Natural Fresh Proteins Directed Hierarchically Porous Nitrogen-doped TiO2 as with High Performance as Photocatalyts and Electrode Materials
2021-04-16ZUOJiaxiangJINXinZENGHui
ZUO Jiaxiang, JIN Xin, ZENG Hui
(Wuhan Institute of Marine Electric Propulsion, Wuhan 430064, China)
Abstract: Fresh EPF proteins from Liangzi Lake were employed as a template to synthesize hierarchically porous nitrogen-doped TiO2 in a single process. The experimental results show that N-TiO2 sample exhibits significantly enhanced visible-light photocatalytic activity in both chemical waste treatment and hydrogen production, and the visible-light photocatalytic activities vary with the concentrations of EPF proteins.The optimal concentration of protein is 600 mg·mL-1 and the degradation of RhB could be almost completed in just 25 min. Furthermore, the performance of as-synthesized TiO2 as an electrode for Li-ion battery can be also regulated by the EPF proteins. Natural extrapallial fluid (EPF) proteins extracted from the same kind of mussels living in different regions have significantly different effects on the performances of TiO2 as electrode materials for Li-ion battery. The present work highlights the unimaginable effects of natural organic matrix on the synthesis of advanced materials with optimized functional properties.
Key words: N-TiO2; photocatalytic activity ; hydrogen production; hierarchically porous structure;extrapallial fluid (EPF) proteins
1 Introduction
In nature, biominerals have received extensive attention because of their perfect nanostructure and particular functions[1-3]. Recently, some scientists began to study the structure-formation process of biological minerals[4-6]. As a result, a new research directions “bioprocess inspired fabrication” was developed[4,7]. It has been found that organic matrices play an important role in the process of biomineralization[8,9]. For instance,natural biological macromolecules such as proteins,peptides, collagen and fibers can act as structuredirecting agents to regulate the structures and functions of materials[10-12]. Compared with single protein or their synthetic analogues, fresh composite proteins in living system show higher efficiency in promoting the formation of materials due to the synergistic effect between complex components[11,13].
Titanium dioxide (TiO2) has a variety of excell-ent physical and chemical properties, which has been widely used in the energy areas such as photocatalyt and lithium-ion battery[14-16]. Due to its inherent large band gap (3.2 eV)[17], the absorption wavelength range of TiO2is mainly in the UV range.In the past years, researchers are devoted to finding methods for extending the absorption range to visible light[17,18]. In particular, nitrogen doping is an efficient route to enhance the photocatalytic activity of TiO2in the visible-light range[19,20]. Meanwhile,some studies have shown that hierarchically porous nanomaterials can improve the photocatalytic activity of nanomaterials[21-23]. However, the process of preparing hierarchically porous N-TiO2materials is often complicated and requires high temperature and/or pressure. Therefore, it is still a challenge to design one-pot synthesis to simultaneously tuning the microstructure, phase stability and light-use efficiency of TiO2at low temperatures[24].
Natural extrapallial fluid (EPF) proteins is a complex protein that regulate the formation of calcium carbonate during the biomineralization process. EPF proteins show strong mineralization activity due to the synergistic effect between different proteins[25].Scientists have extracted fresh EPF proteins from Zhanjiang living mussels, and successfully synthesized nitrogen-doped TiO2with hierarchically porous structure at low temperature. They also point out that the EPF proteins extracted from the same kind of mussels living in different regions have significantly different effects on the photocatalytic performance of TiO2[26]. However, the reason is still unclear. Therefore,a series of systematic studies should be conducted.
In this paper, we further studied this phenomenon to confirm the different effects of EPF proteins in the regulation of the structure and function of TiO2.Fresh EPF protein extracted from mussels living in Liangzihu River was selected as structure-directing agent. Indeed, the results demonstrate that the obtained N-TiO2has different functional properties such as visible-light photocatalytic activity and Li-ion battery.The mysterious relationship between the natural living protein and the functional properties of the material are worth well studied in the future. .
