Self-propagating High-temperature Synthesis of Sm and Zr Co-doped Gd2Ti2O7 Pyrochlore Ceramics as Nuclear Waste Forms
2021-04-16XIEDayanZHANGKuibaoLIWeiweiLUOBaozhuGUOHaiyan
XIE Dayan, ZHANG Kuibao,2*, LI Weiwei, LUO Baozhu, GUO Haiyan
(1.State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology, Mianyang 621010,China;2.National Defense Key Discipline Lab of Nuclear Waste and Environmental Safety, Southwest University of Science and Technology,Mianyang 621010, China)
Abstract: We reported a rapid synthesis of Sm3+/Zr4+ co-doped Gd2Ti2O7 pyrochlore simulated nuclear wastes solidification by self-propagation plus quick pressing technique. With increment excess contents of Sm2O3 and ZrO2 from 0 to 10wt%, the phase composition of the products is a mixed phase of pyrochlore structure and defective fluorite structure by X-ray diffraction (XRD) analysis and Raman spectrum. In addition,the SEM results demonstrate the fracture surface and microstructure of Gd2Ti2O7-based pyrochlore. The densified pyrochlore waste form exhibits high bulk density of 5.56 g·cm-3 and vickers hardness of 11.20±0.2 GPa. The leaching tests show that the elemental leaching rates of Gd, Sm, and Cu after 42 days are 1.92×10-4,1.51×10-4, and 3.90×10-3 g·m-2·d-1, respectively.
Key words: SHS; pyrochlore; immobilization of nuclear wastes; excess
1 Introduction
The nuclear industry has developed rapidly in military and civilian fields since humans control the approach of self-sustained chain nuclear reaction. And the production of radioactive nuclear wastes (RAWs)is increasing with the development of nuclear technology applications. Especially, the high-level radioactive wastes (HLWs) are extremely harmful to the human living environment due to their high radiotoxicity and long half-life[1]. Therefore, how to safely and effectively dispose HLW is a huge problem for humans. In response to this problem, some countries have proposed various methods for the disposal of high-level waste,such as space disposal, deep sea launch and deep geological landfill[2,3]. These countries, including France,the United States and China, have argued through various schemes that it is more reasonable to use deep geological burial after immobilization of nuclear wastes[4].Under deep geological conditions, it is required that the solidified body of nuclear waste can ensure the effective retention of nuclides at high temperature and pressure. So it is necessary to solidify nuclear wastes before burial in deep geology. There are many ways for immobilization of nuclear waste, such as cement solidification[5], vitrification[6,7]and Synroc immobilization[8,9]. In general, cements have been used only for the immobilization of low or intermediate level radioactive wastes[10]. Glass, especially phosphate glass, has poor chemical durability and low thermal stability. When subjected to water and steam at high pressure and temperature after burial in a geological repository, vitrified waste forms transform form a glassy state to a crystalline state, which results in an increase of nuclides leaching rates. Then the low solubility of actinides elements in the glass matrix is also a limiting factor[11-14].
In order to avoid the above problems, scientists began to search for the ideal confinement matrix of HLW. As a second-generation solidified body, Synroc was firstly put forward by Ringwood in 1979 because of its excellent chemical stability and good radiation resistance[15,16]. Pyrochlore is a kind of Synroc, and the ordinary formula of pyrochlore is A2B2O7(where A is trivalent rare-earth ions such as Gd3+, Sm3+,et al,and B is usually Ti4+, Zr4+, and Hf4+,et al)[17,18]. This allows cations with the same valence state or similar ionic radius to be incorporated into the A and B sites. In recent years, the simultaneous doping of zirconate-based pyrochlore at the A and B sites have been widely investigated for the immobilization of HLW due to its superior radiation resistance, stable structural properties, good mechanical properties and low leaching rate of incorporated species[19-21]. Pyrochlore can fix most of the radionuclides in its crystal lattice. However, a crucial factor for pyrochlore immobilization is difficult to obtain highly compacted specimens. In the past,some traditional technologies were employed as solidification of high-level radioactive waste, such as hotpress sintering, cold-press sintering and hot isostatic pressing[22,23]. These technologies are complicated in preparation process, expensive in equipment and raw materials.
