Evalution of Thermal Oxidative Degradation of Trimethylolpropane Trioleate by TG/DTA/DSC
2021-04-16ZHANWenDUANHaitaoLIXinxiangLIJianYUANChengqing
ZHAN Wen, DUAN Haitao, LI Xinxiang, LI Jian, YUAN Chengqing
(1. Wuhan Research Institute of Materials Protection, China Academy of Machinery Science and Technology, Wuhan 430030, China;2. School of Energy and Power Engineering, Wuhan University of Technology, Wuhan 430063, China)
Abstract: In order to evaluate the thermal oxidation degradation behavior of lubricant with different antioxidants, the thermal kinetics equation based on the anlyses of thermogravimetry(TG), differential thermal analysis(DTA), and differential scanning calorimetry(DSC) was established, respectively, to calculate the activation energy of lubricant thermal-oxidative reaction. The thermal analyses of TG and DTA were employed to determine the thermal decomposition properties of ester oils trimethylolpropane trioleate(TMPTO) with butyloctyl-diphenylamine/octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate/amine-phenol combination antioxidant. The activation energy of the lubricating oil adding antioxidant is increased relative to the TMPTO base oil, and the order of activation energy are Ec (93.732 kJ·mol-1)>Ed (88.71 kJ·mol-1)>Eb (58.41 kJ·mol-1)>Ea (46.32 kJ·mol-1). The experimental results show that octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate in TMPTO has favorable resistance to thermal oxidation and decomposition. The thermal analysis method of DSC accurately reflects the heat exchange of lubricant thermal-oxidative reaction. The order of activation energy is calculated to ED (144.385 kJ·mol-1) > EC (110.05 kJ·mol-1) > EB (97.187 kJ·mol-1) > EA(66.02 kJ·mol-1). It is illustrated that the amine-phenol combination antioxidant has the best thermal oxidation resistance, which is the same as what the oxidation onset temperature effected.
Key words: thermal oxidative degradation; thermal kinetics; antioxidants; trimethylolpropane oleate
1 Introduction
Since continuous improvement of high temperature resistance, heavy load resistance and long life cycle of mechanical parts in the fields of automobiles,aerospace and large ships, high reliability and high efficient lubrication under rigorous working conditions is hard to achieve. Improving and monitoring the active performance of synthetic lubricants in mechanical system have became breakthrough direction for researchers[1,2]. Therefore, the development of synthetic lubricant antioxidants and their evaluation of thermaloxidative property are of great significance.
Thermal analysis kinetics is a method of studying the physical and chemical reaction mechanisms of materials using thermal analysis techniques[3]. With the help of mathematical processing, the corresponding reaction kinetic parameter calculation equation can be deduced and the performance of the reactants can be scientifically evaluated. That method has the advantages of quickness, simplicity, few sample consumption,and ignoring to analyze reactant or product structures.Thermal analysis technology has been used to rapid and convenient evaluation of the thermal characteristics of lubricating oils and their antioxidant additives[4,5].Jayadasa NH[6]used thermogravimetry analysis(TG)and differential scanning calorimetry(DSC) to study the thermal oxidative degradation mechanism of coconut oil, sesame oil, sunflower oil and commercial oil ester at low temperature. Jain MR[7]studied the thermal oxidation properties of gear oils by thermal differential scanning calorimetry(PDSC), TGA, rotating oxygen bomb (RBOT) and IP 48, and discussed the effect of different antioxidant additives on the thermal oxidation characteristics of gear oils. Hua JQ[8]used DSC to test the initial oxidation temperature(IOT) and oxidation induction time(OIT) of synthetic oil-soluble molybdate and dioctyldiphenylamine synergistic antioxidants in PAO. The addition of dioctyldiphenylamine resulted in higher IOT and OIT than molybdate. Mads[9]synthesized an organic phosphate ester, and evaluated the thermal oxidative degradation properties of the additive by measuring the initial decomposition temperature of the mineral oil by TG. That results showed the mineral oil added with the organic phosphate present better thermal oxidation performance than the base oil.
Thermal analysis techniques are widely used for the evaluation of thermal properties of lubricants and their additives, but individuals mostly focus on the direct results of thermal analysis, such as (oxidation onset temperature)OOT, OIT, signal maximum temperature (SMT), the Rancimat, initial decomposition temperature (IDT) and integral procedure degradation temperatures (IPDTs)[10-15]. It is generally known that understanding the kinetics of thermal oxidation and thermal decomposition of lubricating oils are the core element that reveals the mechanism of thermaloxidative degradation. The thermodynamic parameters of thermal oxidation and decomposition process of lubricating oils examined by thermal analysis method are as a result of the competition of complex base oil molecular degradation products or numerous parallel reactions. By importing the reaction kinetics into the thermodynamic process, a relatively independent evaluation method can be established for the thermal properties of lubricating oil. Activation energy of the lubricating oil as one of thermal analysis kinetic parameters indicates that how much the degree of lubricating oil reacted. Generally, the greater the activation energy, the less likely the lubricating oil could be degraded. There have been a slice of reports on research work, but the dynamics models are not centralized. Santos JCO[16]used isothermal and nonisothermal thermogravimetric analysis to study the activation energy and dynamic evolution behavior of mineral oil by the Coats-Redfern model method based on the mass loss at unequal temperature and time. That results showed the thermodynamic parameters were highly correlated, and the linear correlation standard obtained had low variation. Aoyagi Yasuhiro[17]used thermogravimetric analysis to evaluate the thermal properties of semi-hindered phenol and thiopropionate antioxidants added to polyol esters by mass loss.The combination of antioxidants can not only effectively inhibit the viscosity of lubricating oil but also significantly reduce the thermal decomposition tendency, and the thermodynamic isotherm method was employed to illustrate the influencing factors of the pyrolysis process.
