Solidification Behavior of in situ TiB2/Cu Composite Powders during Reactive Gas Atomization
2021-04-16JIANGYihuiZHANGMingshuLIYufaHEJiangtanCAOFeiLIANGShuhua
JIANG Yihui, ZHANG Mingshu, LI Yufa, HE Jiangtan, CAO Fei, LIANG Shuhua
(Shaanxi Province Key Laboratory for Electrical Materials and Infiltration Technology, Xi’an University of Technology, Xi’an 710048, China)
Abstract: 2wt% TiB2/Cu composite powders were fabricated in situ by reactive gas atomization. The fabricated composite powder exhibits high sphericity, and the powder sizes range from 5 μm to 150 μm. The morphology of the Cu matrix and the distribution of the TiB2 particles in the composite powders vary with the powder size. The critical transitions of interface morphologies from dendritic-to-cellular and cellular-to-planar interfaces occurs when the composite powder sizes decrease to 34 μm and 14 μm, respectively. Compared with pure Cu droplets, the composite droplets undergo critical transition of the interface morphologies at a smaller droplet size corresponding to a higher cooling rate because the existence of TiB2 particles can cause instability in the advancing solidification front and heterogeneous nucleation. With decreasing powder size, the extent of the TiB2 particle interdendritic segregation decreases as the result of enhanced engulfment of TiB2 particles by the advancing solidification front.
Key words: composite powder; reactive gas atomization; solidification; microstructure evolution
1 Introduction
Selective laser melting is an emerging manufacturing technique used to directly fabricate complex net shape parts. This technique has been used to fabricate various high performance metal matrix composites that can be applied in various industries[1-4]. Because the powder feeding process plays a key role in the selective laser melting technique[5], the quality control of composite powders used for feedstock materials is essential to this process. The production of composite powders can be accomplished by several methods, such as ball-milling[2,6], electrodeposition[7], centrifugal atomization[8], and gas atomization[9,10]. Among these various methods, gas atomization can incorporate the high production rates necessary to reduce powder cost while maintaining the necessary powder characteristics[11].For example, atomized powders exhibit high sphericity, improving the flowability of the powder, which is particularly important for a successful selective laser melting process[2].
The microstructure of metal alloy powders mostly depends on the solidification process of atomized droplets. Therefore, the solidification behavior of atomized metal alloy powders has been extensively studied over the past decades, and much progress has been made toward understanding the evolution of the microstructure of these powders[12-17]. However, prior studies have mainly focused on the solidification behavior of binary[12-15]or multicomponent[16,17]metal alloy droplets,whereas the microstructure evolution of metal alloy droplets involving preexisting ceramic particles during gas atomization has received little attention. Because the presence of ceramic particles in solidifying metal alloys changes the physical conditions of the process compared with the solidification process of metal alloys without any additions[18], the solidification behavior of composite powders is very different.
Two main factors of rapid cooling and interaction between the ceramic particles and matrix are coupled in the solidification process of composite powders.Therefore, interesting phenomena may be expected during rapid solidification[19]. The aim of this work was to analyze the influence of ceramic particles on the solidification processes of atomized metal matrix composite powders. First, in situ TiB2/Cu composite powders were fabricated by vacuum reactive gas atomization. Then, the evolution of the microstructure of the atomized TiB2/Cu composite powders was discussed by applying general solidification theories.
2 Experimental
Two master alloys, Cu-2.8wt% Ti and Cu-1.2wt%B, were first prepared by vacuum induction melting under a protective argon atmosphere. The two master alloys had the same weight and were separately melted in a vacuum medium-frequency induction melting furnace. After a 2 min isothermal dwell, the two molten master alloys were simultaneously driven into a mixing chamber at the same flow rate to synthesize a Cu-TiB2composite suspension by completely reacting the Ti and B. By controlling the atomizing parameters (spray nozzle diameter of 3 mm, atomized pressure of 3 MPa,and melt temperature of 1 573 K), the suspension stream was atomized into fine droplets with nitrogen gas. Fig.1 shows a schematic diagram of the vacuum reactive gas atomization equipment used in this study.Accordingly, composite powders with a nominal composition of 2wt% TiB2/Cu were obtained. Comparative pure Cu powders were prepared with the same atomizing parameters.
