Dynamic Adsorption of Toluene on Hierarchical Porous Carbons with Varying Pore Structure
2021-04-16YANXi
YAN Xi
(SINOPEC Research Institute of Safety Engineering, Qingdao 266071, China)
Abstract: Hierarchical porous carbon material (MMC) was successfully fabricated via hard template synthesis method by carbonization of furfury alcohol within the template (MCM-41). The prepared MMC was studied with characterization methods including scanning electron microscopy (SEM), transmission electron microscopy (TEM), nitrogen adsorption-desorption analyses, and infrared spectral analysis (FTIR). To investigate kinetics of toluene adsorption of hierarchical porous carbon materials, the adsorption performances of these carbon samples with varying pore structure (MC-1, MMC, MMHPC) were analyzed via dynamic adsorption. And the Langmuir model and Freundlich equation were employed to correspond with adsorption isotherms to study the adsorption mechanism. The experimental results demonstrate that the Langmuir model is more appropriate to describe the adsorption process. The capacities of toluene adsorption follow the order of MMC < MMHPC (micro-meso hierarchical porous carbon) < MC-1(microporous carbon). MC-1 has satisfactory absorption performance due to its large pore volume and high ratio of micropores. MMHPC has excellent toluene adsorption performance for proper amounts of surface oxygen containing groups. Long saturation time, interconnected hierarchical pore channels, and large specific surface area make MMC also a promising material for VOCs treatment. These data reveal that the pore channel structure, rational pore distribution, high surface area and reasonable amounts of surface oxygen groups are the main factors contributed to excellent toluene adsorption performance, which proposes theoretical basis for hierarchical porous carbon materials to further engineering application.
Key words: hierarchical porous carbon; toluene; adsorption; pore structure
1 Introduction
With the acceleration of industrialization and global economic integration, air pollution has became a serious problem which attracted global attention[1].VOCs (volatile organic compounds) is one of the main air pollutants due to their toxicity and massive emission, the most common ones are benzene, toluene, xylene[2]. The toxicity caused by VOCs can harm human health[3], correlating with respiratory disease, neurological disease and even cancer. The emitted VOCs can easily volatilize and rapidly evaporate, causing photochemical smog and global warming. There are many technologies such as adsorption[4], absorption[5]member separation[6], combustion[7], biological treatment[8], and photocatalytic oxidation[9]are adopted to treat VOCs and purifying air.
Among them, adsorption is the most advocated method due to its low energy consumption, low operation cost and high efficiency[10]. Thus, researches relative to adsorbents have been carried on in recent years. Dynamic adsorption-desorption of toluene on different carbon materials were studied by Yang Xi[11],who found that the Freundlich model was the most accurate equation for fitting the equilibrium data. Pore structure, volume and ratio of meso-/macro-pores can affect adsorption capacity of activated carbon. In another research on activated carbon[12], CO2/microwave method was applied to form hierarchical pore structure and enhance the surface inertia of activated carbon for excellent toluene adsorption stability. And the Langmuir model was suitable for simulating toluene adsorption. Zhou Huiping[13]developed a novel amino-functionalized spherical mesoporous silica material, and investigated its toluene adsorption capacity. The results showed that the Yoon-Nelson mathematical formula was proper for simulating the experimental adsorption data. It concluded that not only pore volume but also surface chemical groups were the main factors affecting its treatment efficiency of VOCs. However, the kinetics of toluene adsorption on hierarchical porous carbon materials have not been systematically investigated.
This paper reported the synthesis of a promising adsorbent (MMC) for VOCs treatment. The microstructure of MMC was determined by Brunauer-Emmett-Teller (BET) method. And surface groups were analyzed by Fourier transform infrared spectroscopy(FTIR). While toluene is considered as a representative of VOCs, dynamic adsorption of toluene on MMC was studied. MC-1[14]and MMHPC[15]were also synthesized by using MCM-41 as template, while sucrose and phenolic resin as carbon source separately.These three hierarchical porous carbon materials with different morphology features were selected as adsorbents to explore how varying pore structure and surface groups affecting toluene adsorption capacity. Breakthrough curves and toluene adsorption isotherms of these carbon samples were also studied to analyze adsorption kinetics.
