceed;Eindicates the energy released by the formation compound, and the larger the absolute value ofE, the more stable for the formed phase and the stronger the resistance to high temperature.
From the point of the forming heat and binding energy calculated by the first-principles method, the spontaneous formation must conform to the rule that is the forming heat must be negative value, and the absolute value of the forming heat is GdSb > Mg3Gd>SbY > Mg3Sb2> MgY > Mg24Y5, and the absolute value of the binding energy is SbY > GdSb > MgY >Mg3Sb2> Mg24Y5.
Furthermore, from the first-principles calculation,the forming heat and binding energy data are calculated for exploring the reaction mechanism clearly.
From the physical meaning of forming heatHand binding energyE[27], the greater the absolute value,the greater the tendency of spontaneous reaction, and thus the formation will be easier. Therefore, GdSb and Mg3Gd will be formed in order of priority. However,the binding energy of SbY is highest, the binding energy of Mg3Gd and GdSb is relatively high which means the stability after formation is high, all of them can be retained in the system. Then MgY and Mg24Y5will be formed in the system. Because the forming heat and binding energy both low, this phase is difficult to form and also not stable. They will have small amount remaining in the matrix. As for the Mg3Sb2phase,because the content of Sb is very less, it has been consumed by Gd and Y rare earth elements in the initial reaction, the phase Mg3Sb2cannot be formed in the subsequent forming process.
The order of reaction tendency in this system is basically according with the result which calculated by electronegativity principle.
At the same time, due to the melting points of Mg3Gd (640 ℃), MgY (680 ℃), and Mg24Y5(620 ℃)are relatively lower. Mg3Gd is an eutectic compound,which can be incorporated into the melt and remain in the matrix as the main as-cast second phase of rare earth magnesium alloy and a small amount of the second phase of Mg-Y system in the casting process. However,the preferred compounds GdSb and SbY, because of their high melting point (GdSb melting point[24]is 2 142 ℃, and SbY melting point[25]is 1 782 ℃).This result is almost identical with XRD analysis. At the same time, from the data of Table 4, GdSb and SbY also have high binding energy. That means they have better thermal stability under high temperature.These GdSb and SbY will become reinforcing particles to prevent grain boundary sliding under high temperatures. This is the reason that micro-Sb addition can improve the heat resistant of Mg-Gd-Y alloy.
In summary, the addition of Sb in Mg-Gd-Y alloys will result in the preferential reaction of Sb with Gd and Y elements, and it’s difficult to form Mg3Sb2optimized phase[28]. At the same time, it will produce high melting point production GdSb and SbY. These phase has high temperature resistance and thermal stability, which can contribute to this alloy for better mechanical property under high temperature.
4 Conclusions
a) The addition of micro-Sb to Mg-Gd-Y rare earth magnesium alloy further improved its heat resistance property to 250 ℃/362.91 MPa and 300℃/338.26 MPa.
b) The existing form of Sb in Mg-Gd-Y alloy is GdSb and SbY binary intermetallic compounds. It’s distribution mainly exist in the bone-like second phase
c) The reaction mechanism of GdSb and SbY according to the comprehensive judgment of forming heat and binding energy which depended on firstprinciple calculation, the following reaction sequences are GdSb and Mg3Gd, and SbY will be preferentially generated, both GdSb and SbY have high binding energy which shows them high thermal stability.
d) The reaction tendency of Sb in Mg-Gd-Y alloy is ranked as follows: Sb-Gd > Sb-Y > Sb-Mg > Mg-Gd > Mg-Y. A conclusion can be made that Sb and rare earth elements have stronger binding tendency.However normally second phase Mg3Sb2will not reform for Sb has been completely reacted with RE elements.
e) The reason for Mg-Gd-Y alloy after Sb addition has better high temperature resistance is that adding Sb to Mg-Gd-Y magnesium alloys will primarily reform GdSb and SbY with high melting point(GdSb: 2 142℃; SbY: 1 782 ℃) and lead to better thermal stability.The existence of those bone-like phase will also effectively improve the heat resistance property of Mg-Gd-Y magnesium alloy.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Comparative Case Study on Adhesion of Three Common Sizing Agents to Cotton and Polyester Yarns
- Effect of Outer Carbon Layer Thickness of Carboncovered N-doped Hollow Carbon Nanospheres on Its Electrocatalytic Performance
- Ceramification of Composites of MgO-Al2O3-SiO2/Boron Phenolic Resin with Different Calcine Time
- Natural Fresh Proteins Directed Hierarchically Porous Nitrogen-doped TiO2 as with High Performance as Photocatalyts and Electrode Materials
- Dynamic Adsorption of Toluene on Hierarchical Porous Carbons with Varying Pore Structure
- Self-propagating High-temperature Synthesis of Sm and Zr Co-doped Gd2Ti2O7 Pyrochlore Ceramics as Nuclear Waste Forms
