Ceramification of Composites of MgO-Al2O3-SiO2/Boron Phenolic Resin with Different Calcine Time
2021-04-16SHIMinxianTANGQingxiuFANShanshanDONGChuangHUANGZhixiong
SHI Minxian, TANG Qingxiu, FAN Shanshan, DONG Chuang, HUANG Zhixiong
(School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China)
Abstract: The ceramifiable polymer composite of MgO-Al2O3-SiO2/boron phenolic resin(MAS/BPF) with 40wt% of inorganic fillers was calcined at 1 200 ℃ for different time to promote ceramification of ceramifiable composite and improve heat resistance. The effects of different calcine time on the macroscopical morphology, mass loss, phase evolution, microstructure and chemical bond evolution of MAS/BPF composites were characterized by XRD, XPS, and SEM analyses. The experimental results reveal that the increase of calcine time result in the fewer holes, relatively denser and smoother top layer of MAS/BPF composites and protect the interior from deeper decomposition. The final residues of composites are amorphous carbon and C-O-Si-Al-Mg ceramic. And MAS/BPF composites show excellent mass stability, low shrinkage and selfsupporting features after 2 h holding compared with BPF composites without 40wt% of inorganic fillers.
Key words: polymer composites; boron phenolic resin; calcine time; ceramification
1 Introduction
Ceramifiable polymer composites generally contain a polymer matrix and inorganic fillers, and form a self-supporting structure after being heated from ambient service temperature to elevated temperatures,accompanied with critical phase and microstructure changes[1-3]. Many efforts have been made to study the ceramifying mechanism and to improve thermal protection performance of the ceramifiable polymer composites. For example, Huet al[4]investigated the ceramifying process and mechanical properties of silicone rubber/ammonium polyphosphate/aluminium hydroxide/mica composites (SRAAM) after being calcined at various temperatures for 60 min. Hanuet al[5]argued that the addition of certain inorganic fillers to silicone resulted in improving the strength and giving a near-net shape ceramic of the composites fired at an elevated temperature. Their research group also showed that mica and ferric oxide had a stabilizing effect on the thermal stability of the silicon polymer composites,compared with glass frit[6]. Mansouriet al[7,8]reported that when silicone/mica composites were exposed to an elevated temperature (up to 1 000 ℃), a residue ceramic formed with a gradually improved strength as a result of the eutectic reaction between mica and the silica residue. It was verified that the addition of glass frits to the silicone/mica compositions was an effective technique for strengthening the flexural strength of the residual ceramics when heated at lowmedium temperatures, due to lowering the temperature point of the eutectic reaction[9]. Hamdani-Devarenneset al[10]further emphasized that crystallization and char formation in the interior of the residue were the key reasons for the improved ceramic strength as the ceramification process proceeded. Wanget al[11]studied the mechanical and ceramifiable properties of silicone rubber filled with various inorganic fillers. Their results showed that the mechanical properties were increased by incorporating various combinations of different inorganic fillers into the silicone rubber matrix owing to interaction of the frits, fillers and pyrolysis products.
The studies mentioned above are all on ceramifiable polymer composites based on a silicone matrix. This may be attributed to two general reasons.Firstly, a silicon polymer, itself, shows a better thermal resistance than most other polymers. Secondly, the pyrolysis product of a silicon polymer is amorphous SiO2, which can react with fillers or frits incorporated in the ceramifiable composite and be transformed finally to ceramic. On the other hand, polymers based on carbon are consumed completely or very little percent of carbon char is left when fired or heated up to high temperatures. Thus researchers have focused on silicon polymers much more than on carbon-based polymers as the matrix of ceramifiable composites.
However, carbon-based polymers and composites are widely used in many fields and may be subject to severe fire or high temperature environment in use. For example, phenolic resin and composites are used for thermal protection in the aerospace industry,polyurethane foam and polystyrene foam are used for thermal insulation in buildings,etc.Fortunately, some researchers, as described below, have paid attention to ceramifiable polymer composites based on a carbon matrix to improve the thermal resistance at high temperatures.
Al-Hassany[12]obtained a ceramifiable polymer composite by adding various adding various inorganic fillers to poly (vinyl acetate), and he further revealed that the key to superior formation of the ceramic char was based on the inorganic additive systems and on the filler particles and their state of dispersion in the host polymer. Diet al[13]reported that ethylenevinyl acetate (EVA) filled with glass dust (GD), glass fiber (GF), Organically Modified Montmorillonite(OMMT), and melamine cyanurate (MCA) exhibited great flame retardation and ceramifiable capacity. The results showed that the ceramics were successfully formed at various high temperatures. The various high temperatures affected the flame retardation of the composites and the mechanical properties. For instance,the ceramic formed at 800 ℃ had a flexural strength of 13.82 MPa when the EVA/GD/GF/OMMT/MCA had a weight ratio of 35/26/9/5/25. Ferget al[14]investigated a ceramifiable EVA/PDMS composite coating filled with calcium carbonate, aluminum hydroxide, muscovite mica and calcined kaolinite. They showed that the muscovite mica played an important role in keeping the ceramic residue physically stable.