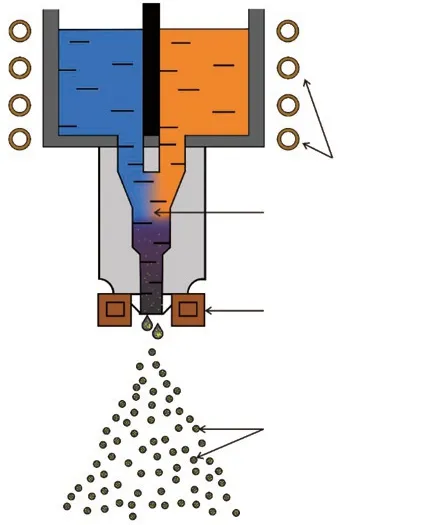
Fig.1 Schematic diagram of reactive gas atomization equipment
The particle size distribution of the in situ-fabricated composite powders was measured by an laser particle size analyzer (BT-9300S, Bettersize Instruments). The phase compositions were determined by X-ray diffraction (XRD, XRD-7000S, Shimadzu). The surface and cross-sectional micromorphologies of the composite powders were then examined by scanning electron microscopy (SEM, JSE-6700F, JEOL).
3 Results
Fig.2(a) displays the SEM observations of the fabricated composite powder morphologies. It can be seen that the composite powder exhibits high sphericity, except for a small amount of irregular powder derived from the collision and/or adhesion of droplets before complete solidification occurred. The fabricated composite powder size, , falls in a relatively large range of 5 μm to 150 μm, and the mean powder size is approximately 50-55 μm. The corresponding XRD patterns in Fig.2(b) shows that the fabricated composite powder is composed of TiB2reinforcement and Cu matrix, which indicates that the in situ reaction is basically complete. Compared with the droplet flight stage after atomization, the mixed liquid at the mixing chamber stage takes a much longer time and remains at a higher temperature. Accordingly, an assumption is made that the in situ reaction is completed before gas atomization,and the liquid mixture at the beginning of atomization was regarded as a dilute suspension of TiB2particles in Cu liquids.
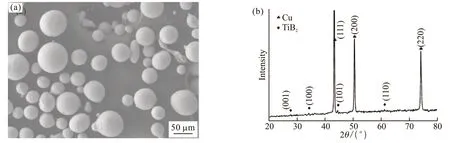
Fig.2 (a) SEM image and (b) XRD pattern of in situ-fabricated 2wt% TiB2/Cu composite powders
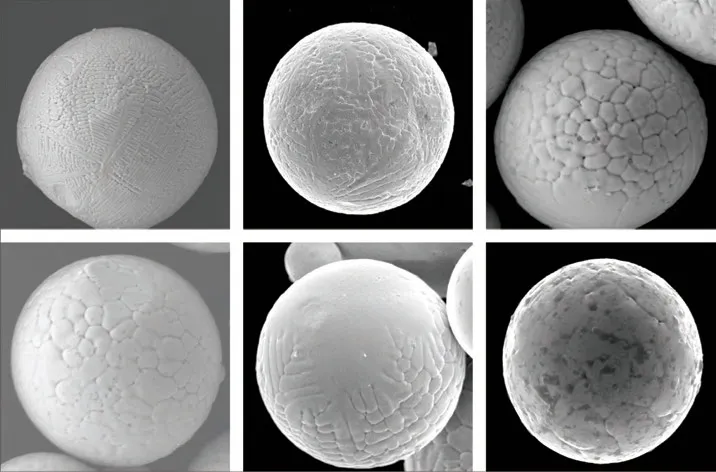
Fig.3 Surface morphologies of in situ-fabricated 2wt% TiB2/Cu composite powders with diameters of (a) 110 μm, (b) 66 μm, (c) 36 μm, (d)32 μm, (e) 14 μm, and (f) 10 μm
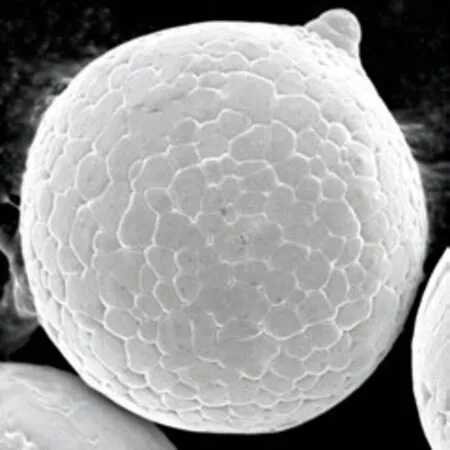
Fig.4 Surface morphology of a pure Cu powder with a diameter of 90 μm
Fig.3 shows detailed observations of the surface micromorphologies of the in situ TiB2/Cu composite powders of different sizes. One distinct feature is that the grain morphology of the Cu matrix varies with powder size. For the fairly large powders (D> 100 μm), the grain morphology exhibits well-developed dendrites with a primary trunk and secondary and even tertiary arms (see Fig.3(a)). For the intermediate sized powders (from 40 μm to 70 μm), the copper matrix grains exist in the form of less-developed dendrites(see Fig.3(b)). As the powder size further decreases(from 14 μm to 36 μm), the matrix phase has a cellular structure (see Fig.3(d)). When the composite powder diameter is smaller than 10 μm, the copper matrix grain has a planar shape, as shown in Fig.3(f). In addition, the coexistence of dendritic and cellular grains in Fig.3(c) and cellular and planar grains in Fig.3(e) can be observed. These observations suggest that the critical morphological transitions from dendritic-to-cellular and cellular-to-planar interfaces occur when the composite powder sizes decrease toD= 36 μm andD= 14 μm, respectively. For comparison, the surface micromorphology of the pure Cu powder is shown in Fig.4.This image shows that the grains in the pure Cu powder with a rather large size (D= 90 μm) can still exhibit a cellular or planar shape. This result indicates that the presence of TiB2particles in Cu droplets significantly impacts the solidification kinetics of the droplets.
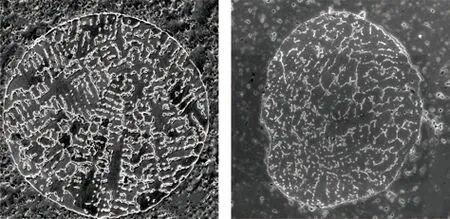
Fig.5 Cross-sectional SEM images of sieved in situ-fabricated 2wt% TiB2/Cu composite powders with diameters in the range of (a) 90-110 μm and (b) 53-75 μm
Fig.5 shows two types of cross-sectional morphologies in the sieved composite powders, where the distribution states of TiB2particles can be observed.In accordance with the surface micromorphology of the composite powder shown in Fig.3(a), the copper matrix in the larger powders (from 90 μm to 110 μm)exhibits a coarse dendritic structure. In this case, there is a clear interdendritic segregation of TiB2particles.For the smaller powders (from 53 μm to 75 μm) shown in Fig.5(b), although segregation of TiB2in the interdendritic regions can be observed, a portion of the TiB2particles can be detected in the inner grain areas. This observation implies that the extent of the segregation of TiB2particles decreases with decreasing powder size.
4 Discussion
The phenomena of the transition from the dendritic-to-planar interface via the cellular interface and the interaction between the advancing solidification front and TiB2particles are strongly associated with the powder size. To illustrate the solidification behavior of composite powders, the cooling rate of the atomized powder was first evaluated; the two phenomena are then discussed accordingly.