2 Experimental
2.1 Materials
MCM-41 (Nankai University, China), MMC,MC-1 and MMHPC (made in laboratory) were degassed before experiments. All the chemical reagents used in this paper were analytical grade purchased from Sinopharm Group. Toluene was utilized as a simulated pollutant,p-toluenesulfonic acid as catalyst, and furfuryl alcohol as carbon source.
2.2 Synthesis
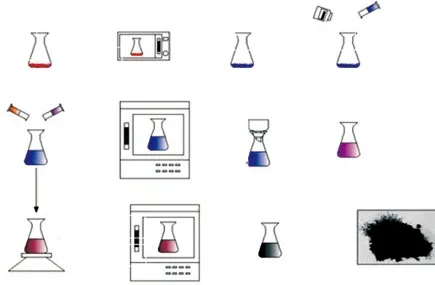
Fig.1 Preparation procedure of MMC
MMC (Fig.1) was prepared via hard template method, using MCM-41 as the framework, p-toluenesulfonic acid as the catalyst, and furfuryl alcohol solution as the carbon source. The template was first added into the catalyst aqueous solution (0.5 mol/L ethanol solution). After continuous stirring for one hour, the uniform mixture was then filtrated by ethanol under vacuum. After drying for 3 h at 80 ℃, the mixture was well mixed with furfuryl alcohol solution by vigorously stirring for 18 h under room temperature. The mixture was then stored in a 60 ℃ oven for six hours, then the oven temperature subsequently increased to 160 ℃ and maintained for 6 h. After drying, the resultant solution was kept in N2at 800 ℃ for three hours. Finally, the template was eroded using 2.5wt% sodium hydroxide solution.
The synthesis method of MC-1 and MMHPC were already reported in previous papers.
2.3 Characterization
Structure and morphological observation of MMC was achieved by a Hitachi S-4800 emission scanning electron microscopy. TEM characterization was conducted on JEM-2100 to obtain high resolution transmission electron micrographs. ASAP 2020-M was utilized to measure nitrogen adsorption-desorption isotherms of carbon samples at 77 K. And Brunauer-Emmett-Teller (BET) equation was applied for evaluating the specific surface areas and pore volumes. According to the Horvath-Kawazoe (HK) algorithm and the Barrett-Joyneer-Halenda (BJH) algorithm, micro- and mesopore size distributions were calculated, respectively.The V-t model was utilized to estimate surface areas and volumes of micropores. And infrared spectra of the samples were achieved on a ThermoNicolet NEXUS infrared spectrometer with the KBr method to investigate the functional groups.
2.4 Dynamic adsorption performance
2.4.1 Adsorption device
Dynamic adsorption performance of these carbon samples were evaluated by a continuous gas introduction device assembled in laboratory. Bubbling reactor was used to generate toluene vapor by continuous air bubbling. Air introduced by air pump was then mixed with toluene vapor in gas mixing apparatus. Gas flow controllers were used to obtain appropriate toluene concentrations. Then the fixed bed reactor is where dynamic adsorption process happened.
2.4.2 Experimental methods
The dehydration and degassing of absorbents were conducted in a vacuum oven at 120 ℃ for at least 12 hours before taken to the adsorption experiments.After adding 1 cm of carbon samples into the fixed bed reactor, the system was operated at 25 ℃. Desired concentrations of toluene were obtained by adjusting air flow rate. Then gas samples were collected by glass injectors at sampling spots. And a gas chromatography equipped with FID (SP-3420A) was used to determine the inlet and outlet concentrations of toluene. The toluene adsorption curves were obtained through dynamic adsorption, whereC0represents the inlet concentration,andCmeans the concentration after treatment. Breakthrough time is whenC/C0=0.1, and saturation time is whenC/C0=0.95. The adsorption capacity of adsorbents(qe) were calculated from column weights difference between initial and saturation adsorption based on the following equation:

where,m0(mg) represents the initial weight of adsorption column, andme(mg) is the column weight after saturation. The adsorption isotherms of toluene on carbon samples were acquired by plotting adsorption capacities versus concentrations, and were fitted using the Langmuir and Freundlich model.