Phenolic resin, another carbon-based polymer,is widely used in many fields due to its fire retardant and thermal protection properties[15-18]. Phenolic resin and its composites can normally be applied under 400℃ and is used at higher temperature primarily as a thermal protection material with great ablation[19,20].Ceramification could improve the thermal properties of a phenolic resin composite, but there have been only a few reports of ceramifiable composites based on the phenolic resin. Wanget al[21]investigated the thermal ceramification of phenolic-based ceramifiable composites modified by nano-aluminum oxide and glass powder at various temperatures. The formation and growth of the ceramic phase after thermal treatment enhanced the thermal stability and ablation performance of the composites at high temperatures.Ding[22]prepared a polymer matrix composite by incorporating microcrystalline muscovite fillers into boron-modified phenolic resin. The results showed that, after being heated at 1 000 ℃, the homogeneous composite structure was converted into a layered structure with a ceramic shell on the surface.
Calcine time would be expected to play a great role in the ceramification of a ceramifiable composite as well as calcine temperature, however, we know of only a few studies that have taken it into considerations.Studies of ceramifiable polymer composites have focused on the effect of the type of fillers on their thermal properties, mechanical properties,microstructure, phase compositions and the ceramifying mechanism under different firing conditions, especially for different firing temperatures for the same calcine time[13,20-22]. The heating temperature has been considered as a key for the eutectic reactions at the interfaces between the fillers and the pyrolysis products of the polymer matrix. In addition, most of research has focused on the ceramification of ceramifiable composites based on a silicone matrix, probably because silica, a pyrolysis product of silicone, may react with inorganic fillers, where is only a few studies are described above have reported the ceramification of ceramifiable composites based on phenolic resin.
Based on our previous work[23], the optimum temperature point of the eutectic reactions for ceramifiable boron phenolic resin composites modified by MgO-Al2O3-SiO2(MAS/BPF) is 1 200 ℃. The present work aimed to study the effect of calcine time on the ceramification of MAS/BPF at this temperature.The samples were calcined at 1 200 ℃ for 0-2 h and then cooled down to room temperature in a muffle furnace. The effects of calcine time on structure evolution, phase evolution and element evolution were investigated.
2 Experimental
2.1 Materials
Boron phenolic resin(BPF, with the boron content of 7% in weight),THC-400 (Shaanxi Taihang Impedefire Polymer Co., Ltd., China) was used as the matrix. Anhydrous ethanol (Sinopharm Chemical Reagent Co., Ltd., China) was used as a solvent.Magnesia, alumina and silica were used as fillers.Magnesia and alumina, both with particle sizes of 30nm, were from Shanghai Chaowei Nanotechnology Ltd., China. Aerosil 200 fumed silica was supplied by Aladdin Industrial Co., China. All inorganic fillers were used as received.
2.2 Sample preparation
Pyrolysis of the BPF and MAS/BPF samples were performed in air using a muffle furnace. Samples were heated from room temperature to 1 200 ℃ at a heating rate of 10 ℃/min, then held at 1 200 ℃ for 0 h , 0.5 h, 1 h, 1.5 h, or 2 h, respectively, and then naturally cooled down to room temperature in the muffle furnace.
2.3 Characterization
The bending strength of residue the composites were measured by were measured by an Instron-1341 universal test machine with loading speed of 2 mm/min.
Mass loss along as a result of pyrolysis was obtained by measuring the weight of the samples before and after heating. The mass loss is defined as Eq.(1):
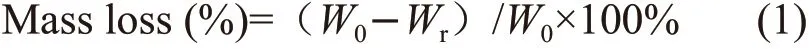
where,W0represents the weight of the original samples,andWrrepresents the weight of the residue.
The crystal phases of the residues after pyrolysis were characterized with a RU-200B/D/MAX-RB rotating anode high power X-ray diffractometer(Rigaku Corp., Japan). XRD data were obtained from 5° to 70° (2θ) at a scanning rate of 10 °/min.
The morphology of the residues after pyrolysis was identified with an field emission scanning electron microscope and energy-dispersive spectrometer(FESEM and EDS, S4800, Hitachi, Japan) at an accelerating voltage of 5 kV. The residues were broken in liquid nitrogen to get cross section samples. The residues samples were sputter-coated with gold before observation. The chemical constituents were also investigated using FESEM and EDS.
X-ray photoelectron spectroscopy (XPS)(ESCALAB 250Xi of Thermo Fisher Scientific Inc.USA), was used to detect the elements and chemical bonds of the residues.of the heat treatment temperature. And the bending strength of MAS/BPF residue is 10.2 MPa at 1 200 ℃.