The oxidation resistance of the antioxidant could be directly judged by examining the activation energy of lubricating oil with different antioxidants. In this paper,based on TG, DTA, and DSC combined with reaction kinetic equations to derive the reaction activation energy calculation of lubricant-related applicability methods, the effects of self-synthesized amines and phenolic antioxidants on thermal oxidative behavior of ester oils trimethylolpropane oleate(TMPTO) were discussed(Fig.1), and a reasonable evaluation method for lubricating oils was proposed.
2 Experimental
2.1 Synthesis of butyl-octyl-diphenylamine
An ionic liquid [Bmim]Cl-0.6AlCl3was prepared as an alkylation catalyst. The reaction was carried out in a three-neck. The optimized experimental conditions were selected shown as follows[18]: the amount of ionic liquid was 20mass% of diphenylamine, the molar ratio of the first stage diphenylamine to 1-chloro-n-butane was 1:1.5, and the solution was stirred and refluxed for 9 hours at 90 ℃ temperature; the molar ratio of the second stage diphenylamine to 1-chloro-n-octane was 1:1.3, and the solution was stirred and refluxed for 7 hours at 120 ℃.
2.2 Synthesis of octadecyl 3-(3,5-di-tertbutyl-4-hydroxyphenyl)propanoate
Potassium tert-butoxide was selected as catalyst for the one-step synthesis process of octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate, of which optimized conditions is as follows[19]: the molar ratio of 2,6-di-tert-butylphenol with methyl acrylate was 1.1:1 in the first stage, the potassium tert-butoxide accounted for 7.5mass% of the 2,6-di-tert-butylphenol,the solution was stirred and refluxed for 4 hours at 110 ℃. The second stage did not separate product and other raw materials after the first stage, what’s more,directly reacted with the alkyl alcohol, and controlled the molar ratio of the added octadecyl alcohol to the 2,6-di-tert-butylphenol was 0.6:1, the solution was stirred and refluxed for 3 hours at 130 ℃. After the reaction had completed, the mixture was cooled to room temperature, and washed with water to remove residual alcohol. The upper layer solution was extracted and dissolved in absolute ethanol, crystallized, and the crystals were filtered to obtain the target product.
2.3 Thermogravimetry(TG), differential thermal analysis(DTA) and differential scanning calorimetry(DSC)
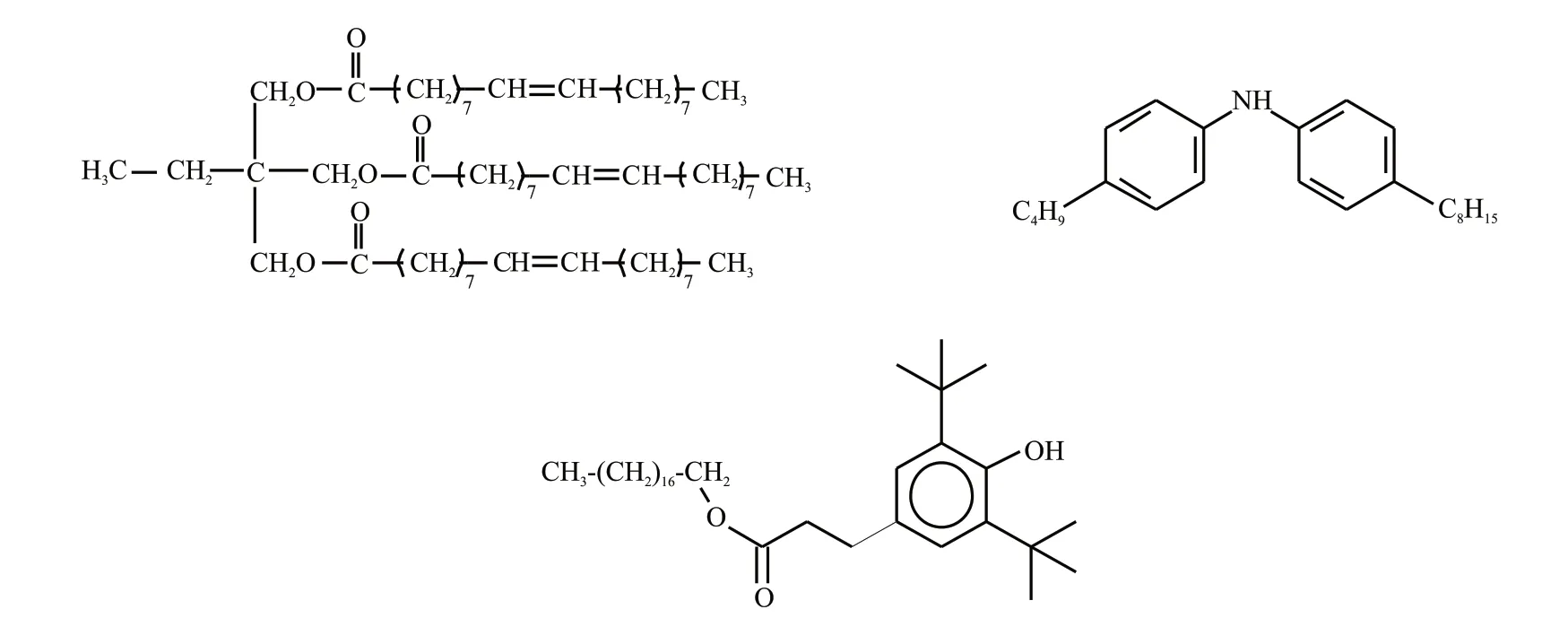
Fig.1 Chemical structures of base oil and antioxthions:(a) Trimethylolpropane trioleate;(b) Butyl-octyl-diphenylamine;(c) Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate
The thermal analysis of the oxidation resistance of the different antioxidants were carried out. The heating rate of the TG-DTA test (STA2500 synchronous thermal analyzer NETZSCH, Germany) includes 10℃·min-1, 15 ℃·min-1, 20 ℃·min-1, oxygen flow rate of 30 mL·min-1. The DSC test (Germany NETZSCH HP204) refers to the standard ASTM E2009 with parameters of heating rate 10 ℃·min-1, oxygen flow rate 30 mL·min-1, and temperature operating from 50℃ to 300 ℃.