2 Experimental
2.1 Extraction of extrapallial fluid protein
Fresh water mussels were purchased from Liangzi lake, Ezhou city, Hubei province, China. The selected freshwater mussels were stored in a glass aquariums for 2 days and filled with oxygen. After the mussels spit out empty dirt, the pipette was inserted into central extrapallial space to suck out the viscous fluid (EPF protein). The isolated EPF protein should be immediately transferred to a sterile centrifuge tube and then stored at -70 ℃. During the experiment, the protein types were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the protein amount was determined by the Bradford assay. Finally, the protein was diluted or concentrated to the required concentration according to the needs of the experiment.
2.2 Synthesis of TiO2 materials
5 mL of EPF protein with different concentrations was added dropwise to 1 mL of tetrabutyl titanate(TBT, Sigma Aldrich) and stirred for 2 h, and then mineralization at room temperature for 4 h. The mineralized product is transferred to Teflon-lined autoclave to undergo the hydrothermal reaction. The temperature is initially raised to 50 ℃ at a rate of 5 ℃/min for 30 min, and then increased up to 150 ℃ at a rate of 5 ℃/min for 12 h. The obtained product was filtered, washed, and dried to obtain nitrogen-doped TiO2powder, which is referred to as MNY-TiO2. In the control experiment, pure water was used instead of EPF protein, and the TiO2powder was used as a control sample, which was denoted as pristine TiO2.
2.3 Characterization of materials
The crystalline phase was determined using X-ray diffraction (XRD, PANalytical X’Pert Pro X-ray diffraction Cu Ka), Raman spectroscopy, and infrared spectroscopy. An scanning electron microscope(FESEM, Hitachi 4800) and a transmission electron microscope (TEM, JEM-2010 HT) were used to characterize the morphology of TiO2samples. X-ray photoelectron spectrometer (VG Multilab 2000)analysis was performed to verify the chemical state of elements. An integrating sphere (Shimadzu, Japan,UV-2550) was used to measure the UV-Vis absorption spectrum of the sample in the wavelength range of 200 to 800 nm, and BaSO4was used as the reflection standard.
2.4 Photocatalytic dye degradation and hydrogen production
The visible-light photocatalytic activity of TiO2in dye degradation was experimentally studied. A 350 W xenon lamp (Shanghai Lanmi Electronics Co., Ltd.) and a 420 nm filter were used to filter the ultraviolet light to provide a visible light source. Experimental details are shown as follows: 0.02 g of TiO2powder was dispersed in a 20 mL RhB aqueous solution with a concentration of 0.01 mM, and then placed in the dark area to achieve the adsorption-desorption balance among TiO2, RhB,and H2O. During the irradiation process, after a fixed time interval, 2 mL of the mixed solution was taken and centrifuged (12 000 rpm, 5 min) to completely remove the nano-TiO2powder suspended in the solution. The concentration was determined using ultraviolet-visible absorption spectroscopy.
Experimental details of hydrogen production are as follows: 50 mg of TiO2is dispersed into 80 mL of methanol aqueous solution in a three-necked flask with a capacity of 100 mL. 0.6wt% precious metal Pt was added by dissolving H2PtCl6into the above mixed solution. photochemical reduction deposition method.After every hour of light exposure, 0.4 mL of gas was extracted from the system with a micro gas sampler and immediately injected into a gas chromatograph(GC-14C, Shimadzu Japan) to measure the amount of hydrogen produced. Before the experiment, the required glass reagents should be washed repeatedly with distilled water to ensure the cleanliness of the instrument.
2.5 Electrochemical measurements
The active materials, super P carbon black and polyvinylidene fluoride with a ratio of 7:2:1 in weight was mixed with N-methyl-2-pyrrolidone (NMP,Aladdin, China) to fabricate the working electrode slurry. The grinded slurry was pasted on Cu foil and dried at 120 ℃ for 12 h under vacuum condition. The average electrode mass loading was about 1.4-1.6 mg.The liquid electrolyte was 1 M lithium hexafluorophosphate dissolved in ethylene carbonate (EC)/diethyl carbonate (DEC) with a 1:1 volume ratio. The separator was the Celgard polypropylene. Lithium metal foil was used as the reference and counter electrode, and was assembled with the working electrode, electrolyte and separator into a coin cell (CR2025-type) in an Argon glovebox. The galvanostatic charge/discharge measurement was conducted on LAND CT2001A multichannel battery testing system within 0.01-3 V (vs. Li+/Li). The electrochemical impedance spectroscopy (EIS) was performed from 100 kHz to 0.01 Hz on Autolab PGSTAT 302N equipment. Cyclic voltammetric (CV) was tested using an electrochemical workstation from 0.01 V to 3 V (vsLi+/Li) with a 0.1 mV·s-1scanning rate.