Fig.2 indicates the heat resistance and structure stability of BPF can be improved by MAS porcelain forming agent, especially in the high temperature stage.Due to the decomposition of resin at 400 ℃-600 ℃, the mass loss rates of BPF at 600 ℃ and 800 ℃ reaches 50.7% and 52.3%, and the mass loss rates of MAS/BPF is 42.2% and 47.3%, respectively. But reactions occur between MAS fillers and cracked carbon at high temperature, which plays a protective role for cracked carbon. As a result, the mass loss of MAS/BPF sample is reduced under this heat treatment condition, with no significant change with heat treatment temperature, and the loss rate is lower than 45%.
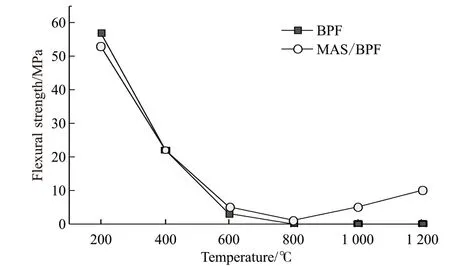
Fig.1 Bending strength of MAS/BPF pyrolysis residues at different temperatures
3 Results and discussion
3.1 Mass and size stability
Fig.1 shows the bending strength of MAS/BPF pyrolysis residues at different temperatures.With the increase of heat treatment temperature,thermal oxidation or structural layout rearrangement of BPF pyrolysis residual carbon occur, hole fusion and internal defects of the material increase, internal structure looses, the mechanical properties of the material decrease sharply and BPF pyrolysis residual has no strength after pyrolysis at high temperature, the bending strength tends to zero after 600 ℃. Meanwhile,the bending strength of MAS/BPF composites has a minimum value of 0.5 MPa at 800 ℃. The ceramic reaction occurs between MAS porcelain forming agent and BPF pyrolysis after 800 ℃, and the bending strength of the high-temperature pyrolysis residue of the MAS/BPF composite increases with the increase
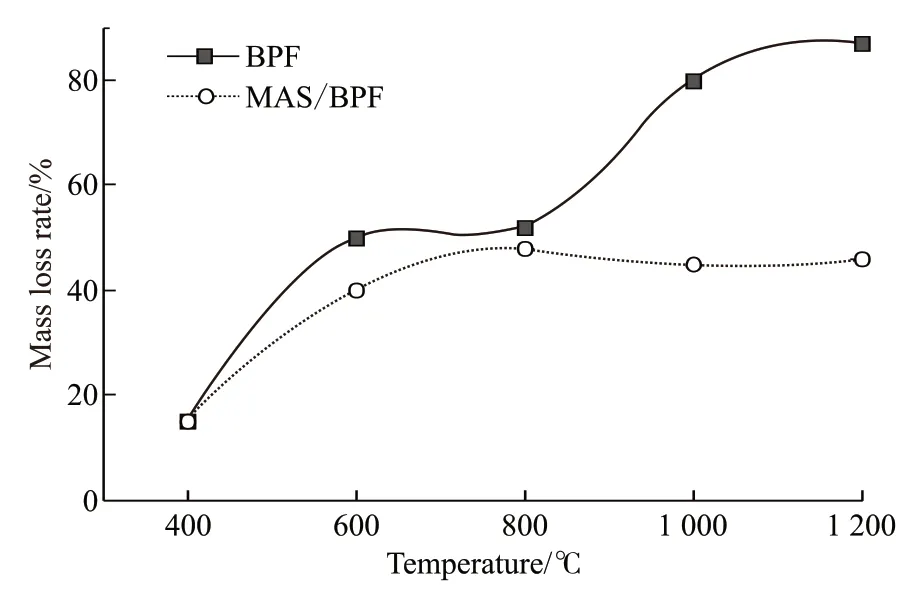
Fig.2 Effect of heat treatment temperature on mass loss rate of MAS/BPF composites

Fig.3 Morphologies of the residues at 1 200 ℃ for (a) 0 h, (b) 0.5 h, (c) 1 h, (d) 1.5 h and (e) 2 h, respectively
Fig.3 shows the morphology of the top surface of the MAS/BPF composite residues after being pyrolyzed at 1 200 ℃ for various calcine time. The residues of the composites show they are self-supported in shape. It is evident that a relatively smooth, white ceramic surface layer is formed after calcining by ceramificaton of the MAS fillers, although the surface is not fully smooth due to the tension and shrinkage of the eutectic phase of the residue when processed in an open air environment without any pressure. Under the top surface layer, a black char is formed, visible in various zones in the images, which comes from the pyrolyzed boron phenolic resin and the products of the fillers. The holes are present in the char which, resulted from the escape of low molecular products of the BPF resin during pyrolysis. As is shown in Fig.3(a) and Fig.3(b), in some areas, these holes are more or less circular in Fig.3 and they are larger and less uniform in the top ceramic layer. However, fewer holes are found on the top surfaces of the residues calcined for longer time, shown in Figs.3(c)-3(e). This may be because of further ceramification and densification of the ceramic surface with a longer calcine time. The well-ceramified top surface is highly heat-resistant and could protect the inner composite from further decomposition during heating.