All of experiment in the paper, lubricant A represents sample of TMPTO (purchased from Lanzhou Institute of Chemical Physics of Chinese Academy of Sciences), lubricant B represents sample of TMPTO with 1mass% of synthetic butyl-octyl-diphenylamine,and lubricant C represents TMPTO with 1mass%of synthetic octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate, lubricant D represents TMPTO with 0.5mass% of butyl-octyl-diphenylamine and with 0.5mass% of octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate.
2.4 Establishment of thermal-oxidative kinetic equation
Non-isothermal modes is the main method selected by thermogravimetric to study reaction kinetics in recent years. Its main advantage is to obtain relevant kinetic data from thermogravimetric curve,which should continuously analyze to thermal reaction kinetics over the entire temperature range of mass loss.Doyle firstly proposed to use the integral method to deduce the thermal reaction kinetics equations, and to separate variate of the basic equation[20]:
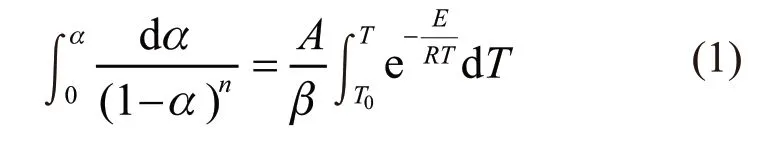
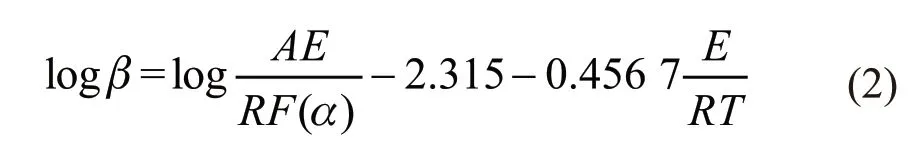
where,Tis the absolute temperature(K),Eis the apparent activation energy(kJ·mol-1),Ris the gas constant(8.314 J·mol-1·K-1),Ais the pre-exponential factor(s-1),αas a fractional mass loss represent the extent of reaction, andβis the heating rate(K·min-1).At the same that heating rate, logβand 1/Tof different rates could be read, the change of activation energy was derived by linear fitting.
A multitude of thermal analysis experiment show heating rate as one of conditions could significantly effect on the DTA curves. When the heating rate increases, the larger the heat effect per unit time magnify, and the peak temperature generally moves toward higher temperature location, and the area of peak accordingly extend. The Kissinger method proposes a quantitative relationship between the heating rate and the peak temperature of DTA curves[21]:
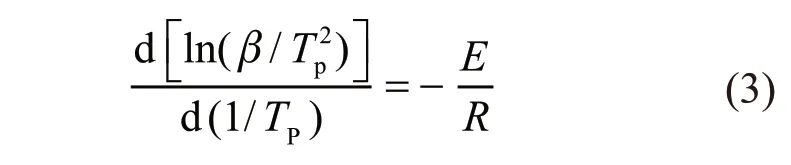
where,Tpis the peak temperature of DTA express in absolute temperature. This equation indicates that the degree of influence of the heating rate on the peak temperature of DTA relates to the activation energy of the reaction. When using differentβ1,β2,…, then the above formula (3) can be transformed to calculate the activation energy equation by differential thermal analysis, which could be calculated from a plot of ln(β/T2)against 1/Tfor a series of experiments.