3 Results and discussion
3.1 Microstructure and chemical analysis of N-TiO2
The SDS-PAGE method was used to confirm the components of the EPF proteins, revealing that there are indeed various kinds of proteins in the extrapallial fluid (Fig.1(a)). In the present work, we investigate the effects of EPF protein on the formation of TiO2. In the control group, the TiO2are common nano-spherical particles (Fig.1(b)) consistent with the results reported in related literature. In contrast, under the control of EPF protein, TiO2sample with 3D network structure(MNY sample) was obtained. As seen from SEM image, it can be observed that the 3D network structure is assembled by nano-TiO2units, and the morphologies change with the concentration of EPF protein. With 300 mg·mL-1of EPF protein, some macropores are formed,and the residual protein is presented as a filament.When the protein concentration is 600 mg·mL-1, the network structure of TiO2develop more regular and perfect. The diameter of long-range periodical pores is 200-400 nm, and the thickness of pore-wall is 100-200 nm. As the protein concentration gradually increases,the residual protein changes from filamentous to flake(Fig.1(e)). It can be concluded that the EPF proteins provide a framework for the formation of TiO2, which acts as the divided spaces where TiO2can nucleate.
In order to further determine the network porous structure, a transmission electron microscope (TEM)was employed. A typical TEM image of the MNY-600 sample presents internal porosity of the particles,indicating the existence of distributed pores inside the wall (Fig.2(a)). The tiny nanoparticles are about 5-8 nm and the network porous structure is obvious.The high-resolution lattice fringes of sample have an interplanar spacing of 0.33 nm corresponding to the (101) plane of anatase (Fig.2(b)), which is the most thermodynamically stable face with low energy and easy to obtain. Therefore, it can be concluded that under the control of EPF protein, TiO2with a hierarchically porous structure can be successfully synthesized at low temperature.
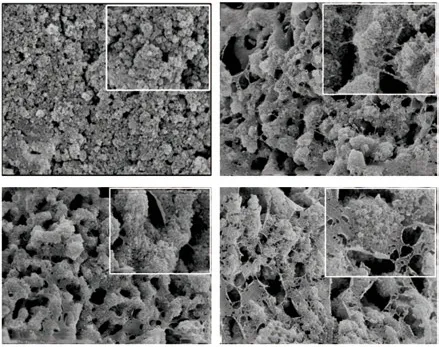
Fig.1 SEM images of MNY samples synthesized with different concentrations of EPF proteins at 150 ℃ for 12 h: (a) 0, (b)300 mg·mL-1; (c) 600 mg·mL-1; (d) 900 mg·mL-1
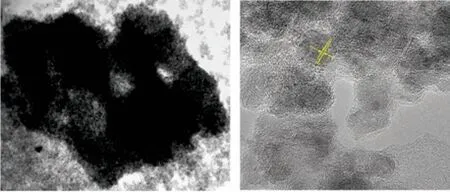
Fig.2 (a) TEM and (b) HRTEM images of MNY-600 particles
X-ray diffraction was used to investigate the effects of EPF proteins on the crystalline phase of TiO2samples (Fig.3). Without protein, the product is mainly anatase phase with a small amount of brookite. The amount of brookite gradually decreases with increased protein concentration, suggesting that the EPF proteins control play an important role in selective of crystal phase during the formation of TiO2. As we know,among the three phases of TiO2, the photocatalytic activity of anatase is superior to brookite and rutile.Therefore, the EPF proteins successfully induce the formation of anatase and suppress the appearance of brookite, which is beneficial to improve the photocatalytic activity of TiO2. Moreover, it can be seen that the intensity and width of diffraction peaks become lower and wider as the protein concentration increases, suggesting that a smaller crystallite size is achieved at higher concentrations.