The mass loss of the BPF and MAS/BPF during pyrolysis at 1 200 ℃ for the various calcine time are measured and are presented in Fig.4. Fig.4 shows that the mass loss of BPF increased almost linearly with the extending of the calcine time. When first heated from room temperature to 1 200 ℃, the mass loss of the BPF sample was 57% and increased to more than 80%when calcined at 1 200 ℃ for 2 h. This indicates that a very low content, lower than 20% content, of polymer residues was left. In contrast, the mass loss of MAS/BPF sample was about 40% when initially heated from room temperature to 1 200 ℃. And only increased to 46% for a heat holding time from of 2 h. So it can be concluded that the MAS/BPF polymer composite with a residue of about 40% content had good thermal stability.
The dimension of a polymer or polymer composite can vary greatly when fired or heated to high temperature because of combustion, pyrolysis and oxidation of the polymer matrix depending on its composition. Even thermally resistant and fire-proof polymer composites can show large size changes in a fire or high temperature environment. In this research,the MAS/BPF sample showed size changes in both the radial and axial directions. As shown in Table 1,shrinkage occurred during the heating process, and the shrinkage in the radial direction being greater than that in the axial direction. In free sintering without pressure,axial shrinkage was less than radial shrinkage due to the action of gravity and surface tension, and the residual carbon produced by BPF pyrolysis at high temperature was further oxidized under aerobic conditions to produce gases such as CO2, which diffused outward and drove the material to move in the longitudinal direction, so that the longitudinal dimension of the sample first expanded and then shrunk, and the radial dimension shrunk, so axial shrinkage was much less than radial shrinkage. The radial shrinkage of the MAS/BPF composite increased from 4.6% when heated from room temperature to 1 200 ℃ to 17.3% when held for 2 h. There was a double increase of axial shrinkage, from an initial 3.6% to 7.4% when the heat holding time was increased from 0h to 0.5 h. However when the holding time was over 0.5h, the shrinkage in the axial direction stabilized at about 8%. The size changes are presumed to result from the pyrolysis of the polymer matrix, loss of some of pyrolyzed products, and the ceramification of the MAS fillers and their densification. The MAS/BPF polymer composite clearly had a better size stability than the control sample of BPF at a high temperature.
Fig.4 Mass loss rate variation with calcine time at 1 200 ℃
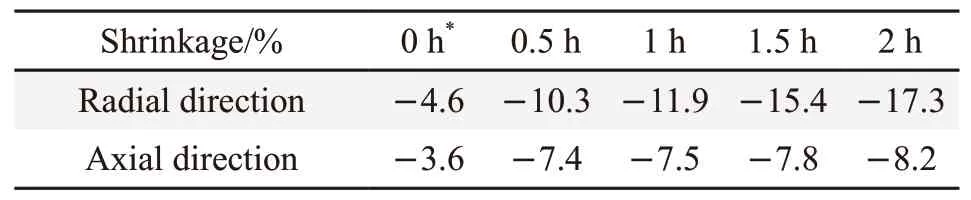
Table 1 Shrinkage of MAS/BPF
3.2 Phase evolution
To verify the ceramification of the MAS, the crystalline phases of the residues after being calcined in an air atmosphere at 1 200 ℃, holding for 0 h, and 1 h ,were analyzed by XRD, as shown in Fig.5. Curve(a) in Fig.5 presents the characteristic diffraction peaks of corundum, periclase, cristobalite, amesite, and considerable amorphous phase in the residue held for 0 h. After being heated to 1 200 ℃ and held for 1 h,all the characteristic diffraction peaks of the MAS disappeared, and only two broad peaks remained. It suggests that all of the fillers had reacted completely to form a non-crystalline residue. The increase in size of those peaks indicated a large amount of amorphous materials was formed in the heating process, which lead to a densified ceramic bulk structure. This special phase structure is succeeded to the good mass stability at high temperature.
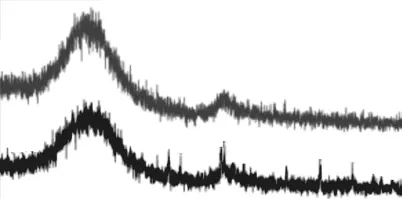
Fig.5 XRD patterns of MAS/BPF residues after holding for 0 h,and 1 h at 1 200 ℃
3.3 Microstructure and element composition evolution
3.3.1 Microstructure and elemental composition evolution of the top layer of the residues
To further investigate the effect of calcine time on the ceramifiable property of MAS/BPF, the microstructures of the residues were observed by FESEM. As shown in Fig.6, observations of the top surface structure of the MAS/BPF residues showed dramatic microstructural changes with the extension of calcine time at 1 200 ℃.