3 Results and discussion
3.1 Physicochemical characteristics of MMC
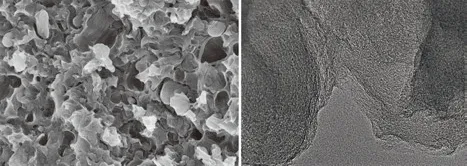
Fig.2 Images of MMC: (a) SEM; (b) TEM
Fig.2 shows the SEM and TEM images of MMC.Fig.2(a) reveals that MMC also possesses stratified and hierarchical porous structure like MC-1[14]and MMHPC[15]. But differences in the surface morphology of the carbons can be observed. MMC has compact and interconnected pore structure, micropores and mesopores growing inside macropores can be observed. While MC-1 has pit-like micropore structure[14], and MMHPC has massive micropores[15]. It can be found that MMC has higher values of average pore size than MC-1 and MMHPC, implying that MMC has few micropores.Fig.2(b) displays the TEM image of MMC. It can be found that MMC also possessed a non-uniform pore distribution like MC-1[14]. While, MMHPC has a partly ordered porous structure[15]. Fig.3 illustrates the N2sorption isotherms of these hierarchical porous carbon materials.
According to IUPAC classification[16], MMC exhibited a type-IV isotherm, implying the porous structure and existence of mesopores. And the H4hysteresis loop existed in the isotherm meaning that MMC is also a typical micro-mesoporous carbon material. There is a steep increase at relative low pressure, which illustrates large amounts of micropores[17,18]. The gradual increase observed is corresponding with mesoporous properties[19]. It can also be obtained in Fig.3 that the specific surface area followed the order of MC-1 > MMHPC >MMC.
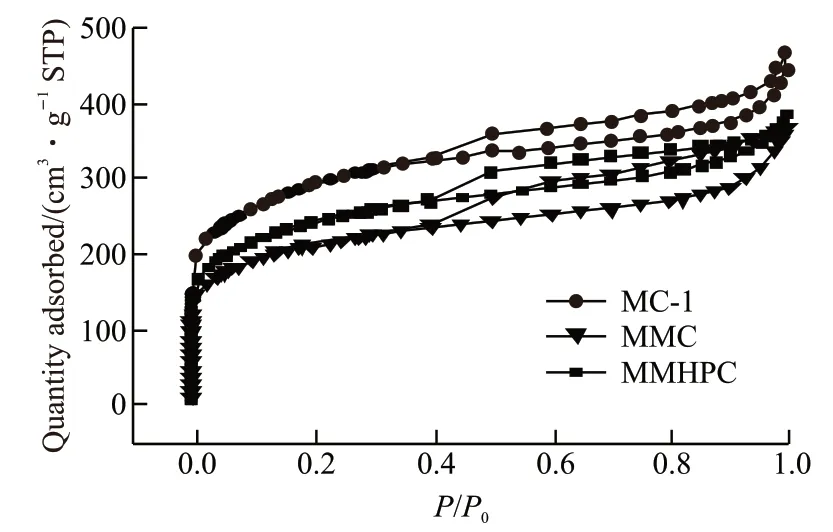
Fig.3 N2 sorption isotherms of carbon samples
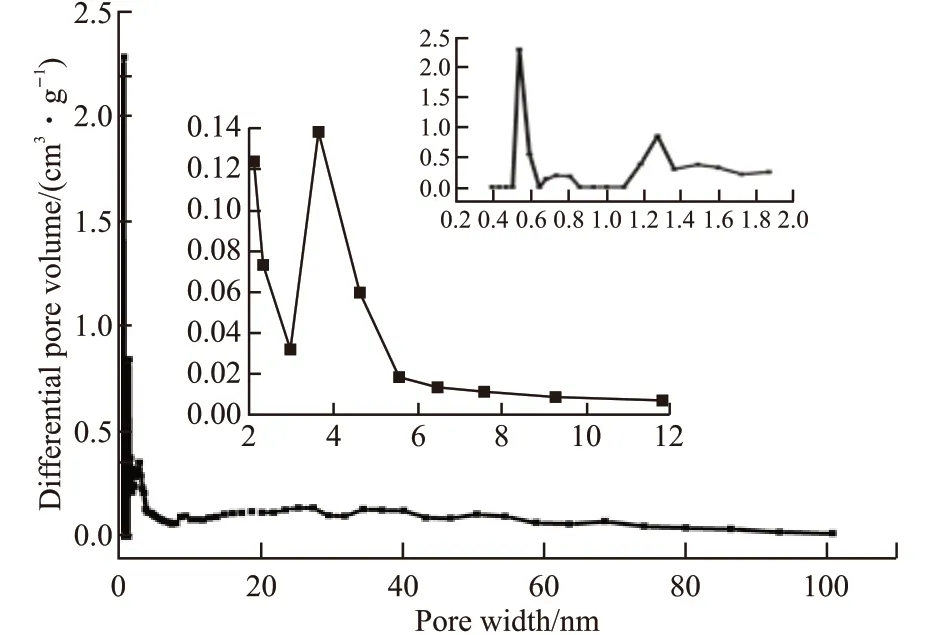
Fig.4 Pore size distribution of MMC
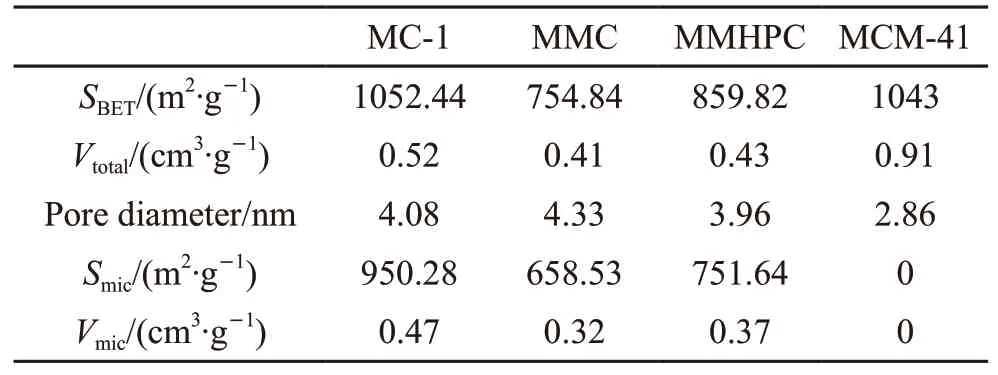
Table 1 Properties of cabons