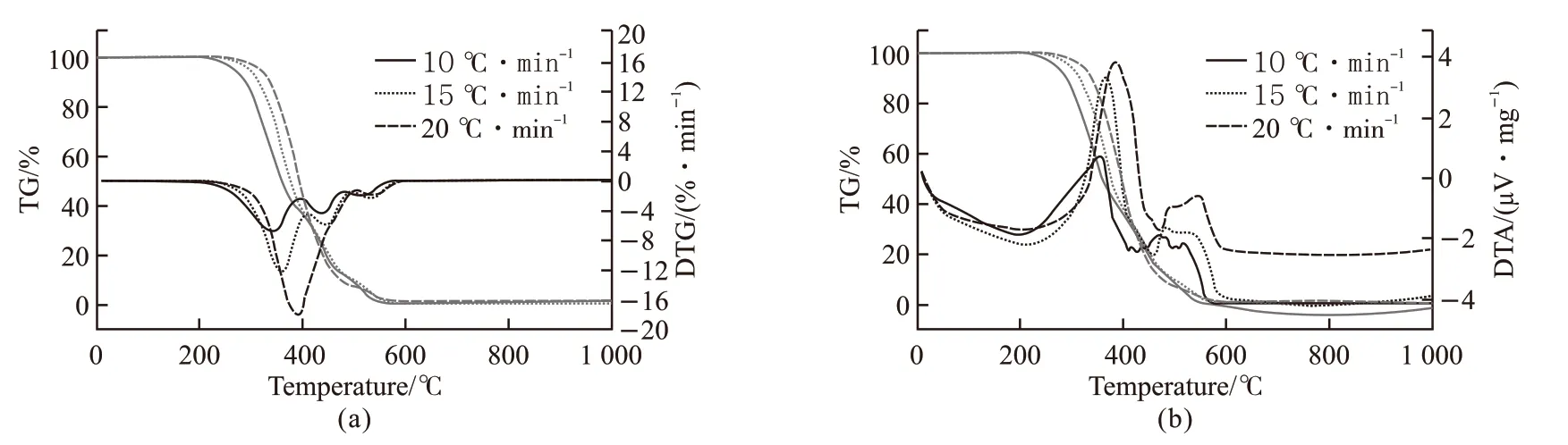
Fig.2 TG/DTG(a) and TG/DTA(b) curves of lubricant A at different heating rates
The main premise of thermal kinetics for examining differential scanning calorimetry is that the degree of reaction should be proportional to the thermal effect as the heating release or absorption, which can be expressed by the following formula:
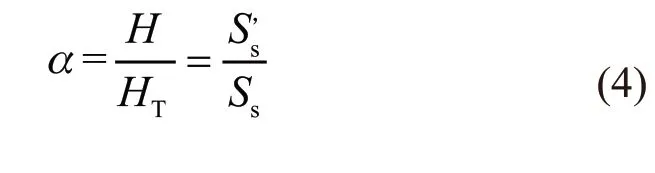
Consequently, the basic equation of reaction kinetics can be rewritten as:
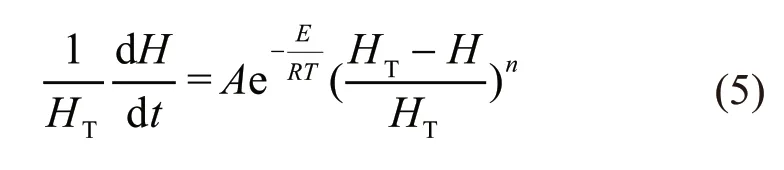
The activation energy namedEcan be calculated by comparing the DSC curves with the same mass loss change of reaction at different heating rates. If that same change occur at the peak temperature, it is further expressed as:
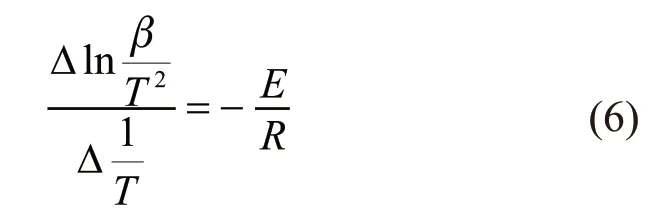
The influence of the heating rateβon the peak temperatureTof DSC relates to the activation energy,which could be calculated by the linear relationship between ln(β/T2) and 1/Testablished by the formula(6).
3 Results and discussion
3.1 Thermal stability of TMPTO with antioxidants by TG/DTA testing
Figs.2(a)-2(b) show that the results of TG-DTGDTA analysis of lubricant A at different heating rates.The mass of the sample decreases with increasing temperature. As the curve of heating rate at 10 ℃·min-1,the TMPTO base oil experienced three weight loss stages. The initial decomposition temperature of the first stage is 287 ℃ until the peak temperature of DTG reaching 341 ℃. This process is mainly caused by intramolecular oxidation of the base oil to form various oxidation products and carbon dioxide, making quiet a few substance directly decomposed or volatilized.Afterwards progressing at the second stage 390-480℃, the base oil undergo deep oxidation, resulting in cross-linked polymerization products, more carbon dioxide and decomposition of hydrocarbons of higher molecular weight. When heating up to 480 ℃ and quickly being the third stage, charking produces maybe get at further deep-oxidation and dehydrogenation until all decomposition or carbonization, the weight loss is slightly slowed. As the heating rate increasing the molecular oxidation accelerated, the initial decomposition temperature shifted large, the thermal oxidation and decomposition of lubricant can be directly completed.
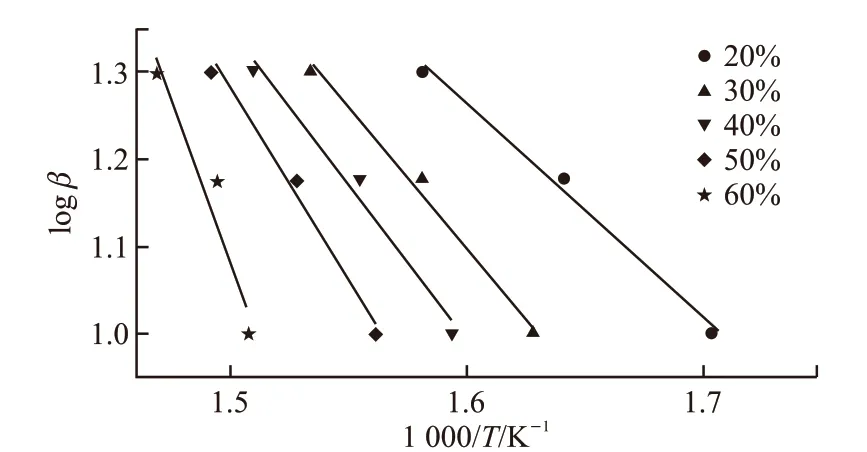
Fig.3 The slope curves by plotting of the activation energy EA1 under different a