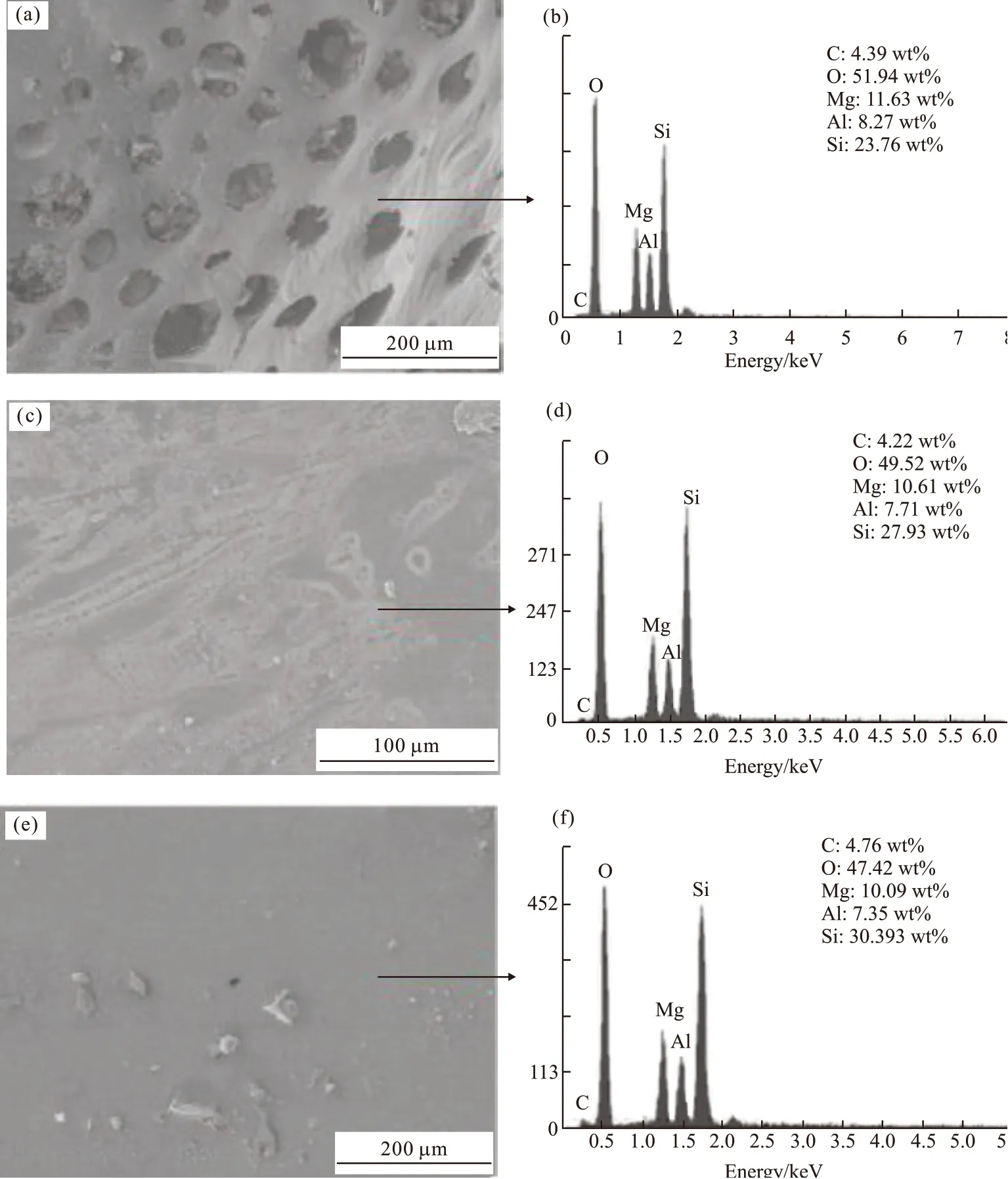
Fig.6 SEM images and EDS of top surface of the residues at various calcine times: (a), (b) for 0 h; (c), (d) for 1 h; (e), (f) for 2 h,respectively
As shown in Fig.6(a), the top layer of the residue heated to 1 200 ℃ and held for 0 h was very porous and, in fact, also very powdery and easy to be smashed.Gas evolution from the thermal oxidation of the carbonized phenolic resin resulted in the porous skin layer of the residue. In addition, with short holding time, the extent of the eutectic reaction was limited.The volume of liquid phase formed as a result of the eutectic reaction was not enough to form strong and hard surface layer when cooled.
With extending the holding time to 1 h, a relatively denser and smoother top layer was obtained,as shown in Fig.6(c). The partially continuous top layer was strengthened by ceramic products of the eutectic reaction between the MAS filler and the pyrolysed residues. The denser and smoother top layer played an important role in preventing the heat damage in the inside of the residue and reducing the mass loss. With further holding for 2 h, as shown in Fig.6(e), the top layer was even smoother and denser (also shown in Fig.3(e), which was caused by further eutectic reaction.
