Rapid Dendrite Growth in Solidification of Highly Undercooled Alloys
2021-04-16ANHongenYANGJineZHANGXiaobingYANGShaopei
AN Hong'en, YANG Jine, ZHANG Xiaobing, YANG Shaopei
(1. College of Mechanical and Electronic Engineering, Huanghe Jiaotong University, Jiaozuo 454950, China; 2. Jiangsu Yingchuang Power Technology Co. ,Ltd., Suzhou 215000, China; 3. Henan Engineering Technology Research Center on Intelligent Manufacturing Technology and Equipment, Jiaozuo 454950, China))
Abstract: Nonequilibrium thermodynamics and transportation kinetics near the propagating solid-liquid interface dominates the rapid solidification process, which is far from a thermodynamically stable state. Rapid solidification process can be described more precisely using quantitative thermodynamic calculation of phase diagram with nonlinear liquidus and solidus and evaluating the nonequilibrium effect in diffusion kinetics.Based on these basic principles, we used a current nonequilibrium dendrite growth model to describe rapid solidification process of deeply undercooled alloys. Evolution of the key fundamental solidification parameters was also evaluated.
Key words: solid solutions; solidification; growth from melt; natural crystal growth
1 Introduction
As a first order phase transformation, solidification consists of three basic processes,i e, nucleation,growth and impingement[1]. Crystalline nuclei can be formed by site saturation nucleation and continuous nucleation as a result of the fluctuation in compositions and structures of undercooled melts. Rapid solidification of undercooled melts is driven by Gibbs free energy difference between the undercooled liquid and solid,and it releases heat spontaneously. In such conditions,the melt composition and temperature around the propagating liquid-solid interface is evolving as the fraction of solid phase increasing, which could subsequently tune the microstructure transition process and solidification kinetics. Deep understanding of dendrite growth is recently improved by advances of experiments and theoretical models. It has been found that not only the growth velocity but also the morphology and the dendrite size are basically influenced by fluid flow effect which strongly affects the heat and mass transfer processes during solidification[2-10]. It has been shown that fluid flow can play an important and dominant role in transport process around the migrating interface by altering the local temperature and concentration gradients, and thus changing the solidification front evolution[11,12]. Fluid flow can be produced by different methodology,eg, by forced convection induced by external force, by natural convection induced by buoyancy force due to thermal and solute gradient and specifically by Marangoni convection induced by surface tension gradient.
In the present study, we obtained equilibrium Ni-Cu phase diagram with nonlinear liquidus and solidus using thermodynamic calculation. We also described nonequilibrium dendrite growth process using a current dendrite growth model, and the temperature rise during recalescence period of deeply undercooled melt under the action of fluid flow was described by a model for predicting maximum recalescence temperature.
2 Theory
Recently, a more stricter and advanced nonequilibrium dendrite growth model as a correction of the BCT model and Galenko’s model was developed, and it considers the nonlinear liquidus and solidus and the relaxation effect in the bulk liquid. According to this model, the bulk undercooling ΔTat the dendrite tip can be divided into four parts:
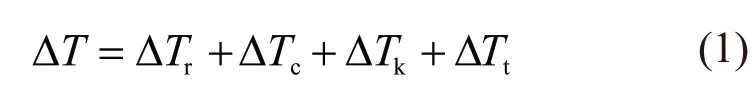
where,ΔTc,ΔTt, ΔTk,andΔTrare thesolutalundercooling, thermal undercooling,interfacial kinetic undercooling and curvature undercooling, respectively.According to this model, the bulk undercooling ΔTat the dendrite tip can be divided into four parts:
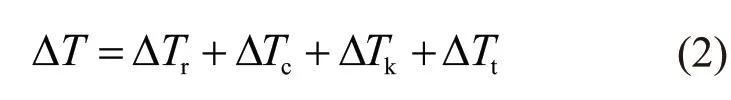
where, ΔTc, ΔTt, ΔTk, and ΔTrare the solutal undercooling, thermal undercooling, interfacial kinetic undercooling and curvature undercooling, respectively. Details of the above model are referred to the Refs.[13-17].Here we give detailed expressions of the undercooling components:
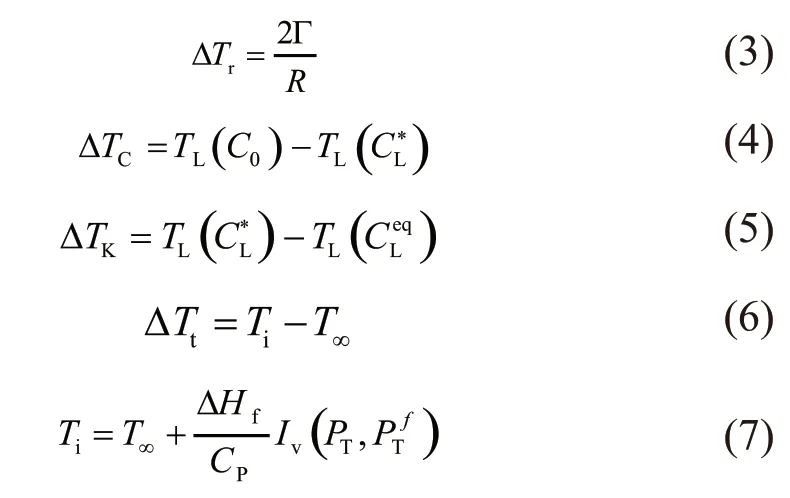
For rapid solidification, the driving force of transformation can be expressed as:
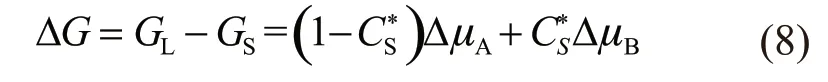
where,GLandGSare the Gibbs free energy of liquid and solid phases, respectively;VDis the solute diffusion speed in liquid phase; Δμare the chemical potential differences of the elements; is the solid composition near the interface.