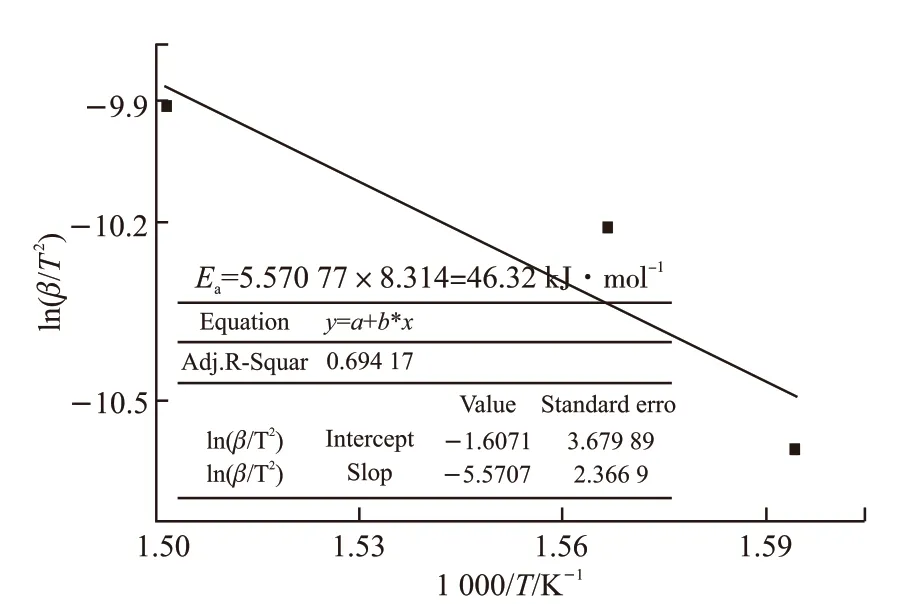
Fig.4 Activation energy Ea calculated from DTA curves by Kissinger method

Fig.5 TG/DTG(a) and TG/DTA(b) curves of lubricant B at different heating rates
To avoid considering deep oxidation, we independently consider the first stage of thermal oxidative decomposition (about loss rate ≤60%) as a reference to explore the degree of oxidation reaction of base oil, calculating the activation energy of that reaction using the established formula (2), and then the corresponding values of multiple heating rates were obtained from Table 1. The change of activation energyEA1was derived by linear fitting in Fig.3. The DTA curves are employed to measure the activation energy of thermal reaction by the formula (3) derived by the Kissinger method. The exothermic peak temperatures corresponding to the heating rates of 10 ℃·min-1,15 ℃·min-1, and 20 ℃·min-1are 354 ℃, 365 ℃, and 393 ℃, respectively. Accordingly, obtaining the slope-5.5707 by linear fitting in Fig.4, the activation energyEawas calculated to be 46.32 kJ·mol-1.
Figs.5(a)-5(b) manifest respectively the results of TG/DTG and TG/DTA analysis of lubricant B at different heating rates. All curves of TMPTO base oil with butyl-octyl-diphenylamine also experienced three weight loss stages. The initial decomposition temperature of the first stage is 308 ℃ until the peak temperature of DTG reaching 359 ℃ as the curve of heating rate at 10 ℃·min-1. This process mainly involve to the evaporation of most volatile components from the low molecular weight of the oil[22], followed hydrocarbons degradation by the oxidation resistance of butyl octyl diphenylamine. The reaction activation energy was calculated using the formula (2) from the TG curves, and then the corresponding values of multiple heating rates were obtained from Table 2. The change of activation energyEB1was derived by linear fitting in Fig.6. The exothermic peak temperatures of DTA corresponding to the heating rates of 10 ℃·min-1,15 ℃·min-1, and 20 ℃·min-1are 359.9 ℃, 379 ℃,395 ℃, respectively. Accordingly, obtaining slope-7.0256 by linear fitting in Fig.7, the activation energyEbwas calculated to be 58.41 kJ·mol-1by the Kissinger method.
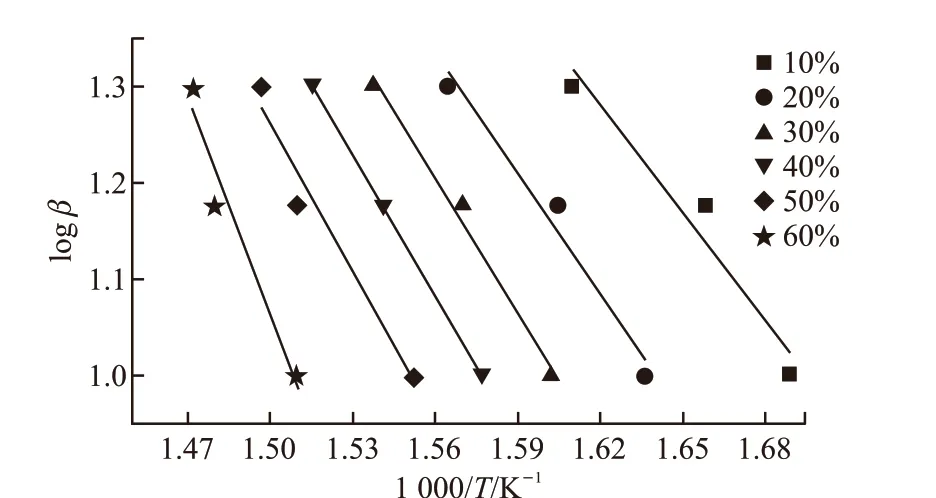
Fig.6 The slope curves by plotting of the activation energy EB1 under different a
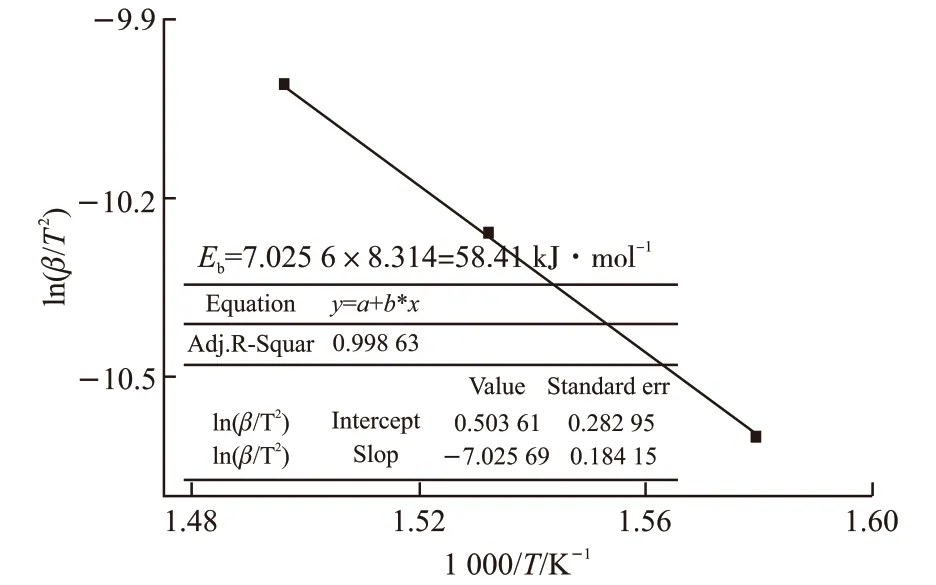
Fig.7 Activation energy Eb calculated from DTA curves by Kissinger method