The elemental composition of the top surface layer of the residues was characterized using EDS, as shown in Figs.6(b), 6(d), and 6(f) for the samples held at 1 200 ℃ for the various times. The main elements of the top layer obtained for the different heating conditions were similar. Carbon (C), oxygen (O), magnesium(Mg), aluminum (Al), and silicon (Si) were contained in all three residues , among which the content of C was relatively low and varied slightly from 4.4% when held for 0h, to 4.2% held for 1h, and to 4.8% holding for 2 h. The slight fluctuation of the content of C indicates that C could not be oxidized fully, even when the heat holding time was increased. According to the elemental composition of the residues of when initially heated to 1 200 ℃, the composition can be designated as a 3.1MgO·Al2O3·5.5SiO2·4(C0.6O), and it can also be defined as a Mg-Al-Si-O ceramic containing carbon.The content of Si in the top layer increased from 24%to 28% and further to 30% when extending the holding time from 0h to 1h, and further to 2 h, respectively,while the content of Al and Mg each decreased slightly.It suggests that the top layer of the residue of the MAS/BPF composite was the product of the reaction of the fillers and a small quantity of carbon left from pyrolysis of the BPF. It can be concluded that there was a relationship between the elemental composition and the heat holding time as a result of migration of the various elements. Si migrates and concentrates on the surface to form a ceramic layer resulting in the MAS/BPF composite becoming more thermal-resistant and able to endure longer at high temperature. This result is well consistent with that in the research by Dinget al[22]on the effect of incorporating microcrystalline muscovite in BPF .
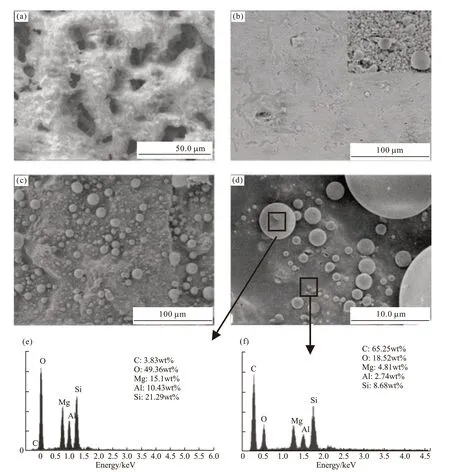
Fig.7 Cross section structures and EDS of the residues at different calcine time:heat holding for (a) 0 h, (b) 1 h, and (c)-(f) 2 h
3.3.2 Microstructure and elemental composition evolution in cross sections of the residue
Similar to the observations of the top surface structure of the residues, the microstructures of the residues in the cross sections are shown in Figs.7(a)-7(d). The microstructures of the cross section show a significant evolution upon extending the heat holding time. Phenolic resin is easily damaged and transformed into CO2and amorphous carbon in a thermo-oxidative environment. As shown in Fig.7(a), an apparent porous floc structure for the cross section of the residue was obtained when just heated to 1 200 ℃. With limited formation of a liquid phase and a short high temperature holding time, the inner part of the residue was difficult to be densified.
With longer heat holding time, coherent and dense microstructures were formed, resulting from the liquid sintering and formation of ceramic materials.When the heat holding time was up to 1 h, the products of the eutectic reaction acted as a binder to form a continuous structure, and large numbers of white beads,presumably liquid at 1 200 ℃ were distributed in the char, as shown in Figs.7(b), 7(c), and 7(d).
The elemental composition of one of the spheres,and the char are shown in Figs.7(e) and 7(f). The main elements of the spheres consisted of O, Mg, Al, and Si,which suggests that the spheres were the ceramified products of the fillers with only a small amount of carbon. It shows that there were certain crystal precipitation, liquid phase composition changes, the increase in the viscosity of the molten liquid causes it to condense into round shape under the action of surface tension. Comparing the elements and their amounts of the inner spheres to those of the top surface layer, the type of element did not change, but changes did occur in their relative amounts. The 3.8% content of C in the sphere was slightly lower than the 4.8% in the top surface layer. However, the contents of 15.1% Mg and 10.4% Al in the sphere were higher than 10.1% Mg and the 7.4% Al in the top surface layer, respectively.The 21.3% Si in the sphere was greatly lower than the 30.4% Si in the top surface layer. It indicates that the Si migrated to the top surface, and the Mg and Al migrated to the inner part of the residue. In general, the top surface and the internal spheres of the residue were ceramified to form ceramic products containing small amounts of carbon.