Self-propagating high-temperature synthesis(SHS), also known as combustion synthesis, is a candidate method that has far-reaching implications for material synthesis[24]. The feature of SHS method is that the reactants once be ignited, the heat produced is self-sustaining for reactions of raw materials, and the process can produce final products spontaneously without demanding any extra heat input. The method has advantages of high reaction speed, as well as low cost, simplified equipment requirement and convenient handling[24,25]. Based on the above advantages, the SHS method has gradually become an attractive method for solidification of wastes. In 1996, Muthuraman Met alhad invented the SHS method for disposal of nuclear wastes[26]. In recent years, disposal of nuclear wastes by SHS method has been attracted considerable interest in the scientific research. Zhang KBet alhave investigated the SHS preparation of zirconolite (CaZrTi2O7)waste forms using Ti as the reductant[27-30]. Peng Let alhave explored the SHS preparation of pyrochlore (Gd2Ti1.3Zr0.7O7) using CuO as the oxidant[13]. Zhang RZet alhave researched synthesis of SrTiO3by SHS for immobilization of HLW[31-33].
In this work, excessive mixtures of Sm2O3/ZrO2were directly added to Gd2Ti2O7starting materials and Gd2Ti2O7-based pyrochlore was prepared by SHS. As Sm and Pu have the same arrangement of extra-nuclear electron, they exhibit similar ionic radii when the ion valence of Sm is equivalent to Pu. Therefore, Sm3+was employed as the surrogate of trivalent actinides and Zr was concurrently doped to improve the radiation resistance of solidied bodies. In order to obtain highly compacted specimen, quick pressing (QP) was introduced in the SHS process. Moreover, the chemical durability of highly densified pyrochlore-based waste form was investigated as well.
2 Experimental
In this study, high purity (99.9wt%) powders of Gd2O3, Ti, ZrO2, TiO2, Sm2O3, and CuO were used as the starting reactants (all these materials were purchased from Shanghai Aladdin Co. Ltd.). The designed SHS reaction is demonstrated as follows[34]:
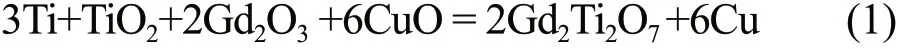
The ingredients (about 20 g) of formula (1) were weighed in stoichiometric proportions and mixed with absolute ethanol in agate can. The mixed powder in the molar ratio Sm2O3/ZrO2= 1:1 was directly put into the raw materials of SHS reaction by different contents(0wt%, 5wt%, 10wt%, 15wt%, 20wt%, and 25wt%).In this study, for convenience of description, they are marked as SZ-0, SZ-5, SZ-10, SZ-15, SZ-20, and SZ-25, respectively. All the reactants were mixed in planetary for 2 hours using agate ball as the mixing medium.Then the mixed reactants were dried at 70 ℃ for 12 hours. Finally, the powders were pressed at 20 MPa usingФ25 mm stainless mould. The obtained cylindrical green bodies would be used for the next ignition.
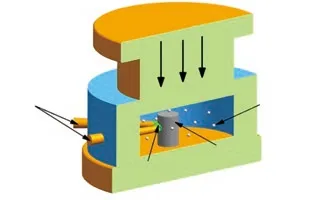
Fig.1 Schematic diagram of the designed SHS/QP process
The SHS/QP process is illustrated in Fig.1, which is identically conducted as our previous studies[12-14].The cylindrical samples were ignited by tungsten filament placed close to one side, which was motivated by a direct current of about 50 A. 100 meshes silica sand was employed as the heat insulator and pressure transmission medium. Before consolidation, the temperature of the reaction system was measured by a W/Re 5/26 thermocouple, which was placed at the center of the green body. The other end of the thermocouple was in contact with the paperless recorder for recording the temperature tendency curves of SHS reaction.