Fig.4 presents the pore size distribution of MMC.It can be observed that MMC has two peaks at around 0.5-0.6 and 1.2-1.4 nm indicating the presence of micropores. And MMC possesses abundant mesopores with peaks at around 3-5 nm. The properties of the adsorbents including specific surface area (SBET), pore volume (Vtotal), micropore surface area (Smic), and micropore volume (Vmic) are listed in Table 1. The descending order of the pore volumn is as follows: MC-1 > MMHPC> MMC, while the order of the pore diameter is MMC> MC-1 > MMPHC. Table 1 presents that MC-1 has the largestSBETandSmic. The value ofSmic/SBETandVmic/Vtotalare calculated. And MC-1 (90.3%) has higher value ofSmic/SBETthan MMC (87%) and MC-3 (87%). And the descending order ofVmic/Vtotalis as follows: MC-1(90.38%) > MMHPC (86.05%) > MMC (78.05%). The results indicate that MC-1 may have better adsorption performance for its appropriate pore distribution.
The IR spectra of these hierarchical carbon samples are shown in Fig.5. It can be found that some peaks existed at same positions in the three curves, which indicates that the carbons may have similar surface chemical groups. The weak adsorption peaks at 797 and 693 cm-1can be ascribed to C-H bending vibrations. And the strong C-O vibrations can be observed in 1 000 to 1 250 cm-1. Peaks in 1 500 to 1 650 cm-1indicating the existence of -COOH group vibrations[20,21]. And by peak area, it can be applied that MMC possesses more-COOH group than MMHPC and MC-1. The wide peak appeared at 3 200-3 670 cm-1can be attributed to O-H strenching[22]. The peak of MC-1 at 3 200-3 670 cm-1is less pronounced than that of MMC and MMHPC, and this may suggest less presence of O-H groups in MC-1[23]. The results suggest that MC-1 possesses the least amount of surface oxygen groups.