The EDS data in Fig.7(f) show that Mg, Al, and Si were still detected in the char, which resulted from permeating and migrating from the liquid phase to the char of pyrolysed BPF. There was 65.3wt% of C remaining in the char although the sample had been heated for 2 h from room temperature to 1 200 ℃ and then held for another 2 h. The content of C in the char was more than 10 times higher than that in the top layer and more than 15 times higher than in the spheres. The morphology study and EDS analysis of the top surface layer and the inner sector indicted that the eutectic reaction was the reaction of the MAS fillers and a small amount of carbon, and the products of the eutectic reactions mainly made up the carbonaceous ceramic phase. We concluded that the ceramified products of the MAS/BPF in the top layer and inner section played a large role to slow down the degradation, oxidization and pyrolysis of the phenolic resin and composite.
The microstructure and elemental composition evolution demonstrated that the quality of the ceramic for the MAS/BPF was gradually improved with the heat holding time. It was obvious that with increasing heat holding time, both the top surface and cross section microstructures of the MAS/BPF evolved accompanied with a phase evolution with time. The microstructure evolution showed that the residue, calcined at 1 200℃ for at least 1 h, was densified and had a smooth top surface layer and bulk structure.
3.4 Chemical bond evolution
The absorption peaks of 3 220, 2 261, 884,and 649 cm-1are the stretching vibration peaks of C-H bond, while 1 626 cm-1and 1 459 cm-1are the stretching vibration peaks of C-C bond. The stretching vibration peak of C-O is 1 196 cm-1, and the absorption peak of resin carbon structure in aromatic ring structure is 1 091 cm-1, in Fig.8(b), 1 394 cm-1is the vibration peak of C=C double bond, 1 091cm-1and 792 cm-1belong to the stretching vibration peak of Si-O bond,and 472 cm-1belongs to the characteristic peak of Mg-O-Al bond. According to the structure analysis of the binder phase, certain chemical reactions occurred in the MAS/BPF composites at 1 200 ℃.
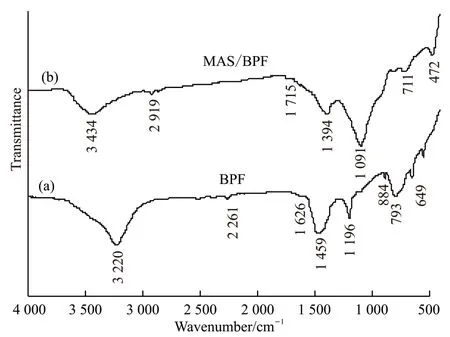
Fig.8 Infrared spectra of residues of BPF and MAS/BPF after 2 h heat treatment at 1 200 ℃
To confirm the reactions and new chemical bond formation between the fillers and BPF, XPS curves of the residue were obtained. As shown in Fig.9(a), C,O, Mg, Al, and Si existed in the residue of the MAS/BPF composite calcined at 1 200 ℃ for 2 h, which is consistent with the results of the EDS analysis.
Fig.9(b) shows five main peaks of binding energy of C1s, 284.6 eV, 285.7 eV, 286.5 eV, 287.7 eV, and 289.2 eV, in which the peak at 284.6 eV is the characteristic peak of C-C bonds, 285.7 eV corresponds to C-Si bonds, 286.5 eV is attributed to C-O bonds and the peaks at 287.7 eV and 289.2 eV are together assigned to the bonds of C=O. As shown in Figs.9(c),9(d), and 9(e), the XPS curves of Mg, Al, and Si are all each fitted with just one curve of the binding energy.The peak at 1 305.1 eV characterizes the Mg-O-Si the montmorillonite bond. 74.8 eV indicates the Al-OSi bond of a silica-alumina compound and 103.2 eV relates to the Si-O bond of a silica-alumina compound.The above results indicate that C, the product of pyrolysed BPF, reacted chemically with O and Si, so the residue were Mg-O-Si, Al-O-Si, and part of Si-C complex ceramics.