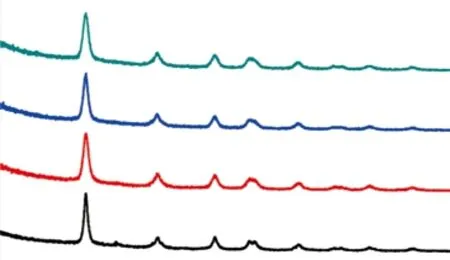
Fig.3 X-ray diffraction patterns of the pristine TiO2 and MNY samples prepared with different EPF protein concentrations
As shown in Fig.4, the surface chemical bonding of the MNY samples is changed. We take the MNY-600 sample as an example, a mixed solution of 6 mol HCl and 1% SDS was used to remove residual protein before testing. XPS survey shows that the TiO2sample mainly contains Ti, O, C, and N elements(Fig.4(a)). 284.6 eV is C1s peak, which is confirmed as a contaminant during sample preparation or testing.As seen in Fig.4(b), two peaks corresponding to Ti2p3/2and Ti2p1/2at 458.5 eV and 464.4 eV are the characteristic peaks of Ti4+on the surface of TiO2.Fig.4(c) shows that the O1s peak can be divided into three peaks of 529.6, 531.1, and 532.2 eV. The peak at 529.6 eV is a characteristic peak of lattice oxygen in TiO2. The chemical state of the obtained TiO2sample is different from that of standard anatase and has certain structural defects. The other two peaks belong to Ti-OH species. Fig.4(d) shows the N1s XPS spectrum with a peak value of about 400.1 eV, which is usually identified as an interstitial nitrogen dopant. The results indicate that the nitrogen atoms of the mantle protein are successfully doped into the lattice gap of the TiO2sample. Nitrogen-doped TiO2will change its valence band, thereby changing the visible radiation absorption performance. Therefore, it is conceivable that the natural EPF protein has a unique affinity for TiO2and serves as a source of interstitial nitrogen doping.
3.2 The effects of EPF proteins on the photocatalytic activity of TiO2
Due to the nitrogen doping, the valence band of TiO2has changed, which usually cause the absorption of visible light. Hence, the photocatalytic activity of TiO2samples prepared with different concentrations of EPF proteins were investigated. Fig.5(a) shows the UVvisible spectra of all the samples. In a control group,the sample has no visible-light absorption. In contrast,the radiation absorption range of N-doped anatase TiO2regulated by EPF proteins (300 μg/mL, 600 μg/mL,and 900 μg/mL) was extended from 380 nm to 700 nm,indicating that the visible-light absorption capability of TiO2is significantly enhanced. As seen from Fig.5(b),compared with pristine TiO2, MNY samples prepared with EPF protein exhibit extremely strong visiblelight photocatalytic activity on dye degradation.With the protein concentration of 600 μg/mL, TiO2sample shows the highest photocatalytic activity and the concentration of rhodamine B (RhB) is decreased to below 20% within 25 minutes. As the protein concentration increases up to 900 μg/mL, the residual protein in the TiO2sample increases, resulting in a decrease in the photocatalytic activity. Compared with other doped TiO2samples reported in previous studies,the obtained TiO2material has a considerable increase in visible-light photocatalytic activity. Thus, it could be concluded that EPF protein from Liangzihu can not only regulate the crystal phase and microstructure of TiO2, but also the photocatalytic performance.