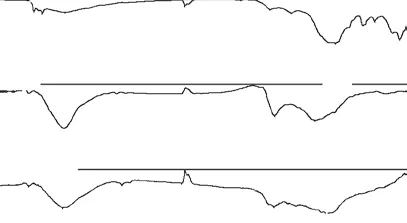
Fig.5 IR spectra of carbons
3.2 Adsorbing toluene properties of adsorbents
Fig.6 depicts the toluene adsorption curves over different absorbents, when the inlet concentration of toluene is 1 750 mg/m3. The curves are existed basically as an S type curve. From Fig.6, it can be observed that the order of saturation time is as follows: MMC(320 min) > MC-1 (269 min) > MMHPC (207 min),which is in accordance with the order of average pore diameter. The curve of MMC is flatter than that of the other adsorbents, indicating that the adsorption rate constant of MMC is the smallest. It can be ascribed to the truth that the more complex the porous structure is,the lower the mass transfer rate is[24]. With regular pore structure, toluene may spread more easily in MMHPC and MCM-41. This indicates that pore structure, high surface area and long saturation time make MMC a promising material for toluene adsorption. However, the adsorption capacity of MMC was smaller, for the micropore volume and surface area of MMC was smaller than that of the other two carbons. This means that pore structure is also responsible for adsorption capacity, in addition to total pore volume and specific surface area[25]. Even though MMHPC has smaller microporous specific surface area and microporous volume than MC-1 and MMC, MMHPC with more surface oxygen groups have higher toluene adsorption capacity (358.8 mg·g-1). And it may due to the fact that the oxygen groups can increase surface polarity of MMHPC, which changes the electron cloud density of toluene molecule[26]. The decrease of dispersion force between toluene and MMHPC caused by this process would enhance the adsorption. This signifies that specific surface area and pore volume are not the only factors affecting adsorption capacity, so does the surface oxygen containing groups[27].
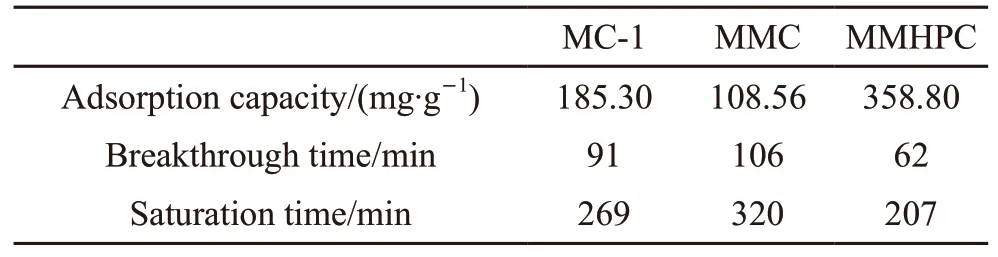
Table 2 Toluene adsorption capacities of adsorbents
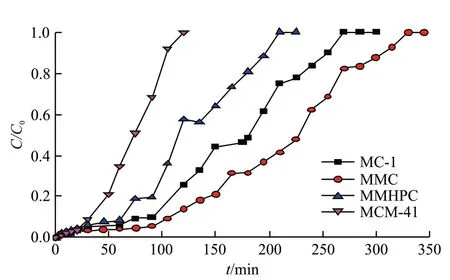
Fig.6 Adsorption curves for toluene over different absorbents
3.3 Dynamic adsorption performance
3.3.1 Breakthrough curves of MMC
Fig.7 illustrates the breakthrough curves of MMC under toluene inlet concentrations (C0) varying form 389 mg/m3to 3 869 mg/m3. The parameters related to adsorption performance of carbon samples including adsorption capacity, penetration time, and saturation time are presented in Table 3. Fig.7 shows that the five obtained adsorption curves presenting identical curvilinear trend. And the saturation adsorption capacity increased with initial toluene concentration. The curves can be divided into three stages. In the first phase, a small amount of toluene can be detected for the existence of abundant micropores[28]. There is a rapid increase of the curves after breakthrough time, and this can be attributed to almost complete toluene adsorption at the second stage. And in the third phase, the curve became flatter before saturation.