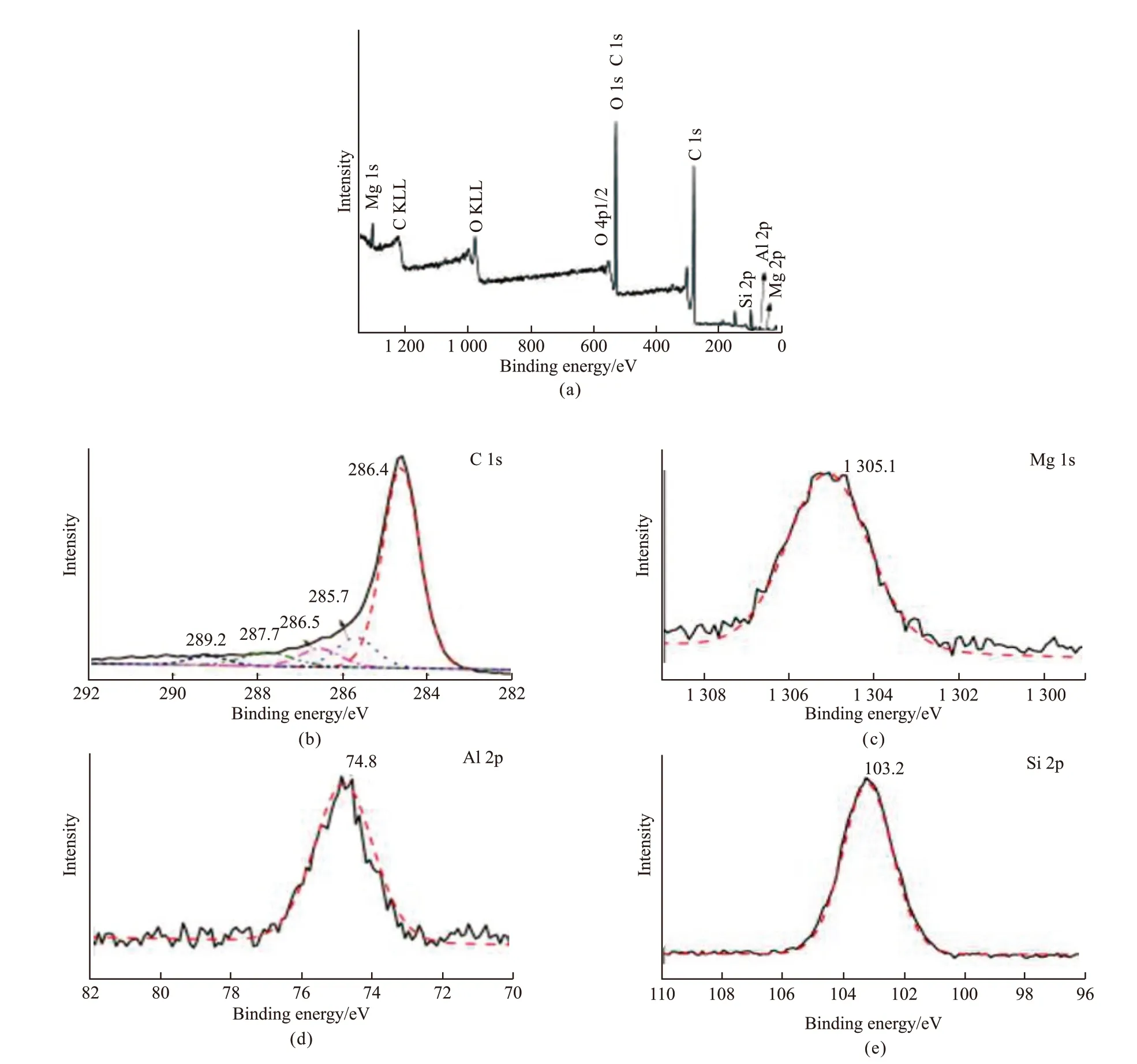
Fig.9 XPS curves of residue of MAS/BPF composite calcined at 1 200 ℃ for 2 h
4 Conclusions
The macroscopical morphology, mass and size stability, phase evolution, microstructure and elemental composition evolution and chemical bond evolution of MAS/BPF during a ceramifying process at 1 200 ℃for various calcine times were investigated. A ceramic surface layer was formed by ceramification of the fillers and residues during the heating, and fewer holes were found on the top surface of the residues calcined for times longer than 1 h. The MAS/BPF polymer composite showed good mass and size stability. The XRD patterns showed amorphous phase formed in the heating process, which lead to a nearly densified ceramic-like bulk structure. The relatively dense and smooth top layer played a very important role in preventing heat and oxygen penetration into the inner part of the residue. When the heat holding time was 1 h or 2 h, a continuous matrix in the cross section of the residue was formed and large numbers of spheres were distributed in the char. As to the evolution of the elemental composition, Si migrated and concentrated on the top surface to form a ceramic layer to make the composite more thermal-resistant, however, 65.25wt%of C, more than 10 times higher than that in the top layer, was left in the inner char although the sample had been heated for 2 h and then held for another 2 h at 1 200 ℃. Carbon, the product of the pyrolysed BPF, reacted chemically with O and Si. In summary,the MAS/BPF composite can be ceramified at a high temperature, resulting in the formation of a smooth,dense ceramic top layer. Importantly, this top layer protected the inner char from further decomposition.The ceramifiable MAS/BPF composite may be a new type of thermal resistant composite.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Comparative Case Study on Adhesion of Three Common Sizing Agents to Cotton and Polyester Yarns
- Effect of Outer Carbon Layer Thickness of Carboncovered N-doped Hollow Carbon Nanospheres on Its Electrocatalytic Performance
- Natural Fresh Proteins Directed Hierarchically Porous Nitrogen-doped TiO2 as with High Performance as Photocatalyts and Electrode Materials
- Dynamic Adsorption of Toluene on Hierarchical Porous Carbons with Varying Pore Structure
- Self-propagating High-temperature Synthesis of Sm and Zr Co-doped Gd2Ti2O7 Pyrochlore Ceramics as Nuclear Waste Forms
- Solidification Behavior of in situ TiB2/Cu Composite Powders during Reactive Gas Atomization