Similarly, the results of TG/DTG and TG/DTA curves of lubricant C at different heating rates are described in Figs.8(a)-8(b). All curves of TMPTO base oil with octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate presented three weight loss steps. In the heating rate of 10 ℃·min-1, the initial decomposition temperature of first step is 303 ℃, and the peak temperature of DTG gets 351 ℃. This step is the most important one to determine the thermaloxidative stability of the lubricants. Intramolecular oxidation of the base oil could form various oxidation compounds as carboxylic acids and ketones, and occur to elimination of low molecular weight products followed by hydrocarbons degradation. The trend of activation energyEC1was plotted by linear fitting in Fig.9 using the formula (2) from the values of TG obtained Table 3. The exothermic peak temperatures of DTA corresponding to the heating rates of 10 ℃·min-1,15 ℃·min-1, and 20 ℃·min-1are 355.6 ℃, 367 ℃,378 ℃, and respectively. Accordingly, obtaining slope-11.274 by linear fitting in Fig.10, the activation energyEcwas calculated to be 93.732 kJ·mol-1by the Kissinger method.

Fig.8 TG/DTG(a) and TG/DTA(b) curves of lubricant C at different heating rates
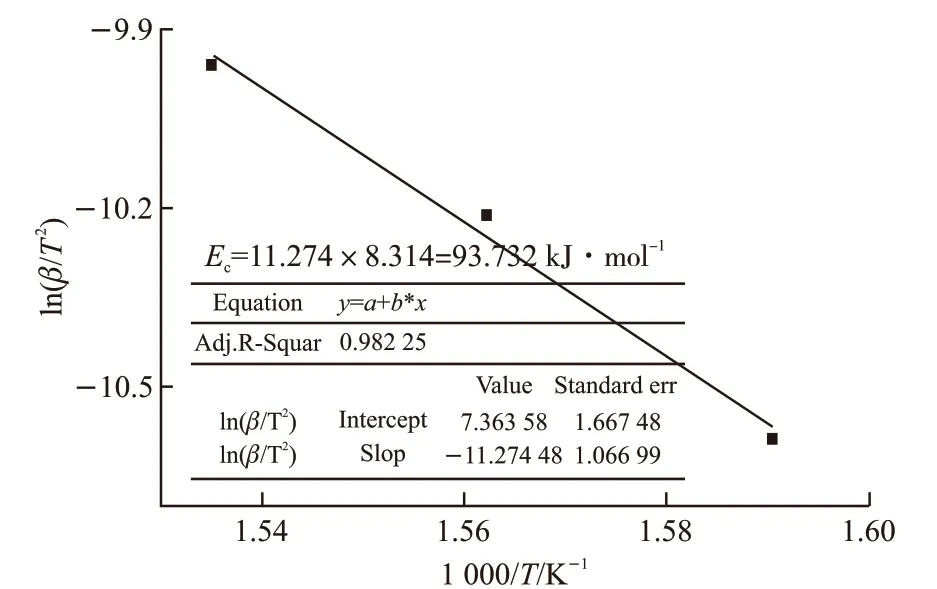
Fig.9 The slope curves by plotting of the activation energy EC1 under different a
Furthermore, Figs.11(a)-11(b) display the results of TG-DTG-DTA analysis of lubricant D at different heating rates. Curves of TMPTO with amines-phenolic combination antioxidants presented three weight loss steps. The initial decomposition temperature is 311℃ which have been greatly improved than others,meanwhile the peak temperature of DTG reaches 341℃ in the heating rate of 10 ℃·min-1. During thermaloxidative degradation of the most important one step,the base oils may initially form the hydroperoxides that go on to degrade by a number of pathways to secondary oxidation products by classical free radical chain reaction[23]. The slope curves of the activation energyED1under different α is plotted in Fig.12 using the formula (2) from the TG-DTG obtained Table 4. The exothermic peak temperatures of DTA corresponding to the heating rates of 10 ℃·min-1, 15 ℃·min-1, and 20℃·min-1are 366 ℃, 375 ℃, and 390 ℃, respectively.Accordingly, obtaining slope -10.67 by linear fitting in Fig.13, the activation energyEdwas calculated to be 88.71 kJ·mol-1by the Kissinger method.