The sintered specimens were milled to fine powders for X-ray diffraction analysis (XRD, X’Pert PRO,PANalytical BV, Almelo, Netherlands) to explore the phase composition. To obtain highly densified specimen, the ignited samples were compressed at 50 MPa using hydraulic quick pressing (QP). After holding for 60 s, the samples were cooled to room temperature naturally. The solidified specimens were cut and polished to investigate the microstructure and elemental distribution using field-emission scanning electron microscopy(FESEM, Zeiss Ultra-55, Oberkochen, Germany) and energy-dispersive X-ray spectroscopy (EDX, ULTRA 55, ZEISS, Oberkochen, Germany). The density and hardness of sintered samples at different pressurization time were measured using the Archimedes method and vickers hardness tester. The densified sample was heat treated at 900 ℃ for 3 hours to explore the grain size of the surface. Chemical durability of selected sample was tested using the standard MCC-1 method[35]. The leaching rates of rare-earth ions (Gd3+and Sm3+) were evaluated according to inductively coupled plasma-mass spectrometry (ICP-MS, iCPA 6500, ThermoFisher,Waltham, MA, USA). The normalized elemental leaching rates were calculated, shown as the following equation:
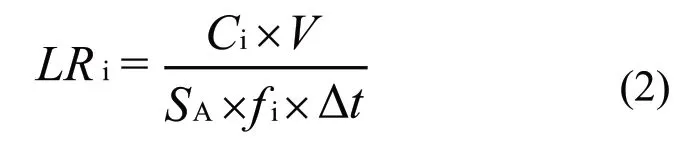
where,fi(wt%) is the mass fraction of elementiin the sample,SA(m2) is the geometric surface area,V(m3) is the volume of the leachate, Δt(d) is the interval soaking duration, andCi(g·m-3) is the concentration of elementiin the solution.
3 Results and discussion
3.1 Temperature and XRD analysis
The temperatures of SZ-0, SZ-5, SZ-10, SZ-15,and SZ-20 samples were measured and depicted in Fig.2(a),which exhibits the maximum temperature decreases with the increment of SZ content. When the SZ content exceeds over 20wt%, the SHS reaction could not be self-sustainable. The highest temperature of SZ-0 is shown to be 1 579 ℃, while the SZ-20 sample exhibits the highest temperature of 359 ℃ and the SHS reaction time is delayed from 15 s to about 45 s.This phenomenon may be explained that the gradual disordering of A- and B-site cations to a defect fluorite structure with increasing SZ contents. According to Heleanet al[36], more heat is absorbed in the combustion process.
The phase composition of samples with different contents of SZ were determined by XRD as depicted in Fig.2(b). The XRD patterns reveal that the major phase of all samples is Gd2Ti2O7pyrochlore (PDF No.73-1698). There is no change of the XRD patterns when adding 10% content of SZ, which the phase composition represents ordered pyrochlore phase (corresponding to (1 1 1), (3 1 1), and (3 3 1)) and disorder fluorite structure (corresponding to (2 2 2), (4 0 0), (4 4 0), and(6 2 2)). However, weak traces of Gd2TiO5and TiOxappear in SZ-15 from Fig.2(b). Moriet alhave explained that the presence of Gd2TiO5or TiO2in the Gd2Ti2O7pyrochlore is related to the effect of vacancies or excess of Gd3+ions in the 16 d sites, respectively[37]. Therefore, the SZ-10 sample was selected for the following research. In addition, the characteristic peak (2 2 2) of Gd2Ti2O7pyrochlore was singled out and magnified as attached in the behind of Fig.2(b). The peak gradually shifts to a lower angle. According to the Bragg equation, this phenomenon can be attributed to the increment of lattice parameter. This elucidates that Zr4+(0.720 Å) with a larger radius at the B-site replaces Ti4+(0.605 Å), which indicates successful replacement[13].