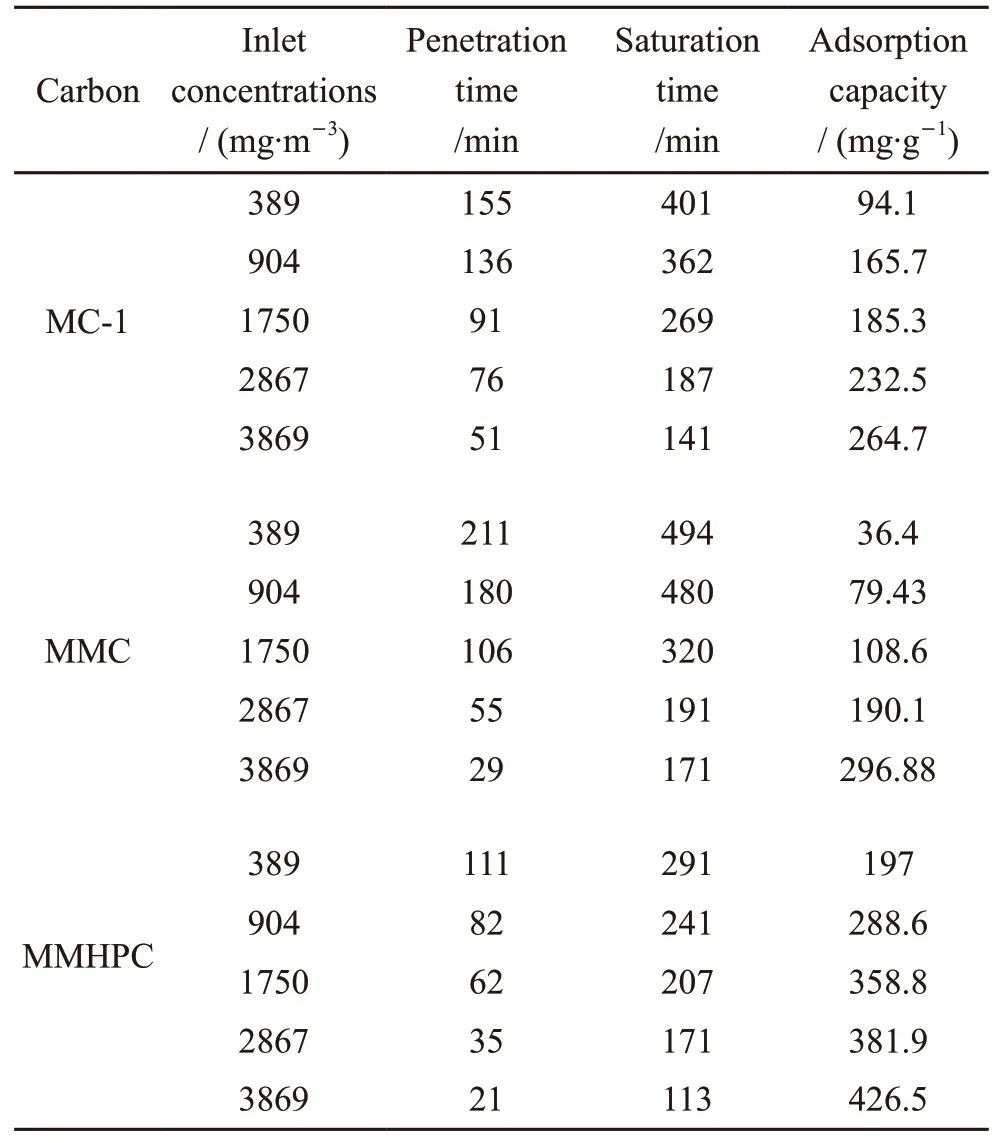
Table 3 Toluene adsorption performance of different adsorbents under varying concentrations
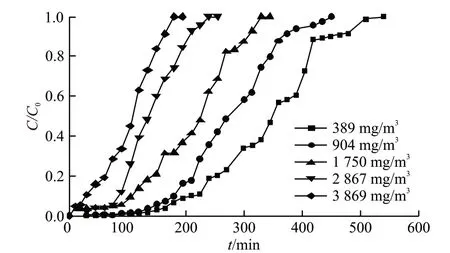
Fig.7 Breakthrough curves of MMC under different initial concentrations
Table 3 reveals that toluene adsorption performance varies with different hierarchical carbon samples. It can be observed that the penetration time and the saturation time have the following order: MMC >MC-1 > MMHPC. But when the initial concentration of toluene is greater than 2 867 mg/m3, the descending order is as follows: MC-1 > MMC > MMHPC. This may because MC-1 have larger micropore surface area and micropore volume than MMC[29,30]. Thus, MC-1 with proper micropore size and suitable micropore volumn may have higher toluene adsorption capacity under high toluene concentrations.