3 Results and discussion
For Ni-20at% Cu alloys, when an initial undercooling ΔT< 35 K, the solute undercooling ΔTcis larger than other undercooling components, so the dendrite growth in the undercooled melt is mainly controlled by the solute diffusion in front of the dendrite tip and the crystal growth velocity is very low, therefore, coarse dendrites can be resultant. As initial undercooling continuously increasing, the effect of thermal diffusion on the dendrite growth becomes strong. In the range of 35 K < ΔT< 65 K, ΔTtstarts to exceed ΔTc, which reaches a maximum at ΔT= 42 K where the dendrite tip also reaches its maximum (Fig.1(a), Fig.2(a)) and is the most unstable due to the maximum solute undercooling. In this undercooling range, the action of solute undercooling ΔTcand thermal undercooling ΔTtis on the same level. When ΔT> 67 K, ΔTtincreases rapidly and exceeds ΔTcsubstantially, the dendrite tip radius accordingly decreases and the crystal growth rateVincreases rapidly (Fig.1(a), Fig.2(a)). The increasing action of thermal diffusion results in directionality of dendrites. Solute diffusion is replaced by the thermal diffusion to predominantly control the dendrite growth process, which indicates a transition from the equilibrium of a solidification controlled by the solute gradient to a thermally controlled growth owing to a relaxation of diffusion equilibrium at the solid/liquid interface.Therefore, the higher the initial undercooling and the thermal undercooling, the more convenient it becomes for dissipating of the latent heat at the dendrite tip during the crystal growth. With increasing undercooling, the interface temperature increases (Fig.2(a)) and the growth velocity becomes large enough to makekobviously deviate fromk0. When ΔT> 242 K, the migration velocity of the solid/liquid interface exceeds the solute diffusion speed VD, complete solute trapping occurs and the dendrite growth is completely controlled by thermal diffusion. Under this condition, the crystal growth velocityVincreases linearly with undercooling ΔT(Fig.2(a)) and the solute segregation is completely avoided (Fig.3(b)). It is well known that, in single phase alloys, the condition of diffusion equilibrium is gradually becoming less important with the increase of solidification velocity and undercooling in front of the dendrite tip. Therefore, solute rejection is reduced(Fig.2(b)) and solute undercooling decreases as the interface concentration approaches the melt composition. This ultimately causes partitionless solidification(Fig.2(b)), which is solely controlled by thermal gradient.