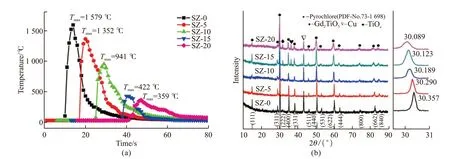
Fig.2 (a) Real temperature curve of all samples during SHS reaction; (b) XRD patterns of as-prepared samples
3.2 Raman spectroscopy analysis
Raman spectroscopy is an effective technique to identify the internal bonds of pyrochlore. Based on point group theory, there are a total of six Raman active modes in Gd2Ti2O7pyrochlore, which consists of A1g, E1g, and 4F2g[38]. The Raman spectra exhibits two intense characteristic bands in Fig.3. One of the intense band located at about 310 cm-1is attributed to the O-A-O bending mode, which contains two modes with close frequency, E1g(about 325 cm-1) and F2g(about 309 cm-1). Another intense peak observed at about 515 cm-1, A1g, which is related with A-O stretching[39]. The other three F2gmodes are so weak that the bands can not be clearly observed. It is difficult to confirm the location of three remaining F2gmodes because the literature data are not consistent, while the bands mainly appear around 200-230 cm-1, 510-530 cm-1, and 580-610 cm-1[39,40].
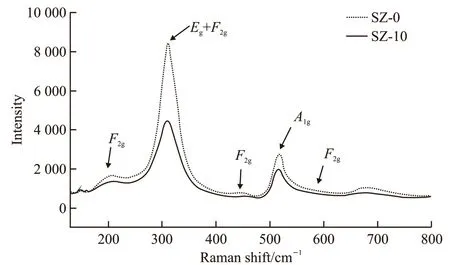
Fig.3 Raman spectroscopies of the SZ-0 and SZ-10 samples
It is interesting to note from Fig. 3 that the vibration intensity of E1g+ F2gbands and A1gbands shift to slightly lower wavenumbers from 520 cm-1(A1g), 314 cm-1(Eg+ F2g) to 514 cm-1and 308 cm-1, respectively.Moreover, there are some changes in broad and intense bands of the SZ-0 and SZ-10 samples. We speculate that the results represented in Fig.3 may be caused by the addition of simulated nuclides. According to previous literatures[40-43], this phenomenon may be explained as some ions of simulated nuclides occupy the A and B sites of pyrochlore structure, leading to changes in crystal structure and oxygen ions’ environment of Gd2Ti2O7. In general, the pyrochlore structure of co-doped Gd2Ti2O7keeps the original form of crystal structure.
3.3 The mechanical properties and microstructure analysis
In the long-term geological disposal environment,the solidified matrix will suffer from the erosion of groundwater, which will destroy its stability and change the leaching rate of nuclides. The compact specimen plays an important role in immobilization of nuclear wastes, especially for HLW. High density samples have significant impact on mechanical properties and chemical durability. In this study, we have produced highly compacted specimen using rapid hydraulic pressing.The results of the bulk density and vickers hardness of SZ-10 sample at different pressure times are illustrated in Fig.4, which range from 4.30 to 5.56 g·cm-3and from 8.90±0.2 GPa to 11.20±0.2 GPa, respectively.For Vickers hardness, we have measured multiple time under the same pressure to obtain the average values.After the sample being compressed at 22 s and 24 s,the green body does not fully react, which results in the gas not being able to escape from the sample and reducing the compactness. When the pressurization time is 28 s, the sample has completely reacted. Some gas is retained inside the sample because there is no timely pressure on the sintered sample in the red hot state.The test result of 26 s compressed sample demonstrates the highest density of 5.56 g·cm-3and high vickers hardness of 11.20±0.2 GPa. This result is close to the mechanical properties of Gd2(Ti2-xZrx)O7pyrochlore prepared by SHS/QP technology[13,34].
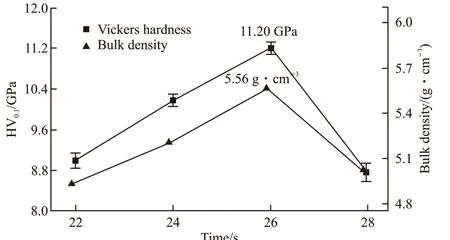
Fig.4 Vickers hardness and bulk density of SZ-10 sample with different pressure time
Figs.5(a)-5(d) exhibit the SEM images of microscopic surface of SZ-10 sample under a series of pressurization times. With the delay of pressurization time, the area and amount of pores in the samples’ surface are firstly decreased and then increased. Figs.5(a)-5(b), and 5(d) illustrate that there are lots of pores in the 22 s, 24 s, and 28 s samples, which are attributed to the pressurization time. When the sintered sample is pressurized at different time, part of the gas is trapped inside the sample to generate pores. It is evidently observed from Fig.5(c) that the pores of SZ-10 sample at 26 s are lower than other time. This may be due to most gas just escaping from the inside of the sample when the densification time is 26 s. Therefore, the most compact sample was obtained at 26 s. This result is in consistent with the mechanical properties of the sample. In addition, Fig.5 shows the micro area of the sample, so no copper appears in the visible range of the field view.The specimen in 26 s was cut and polished for subsequent characterizations.