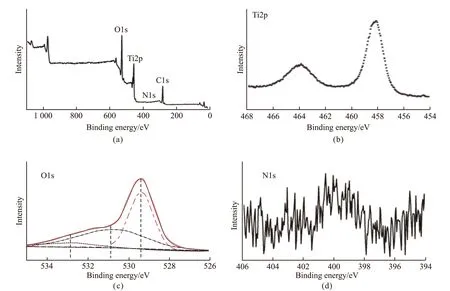
Fig.4 XPS spectra of the MNY-600 sample: (a) The survey XPS spectrum; (b) Ti 2p; (c) O 1s; (d) N 1s
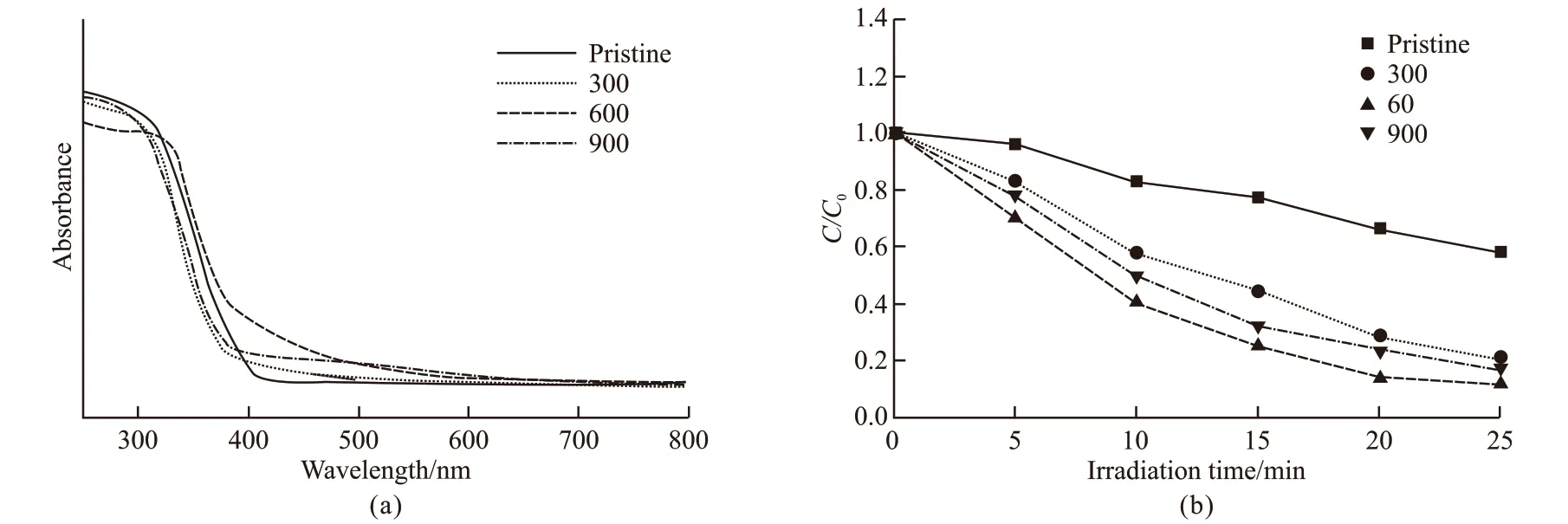
Fig.5 (a) UV-Vis absorption spectra of the pristine TiO2 and MNY samples; (b) Comparison of the visible-light photocatalytic activity for the pristine and MNY samples for the degradation of 0.01 mM RhB
We also conducted the photocatalytic water splitting experiment using different TiO2samples under visible-light irradiation. As shown in Fig.6 no hydrogen is observed in the control group. Because of its inherent band gap, pristine TiO2cannot absorb visible light,thereby cannot excite electron-hole pairs to carry out oxidation-reduction reactions to decompose water.In contrast, the nitrogen-doped TiO2prepared with EPF proteins show photocatalytic performance on hydrogen production under visible light. Moreover,the concentration of EPF proteins is also an important factor that affects the efficiency of H2production. As the protein concentration increases, the H2-production rate increases first and then decreases. When the protein concentration is 600 μg/mL, the rate of H2production is 94 μmol·g-1·h-1. It can be concluded that the hierarchically macro-/mesoporous structure of MNY-TiO2induced by EPF proteins causes the large specific surface area and act as an electron collector and transporter to inhibit the recombination of electrons and holes, which in turn contributes to the photocatalytic hydrogen production performance.