Fig.10 Activation energy Ec calculated from DTA curves by Kissinger
Fig.11 TG/DTG(a) and TG/DTA(b) curves of lubricant D at different heating rates
Fig.12 The slope curves by plotting of the activation energy ED1 under different a
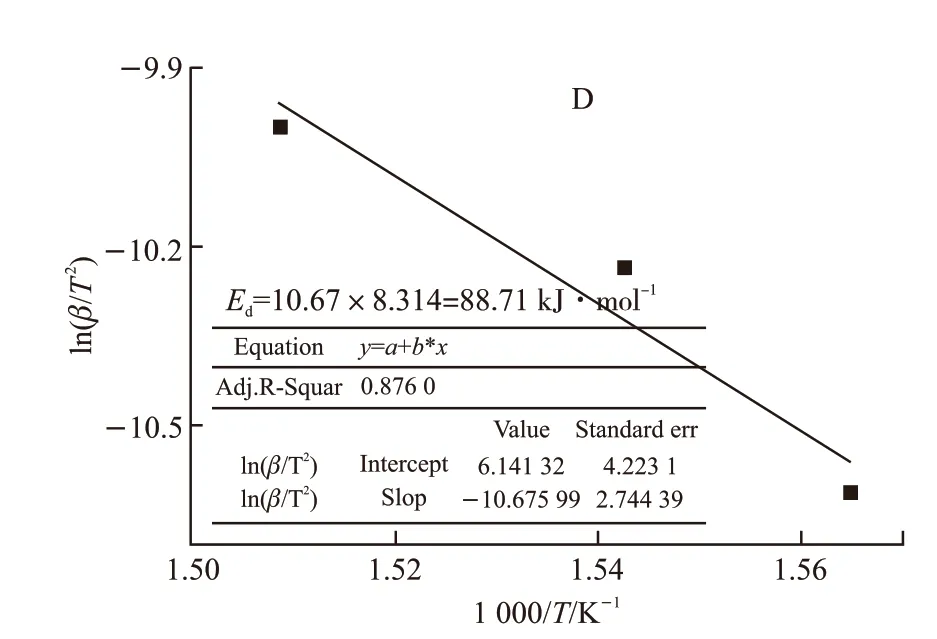
Fig.13 Activation energy Ed calculated from DTA curves by Kissinger method
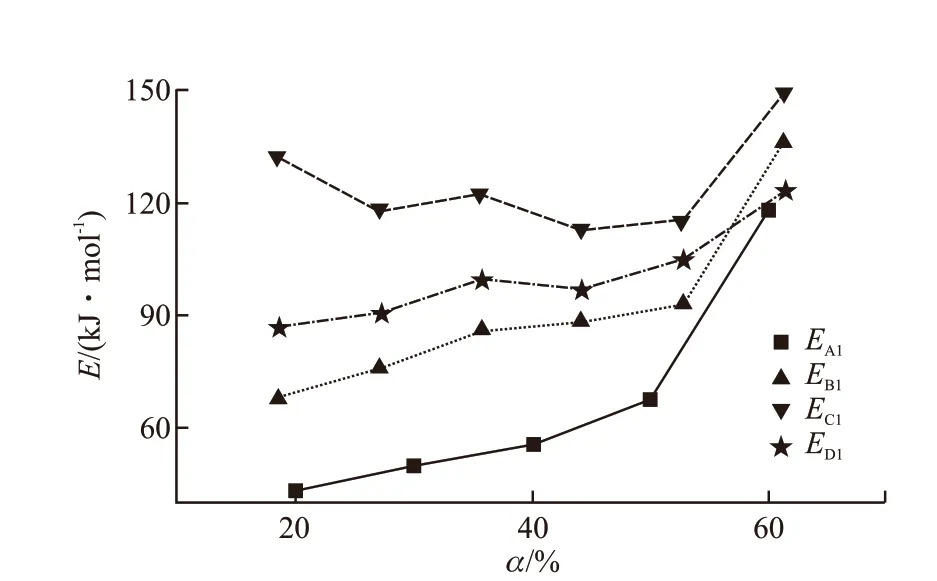
Fig.14 The trend of activation energy of lubricants A/B/C/D was plotted by thermogravimetry
Finally, the calculation results combined TG and DTA are summarized by solving equations using the reaction kinetic activation energy, of which the lubricants A/B/C/D demonstrated in Fig.14 and Fig.15.The activation energy of the lubricating oil adding antioxidant is obviously increased relative to the TMPTO base oil, coincidently, the order of activation energy areEc>Ed>Eb>Eaby TG or DTA.That indirectly indicates octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate has the best resistance to thermal oxidation and decomposition, the aminephenol combination antioxidant better than butyl-octyldiphenylamine. Referring to the specific trend of Fig.14,as the oxidative decomposition loss grow, the activation energy gradually increased. Moreover, in the late stage of thermal oxidation and decomposition, butyl-octyldiphenylamine resists oxidative decomposition more than the combined antioxidant, closing to octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate.It`s universally acknowledged that the initial reaction requires a large activation energy to induce more free radical formation, the antioxidant could effectively transfer the free radical at the phase of chain growth reaction, the accumulated molecular reaction would be relatively stable. Until the molecules of lubricating oil undergoes deep thermal oxidation and decomposition,the multiple activation energy increases accordingly[24].
3.2 Thermal stability of TMPTO with antioxidants by DSC testing
Fig.15 displayed the results of DSC analysis of lubricants A/B/C/D at different heating rates.As the heating rate increases, the DSC curves of identical lubricant shift outward, and the shape of exothermic peaks are quite consistent except the area of exothermic peaks increase accordingly. It's known that the heat exchange in the thermal reaction of the lubricant molecules lag to the temperature rising, and the heat release increase at the same time. Comparing the results of Lubricants with or without antioxidants simultaneously at heating rate of 10 ℃·min-1,theoxidation onset temperatures of lubricants A, B, C,D are 202 ℃, 217 ℃, 207 ℃, and 222 ℃, respectively.Several antioxidants increased the initial oxidation temperature of the TMPTO base oil, and the aminephenol combination antioxidants worked best, followed by butyl-octyl-diphenylamine.