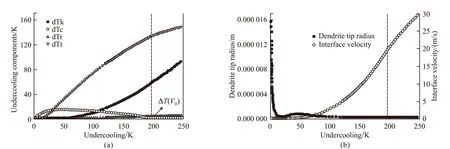
Fig.1 (a) Evolution of undercooling components and (b) Dendrite tip radius TR and dendrite growth velocity V as a function of undercooling
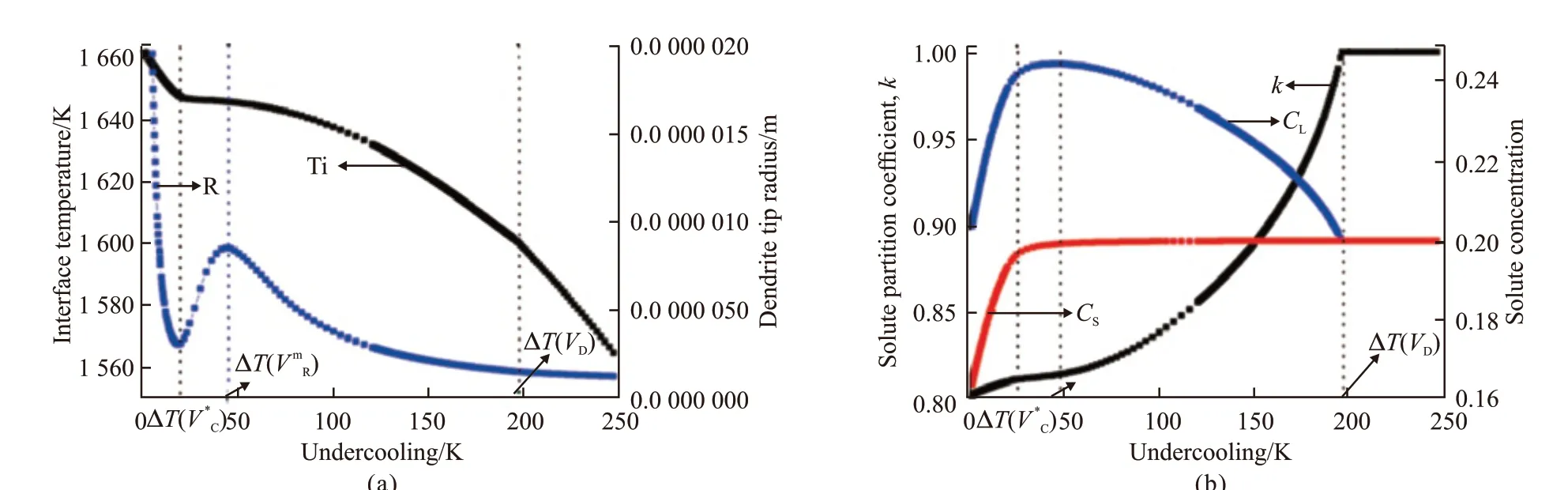
Fig.2 (a) The dendrite tip radius R and interface temperature Ti; (b) Solute partition ratio k, solute concentration of liquid CL and solid CS as a function of undercooling
In general solidification conditions, the temperature and composition on the liquid-solid interface lies in a local thermodynamic equilibrium state. During rapid solidification, when the interface migration velocity overcomes the solute diffusion velocity at the liquid side of the interface, although there exists a substantial driving force for the solutes at the interface to diffuse into the melt, it’s too late for the solutes to diffuse into the melt, and the solute is trapped by the solid/liquid interface. Solute trapping effect will lead to the formation of supersaturated solid solutions with no solute segregation. When solidification starts, dendrite growth is very fast as a result of the very high thermodynamic driving force for solidification, which is stored as Gibbs free energy difference in an undercooled melt. Thus the dendrite growth is far from equilibrium and the non-equilibrium coefficient equals to one as a result of complete solute trapping. As solidification continuing,the migrating interface velocity and the non-equilibrium solute redistribution coefficient decrease continuously due to the decreasing of the solidification driving force. As the dendrite growth velocity decreasing to zero, the non-equilibrium solute redistribution coefficient will decrease to its equilibrium value and solidification will stop. Meanwhile, the liquid-solid interface temperatureTiwill increase to the maximum recalescence temperatureTR, correspondingly.
4 Conclusions
Based on experimental study and theoretical analysis, the maximum recalescence temperature and rapid solidification behavior of highly undercooled Ni-20at%Cu alloys are studied. We can make the following main conclusions : According to the theoretical model calculation results, as the initial bulk undercooling is smaller than 242 K, the liquid-solid interface migration velocity during rapid solidification increases exponentially as undercooling increasing. When initial bulk undercooling exceeds 242 K, complete solute trapping occurs and the liquid-solid interface migration velocity increases linearly as undercooling increasing.According to the theoretical model calculation results,upon rapid solidification proceess, the driving force for solidification deceases to zero accordingly, the interface migration velocity decreases to zero and the solute partition coefficient decreases to the value of the equilibrium solute partition coefficient.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Comparative Case Study on Adhesion of Three Common Sizing Agents to Cotton and Polyester Yarns
- Effect of Outer Carbon Layer Thickness of Carboncovered N-doped Hollow Carbon Nanospheres on Its Electrocatalytic Performance
- Ceramification of Composites of MgO-Al2O3-SiO2/Boron Phenolic Resin with Different Calcine Time
- Natural Fresh Proteins Directed Hierarchically Porous Nitrogen-doped TiO2 as with High Performance as Photocatalyts and Electrode Materials
- Dynamic Adsorption of Toluene on Hierarchical Porous Carbons with Varying Pore Structure
- Self-propagating High-temperature Synthesis of Sm and Zr Co-doped Gd2Ti2O7 Pyrochlore Ceramics as Nuclear Waste Forms