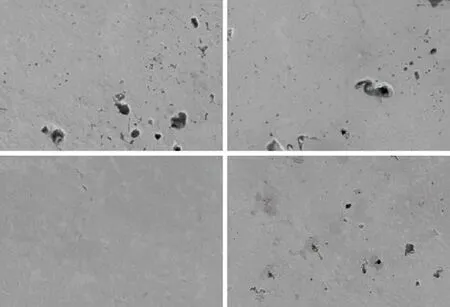
Fig.5 Surface microstructure images of the SZ-10 sample with different pressure time: (a) 22 s, (b) 24 s, (c) 26 s, and (d) 28 s
In order to further explore microstructure of the SZ-10 sample at 26 s, we have observed the fracture surface of the sample as well as surface morphology after heat treatment (900 ℃, holding time 3 h). The grains of the fracture surface can be clearly found from Fig.6(a), and the particle size of the crystallite grain is shown in Fig.6(b). In addition, Fig.6(a) shows that the grain size of the particles on the fracture surface is not uniform, which can be confirmed by Fig.6(b). Particle size distribution shows the average particle with size up to around 2.85 μm and the largest grain is 4.72 μm and the smallest is 1.04 μm. Fig.6(c) is the surface of the sample at 26 s and there is no grains on the surface.Fig.6(d) shows the transformation in surface after heat treatment, which can observe clear grain boundaries and the real grain sizes is about several microns. An interesting feature noticing that the heat-treated sample grows fine particles on the surface (around 200 nm).This indicates that temperature has a significant effect on the grain size.
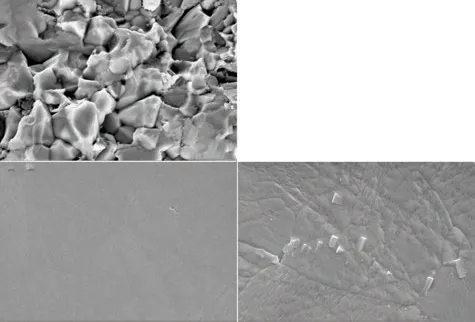
Fig.6 (a) The fracture surface of the sample at 26 s, (b) the particle size distribution of (a), (c) surface morphology of the sample at 26 s, (d) surface (c) after heat treatment
Elemental analysis was carried out to study the distribution of elements in Gd2Ti2O7pyrochlore with Sm2O3/ZrO2. The SZ-10 bulk sample was analyzed bySEM-EDX as shown in Figs.7(a)-7(f). The area of the sample surface in Fig.7(a) was selected for elemental analysis. The EDX spectra from Figs.7(b)-7(e) represent that there are all elements of Gd, Sm, Ti, and Zr in the selected region. EDX mapping results reveal the homogeneous distribution of Gd, Sm, Ti, and Zr.In addition, as can be seen from Fig.7(f), the Gd, Sm,Ti, and Zr peaks were identified by EDX spectra and their atomic ratios are measured to be 17.65%, 2.28%,17.01%, and 2.16%. The atomic ratios of Gd and Sm,Ti, and Zr are very close, which is in consistent with the addition of excess Sm2O3/ZrO2to the raw material of Gd2Ti2O7pyrochlore. According to the atomic ratio of elements, we can calculate the chemical formulation of pyrochlore matrix phase is Gd1.81Sm0.23Ti1.75Zr0.22O7,where Gd site is replaced by Sm and Ti site is replaced by Zr. This chemical formulation conforms to the pyrochlore formula of A2B2O7.