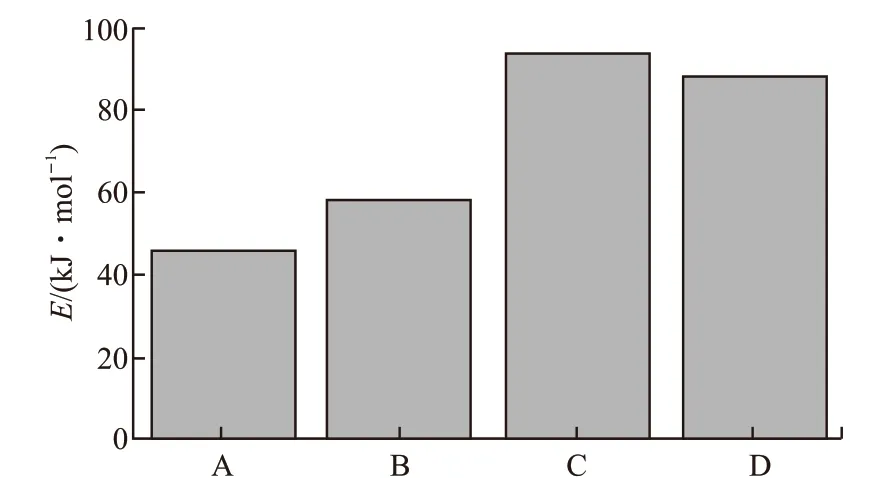
Fig.15 Activation energy of lubricants A/B/C/D calculated by DTA
DSC accurately reflecting heat exchange of the thermal oxidation reaction is different from TG/DTA.In the initial stage of the thermal reaction of identical lubricant at three different heating rates, the change of mass loss at the exothermic peaks are theoretically similar, which always illuminated that radical chain growth reaction in the lubricating oil molecules, the dehydrogenation and the radical transfer reaction may reach to the equilibrium state. The formula (6) would be used to calculate the reaction kinetic activation energy of the lubricating oil. The relevant parameters of Table 5 are fitted to obtain in Fig.16, and the calculation results of the activation energy of lubricants A/B/C/D are plotted in Fig.17. The order of activation energy isED(144.385 kJ·mol-1) >EC(110.05 kJ·mol-1) >EB(97.187 kJ·mol-1) >EA(66.02 kJ·mol-1). It's indicated that the amine-phenol combination antioxidant have the better thermal oxidation resistance than other single antioxidant, which the result is the same as what the initial oxidation temperature effected.
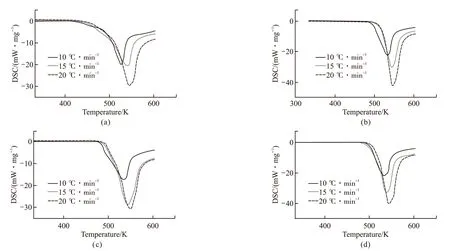
Fig.16 DSC curves of lubricants A/B/C/D at different heating rates
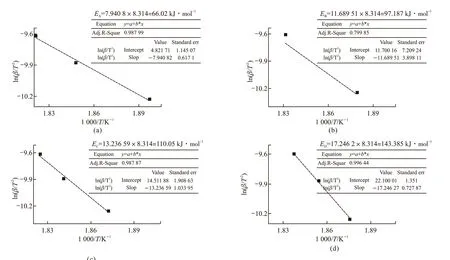
Fig.17 Activation energy of lubricants A/B/C/D calculated from DSC curves by linear fitting
Lubricating oil compound are composed of numerous base oil molecules, and the effect of antioxidant mainly inhibits the initial thermal oxidation reaction of base oil. In this paper, only the oxidation reaction in the initial stage of thermal analysis is investigated. The mass loss change of reaction at different rates taken place of the exothermic peak is approximately consistent. The activation energy of reaction can be measured by the above DSC curves to evaluate the thermal oxidation capacity of the antioxidant in base oil, In general, the greater the activation energy of thermal oxidation reaction in lubricating oil, the better its resistance to thermal oxidation and decomposition.
4 Conclusions
The reaction kinetic activation energy of lubricating oil was established by TG and DTA thermal analysis solving equation. The data showed that the activation energy of the lubricating oil adding antioxidants were significantly increased relative to the TMPTO base oil, and indirectly verified octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate has favorable resistance to thermal oxidation and thermal decomposition. Possibly being difference error of quality or temperature between the sample and reference material in the TG and DTA experiment,the repeatability may be poor, and the calorimetry quantify maybe also complicated. DSC could directly reflect the heat exchange of thermal oxidation reaction.The calculation of the activation energy of reaction kinetics shows that the amine-phenol combination antioxidants have the best thermal oxidation resistance,For conventional evaluation of the thermal oxidation performance of lubricating oils, DSC can be suggestive of calculating the reaction activation energy. The initial reaction activation energy measured by TG/DTA could be employed as a method judging the thermal oxidation and thermal decomposition properties of lubricating oils in a harsh working environment.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Experimental Study on Viscosity Characteristics of Expanding Polymer Grout
- Thermal-responsive Photonic Crystals based on Physically Cross-linked Inverse Opal Nanocomposite Hydrogels
- Transformation Characteristics and Microstructure of Rail under Low Stress during Continuous Cooling
- Formation Mechanism and Existing Form of Sb in Heat Resistance Mg-Gd-Y-Sb Alloy
- Rapid Dendrite Growth in Solidification of Highly Undercooled Alloys
- Research of Ultrafine Cemented Carbides for PCB Microdrills