3.3.2 Toluene adsorption isotherms
The adsorption isotherms on hierarchical porous carbon samples are depicted in Fig.8. MMC reveals a type-IV isotherm. Gradual increase can be observed at the beginning of adsorption isotherms for microporous structure in pore channels, then MMC shows an obvious increasing for toluene adsorption efficiency at the high-concentration region, implying the capillary condensation appeared when toluene molecules were adsorbed in the carbon which had large mesoporous volume. While the isotherms of MC-1 and MMHPC display a type-I isotherm. At the low-concentration region, the abrupt rise of the curves reveals the high efficiency of toluene adsorption, which can be attributed to the abundant existence of micropores. As the adsorption capacity of the micropores in carbon samples reaches to its maximum point, the curves show a gradual increase, followed by a more rapid increase near saturation.
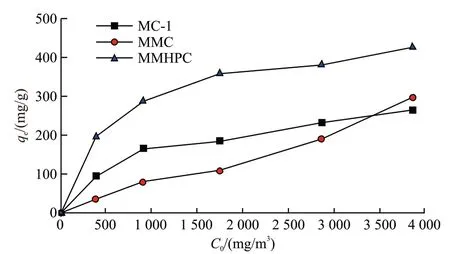
Fig.8 Adsorption isotherms on carbon samples
3.3.3 Adsorption kinetics
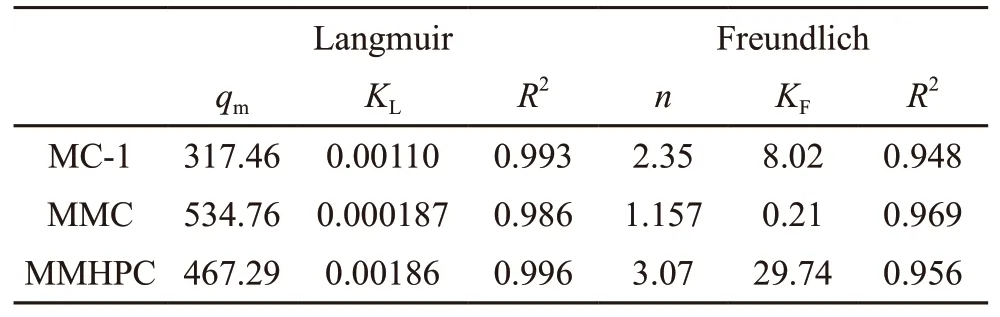
Table 4 Fitting parameters for toluene on carbon samples
The Langmuir equation and the Freundlich equation are both selected to describe toluene adsorption data. And the fitting parameters are calculated and summarized in Table 4. It shows that the order ofR2fitted by these two models is Langmuir > Freundlich,illustrating the adsorption surfaces of these hierarchical porous carbon are single molecular layer adsorption,which implies physical adsorption process. In addition,there is difference on the adsorption behaviours of the three samples, meaning adsorption capacity of adsorbents is significantly affected by pore structure.