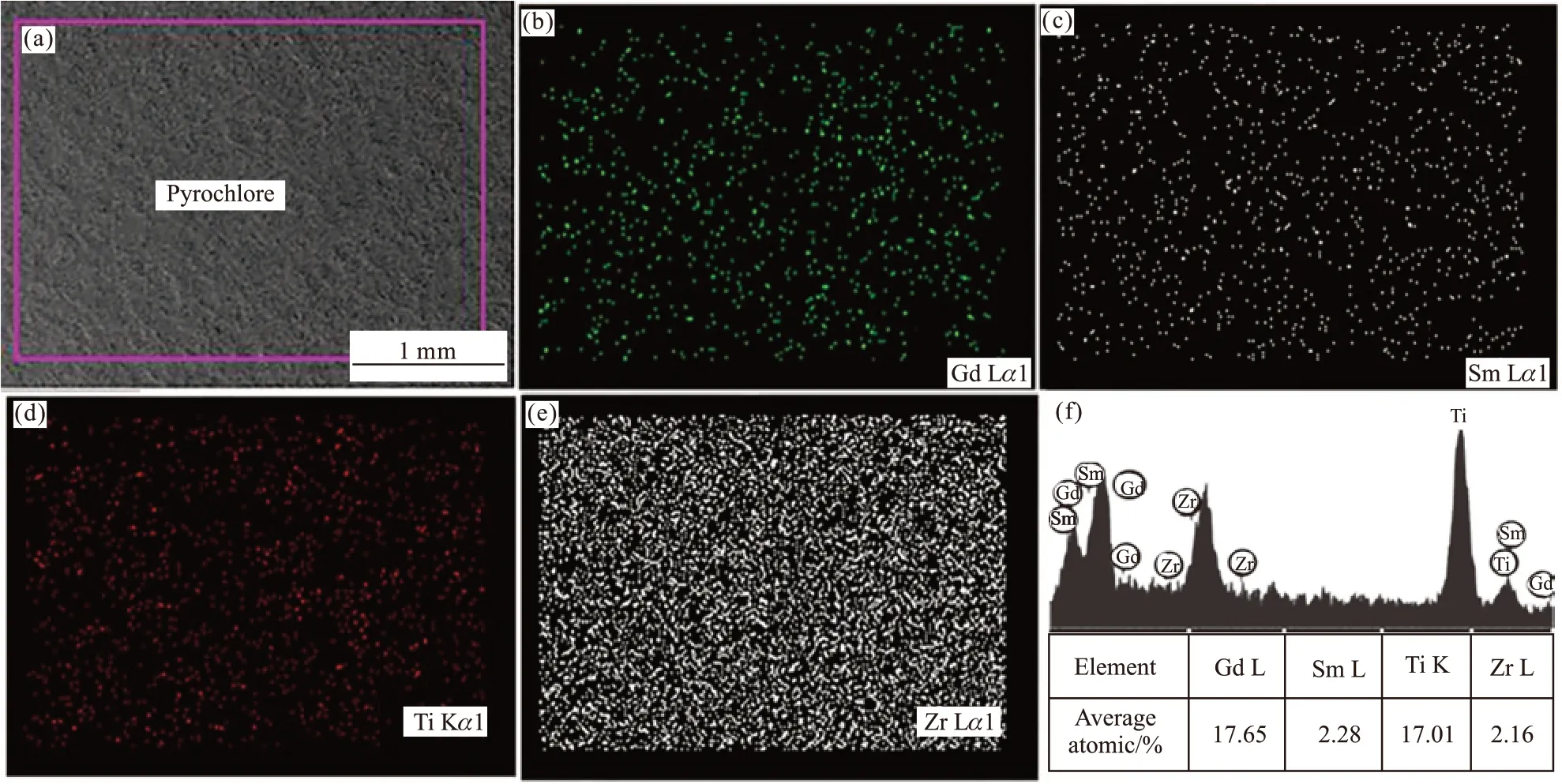
Fig.7 EDX mapping images of consolidated SZ-10 specimen with pressure time 26 s: (a) Representative SEM image and respective elemental mapping images of (b) Gd, (c) Sm, (d) Ti, (e) Zr and (f) element surface area analysis
3.4 Aqueous durability of the SZ-10 sample
Aqueous durability has great significance for leaching performance of nuclear waste forms[44]. In this work, the MCC-1 leaching method was used to evaluate chemical durability of the SZ-10 compact sample.Fig.8 indicates the 1-42 days leaching results as the normalized leaching rates of rare-earth elements Gd and Sm, as well as metallic element Cu, are depicted in it.It can be noted that Cu exhibits relatively high leaching rates. The normalized leaching rate of Cu (LRCu) value decreases gradually with the extension of soaking time and the LRCuvalue on 42 days is 3.90×10-3g·m-2·d-1.Fig.8 shows that the leaching rate of the elements fluctuates within a reasonable range. The values of rare-earth elements reach the highest on the 3rd day and subsequently continue to decline. This phenomenon may be attributed to the decrease of surface mechanical properties after soaking the solidified substrate for a period of time, resulting in the increase of nuclide leaching rate in the solidified specimen. The LRGdand LRSmvalues are 1.80×10-4and 2.48×10-4g·m-2·d-1after 1 day, 8.69×10-4and 5.13×10-4g·m-2·d-1after 3 days,as well as 1.92×10-4and 1.51×10-4g·m-2·d-1after 42 days. The 42 days LRGdand LRCuresults are lower than the leaching rates of Gd and Cu of ZrO2incorporated Gd2Ti2O7pyrochlore synthesized by SHS[13]. Compared with the Gd0.59Nd1.39Ti1.33Zr0.69O7waste form prepared by SHS, the leaching rates of Cu and Gd after 42 days are very close in this study[12]. Generally speaking, the elemental leaching rates of Synroc solidified nuclear wastes are significantly lower than typical vitreous products[45,46].