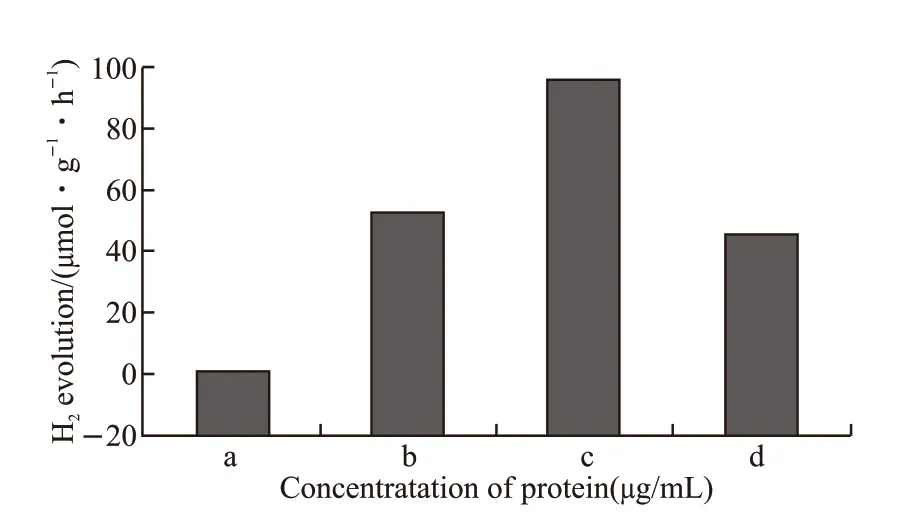
Fig.6 Hydrogen evolution through photocatalytic water splitting with the TiO2 samples under visible-light irradiation: (a)Pristine; (b) MNY-300; (c) MNY-600; (d) MNY-900
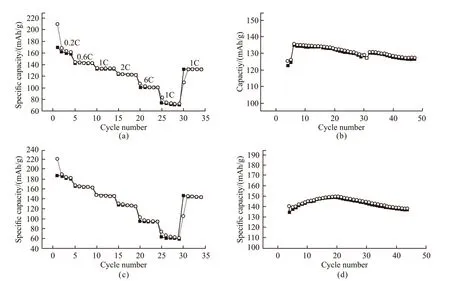
Fig.7 The rate capability and cycling stability of TiO2 samples prepared with 600 μg/mL EPF proteins: (a, b) Liangzihu protein; (c, d)Zhanjiang protein
3.3 The performance of TiO2 as electrode for lithium-ion battery
The performance of as-synthesized TiO2on the Liion battery were also studied. It is found that the TiO2samples prepared with EPF proteins from shell living in diverse regions show different rate capabilities and cycling stabilities as electrodes. For instance, the rate capability of TiO2sample regulated by EPF proteins from Liangzihu Lake is 132 mAh/g at 1 C, which is lower than that of sample prepared with EPF proteins from Zhanjiang (Fig.7). The results also confirm the different effects of EPF proteins in the regulation of the structure and function of TiO2.
4 Conclusions
Previous scientists provide a clue that EPF proteins extracted from the same kind of mussels living in different regions have significantly different effects on the photocatalytic performance of TiO2. In this paper, this phenomenon is further studied in detail by using fresh EPF protein from Liangzi Lake to tune the microstructure of TiO2at low temperature. The results show that the TiO2sample exhibits significantly enhanced visible-light photocatalytic activity in both chemical waste treatment and hydrogen production owing to the hierarchically macro-/mesoporous structure and nitrogen doping, and the visible-light photocatalytic activities vary with the concentrations of EPF proteins. On the other hand, it is also found that the TiO2samples prepared with EPF proteins from shell living in diverse regions show different rate capability and cycling stability as electrodes for Li-ion battery. This paper provide new ideas and ways for the synthesis of functional composites at low and /or room temperature.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Comparative Case Study on Adhesion of Three Common Sizing Agents to Cotton and Polyester Yarns
- Effect of Outer Carbon Layer Thickness of Carboncovered N-doped Hollow Carbon Nanospheres on Its Electrocatalytic Performance
- Ceramification of Composites of MgO-Al2O3-SiO2/Boron Phenolic Resin with Different Calcine Time
- Dynamic Adsorption of Toluene on Hierarchical Porous Carbons with Varying Pore Structure
- Self-propagating High-temperature Synthesis of Sm and Zr Co-doped Gd2Ti2O7 Pyrochlore Ceramics as Nuclear Waste Forms
- Solidification Behavior of in situ TiB2/Cu Composite Powders during Reactive Gas Atomization