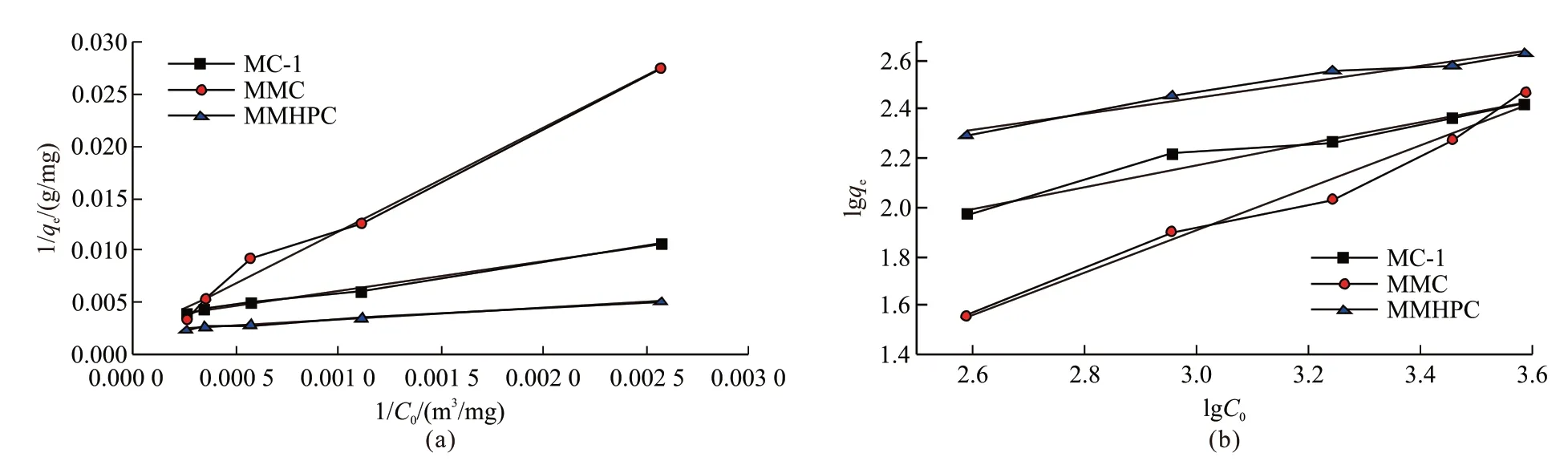
Fig.9 Fitting curves of carbon samples: (a)Langmuir fitting; (b) Freundlich fitting
4 Conclusions
Template synthesis technique is used to obtain MC-1, MMC and MMHPC as adsorbents for toluene adsorption. This paper introduced the synthesis process of MMC, then analyzed kinetics and behaviors of toluene adsorption on these carbon samples by dynamic adsorption. The results show that these mirco-meso hierarchical porous carbons are qualified candidates for toluene treatment, and MMC is a promising material for toluene adsorption for its high surface area(754.84 m2·g-1), long saturation time and excellent adsorption capacity (108.56 mg·g-1). The equilibrium amounts of toluene adsorbed on carbon samples have the following order: MMHPC (358.80 mg·g-1) > MC-1(185.30 mg·g-1) > MMC (108.56 mg·g-1), which is not in accordance with the order ofSBET,Vtotal,SmicorVmic(MC-1> MMHPC > MMC). And MC-1 has the largest micropore content but the least amount of surface oxygen groups. This indicates that not only pore surface area, pore volume, and micropore content but also surface oxygen groups can affect adsorption capacity. The order of saturation time is as follows: MMC> MC-1 > MMHPC, which may contribute to the complex and interconnected pore structure of MMC. This means that pore structure is also an important factor for toluene adsorption performance. Thus, lab-synthesized hierarchical porous carbon materials with high surface area, appropriate developed pore structure, high microporous ratio, and moderate amounts of surface oxygen containing groups are promising adsorbents for treating organic pollutants with small molecules. The data obtained by dynamic toluene adsorption experiments was well fitted by the Langmuir equation, meaning adsorption behaviors of toluene on hierarchical porous carbon materials were physical adsorption. In conclusion, to optimize adsorption performance of existed adsorbents or synthesize high-performance new adsorbents for engineering application, apporpriate porous structure,well-developed pore channels of proper size, and surface oxygen groups are the main factors to research on.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Experimental Study on Viscosity Characteristics of Expanding Polymer Grout
- Thermal-responsive Photonic Crystals based on Physically Cross-linked Inverse Opal Nanocomposite Hydrogels
- Evalution of Thermal Oxidative Degradation of Trimethylolpropane Trioleate by TG/DTA/DSC
- Transformation Characteristics and Microstructure of Rail under Low Stress during Continuous Cooling
- Formation Mechanism and Existing Form of Sb in Heat Resistance Mg-Gd-Y-Sb Alloy
- Rapid Dendrite Growth in Solidification of Highly Undercooled Alloys