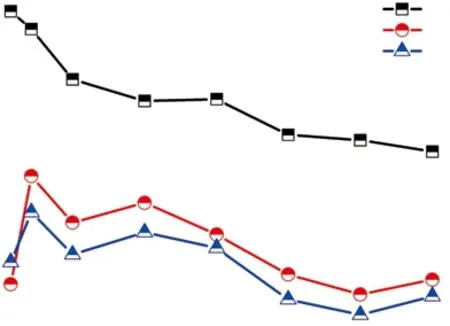
Fig.8 Normalized leaching rates of Gd, Sm, and Cu of SZ-10 from days 1-42
4 Conclusions
In this study, high density titanium-based pyrochlore compounds for simulated radioactive wastes were produced using SHS/QP technique within a short time. Excessive up to 10wt% Sm2O3/ZrO2was successfully immobilized into Gd2Ti2O7pyrochlore. The Gd and Ti sites in Gd2Ti2O7are replaced by Sm and Zr,respectively. The final chemical formulation of SZ-10 sample is calculated as Gd1.81Sm0.23Ti1.75Zr0.22O7. The solidified body of SZ-10 sample exhibits good bulk density of 5.56 g·cm-3, high vickers hardness of 11.20±0.2 GPa, as well as excellent chemical aqueous durability.The 42 days normalized leaching rates of Gd, Sm and Cu are characterized to be 1.92×10-4, 1.51×10-4, and 3.90×10-3g·m-2·d-1. The Gd2Ti2O7pyrochlore that synthesized by SHS technology is a promising substrate for solidification of nuclear wastes.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Comparative Case Study on Adhesion of Three Common Sizing Agents to Cotton and Polyester Yarns
- Effect of Outer Carbon Layer Thickness of Carboncovered N-doped Hollow Carbon Nanospheres on Its Electrocatalytic Performance
- Ceramification of Composites of MgO-Al2O3-SiO2/Boron Phenolic Resin with Different Calcine Time
- Natural Fresh Proteins Directed Hierarchically Porous Nitrogen-doped TiO2 as with High Performance as Photocatalyts and Electrode Materials
- Dynamic Adsorption of Toluene on Hierarchical Porous Carbons with Varying Pore Structure
- Solidification Behavior of in situ TiB2/Cu Composite Powders during Reactive Gas Atomization