4.1 Cooling rate of composite droplets
A model of the thermal history of a droplet during gas atomization has been proposed by Lee and Ahn[12].In this model, the cooling of an alloy droplet is divided into five thermal regions: liquid phase cooling, nucleation and recalescence, segregated solidification, eutectic solidification, and solid-state cooling. Because the liquid phase of the suspension before atomization is assumed to be pure Cu liquid in this work, the segregated solidification and eutectic solidification stages are absent during the cooling of TiB2/Cu composite droplets.Therefore, the remaining three thermal regions can be expressed in a unified form[20]
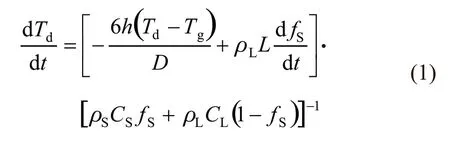
where,Tdis the droplet temperature,tis the time,his the convective heat transfer coefficient,Tgis the gas temperature,ρandCare the density and specific heat of the solid (S) and liquid (L) phases,Lis the latent heat, andfSis the solid frication. Here,hwas calculated by the Ranz-Marshall criterial equation[21], in which the Reynolds number, Re, and Prandtl number,Pr, were used. The Reynolds number was assumed to be a constant by using a stable droplet velocity,vd, and gas velocity,vg,i e, Re=ρgD|vg-vd|/μg, withρgas the gas density andμgas the gas dynamic viscosity[22], and the Prandtl number can be calculated from the physical property parameters of nitrogen gas,i e, Pr=μg/agwith ag as the gas thermal conductivity[20]. To describe the solidification kinetics,fSwas then calculated by the method described in the Ref.[12]. Then, based on the physical property parameters of TiB2and Cu, the parameter values of the 2wt% TiB2/Cu composite suspension and solid were obtained in terms of the mixing rule.
The thermal histories of droplets with different diameters starting solidification at chosen nucleation temperatures,TN, can be calculated (see Fig.6). The recalescence phenomenon associated with the release of the heat of crystallization is observed. A recalescence plateau is also observed for the relatively small nucleation temperature. The duration of cooling and solidification decreases with decreasingTN. However,compared to droplet size, nucleation temperature has a much weaker effect on the time duration, as the time duration for the larger droplets (D= 100 μm) is approximately 2 orders of magnitude longer than that of smaller droplets (D= 10 μm). Therefore, the smaller droplets experience a higher cooling rate, resulting in a higher advancing interface velocity.
According to classical theory, the relationship between the average cooling rate,Φ, and secondary dendrite arm spacing,λ, can be described by[23]

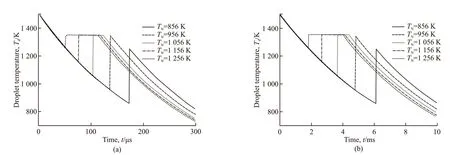
Fig.6 Cooling curves of 2wt% TiB2/Cu composite droplets with diameters of (a) 10 μm and (b) 100 μm, starting solidification at different nucleation temperatures
where,αandβare the constants. According to the SEM observations, the values ofλwere measured for different powder sizes (see Fig.7). The average cooling rate corresponding to different powder sizes can also be calculated from Eq.(1) (see Fig.7). Based on linear fitting of the data points (lnΦ, lnλ), a= -0.312;β= 56.4 can be obtained. The values of a andβobtained for the gas atomization of 2wt% TiB2/Cu composite are comparable with those of other alloys,eg, a = -0.33 andβ=54.4 for martensitic steel[23], and a = -0.323 andβ=34.0 for CuSn6 copper alloy[24], which suggests that the thermal histories of the composite droplets calculated by Eq.(1) are reasonable.
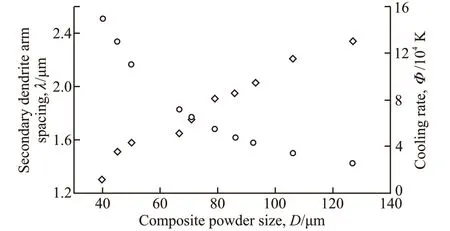
Fig.7 Secondary dendrite arm spacing (diamonds) and cooling rates (circles) of 2wt% TiB2/Cu composite droplets with different diameters
4.2 Evolution of Cu grain morphology with powder size
Due to rapid cooling and the small volume of the atomized droplets, the nucleation event occurs at a large undercooling. Generally, the smaller droplets are prone to crystallize at a larger undercooling. Therefore, the solidification of atomized droplets is classified as rapid solidification that is far from equilibrium. According to section 4.1, the average velocity of the advancing solidification front increases with decreasing droplet size.Based on the M-S theory[25], with increasing advancing solidification front velocity, the morphology transitions from dendritic-to-cellular and cellular-to-planar interfaces in the composite powder indicate that absolute stability was gradually achieved.
For a composite powder withD=36 μm, the coexistence of dendritic and cellular structures (Fig.3(c))indicates that the critical transition velocity from the dendritic-to-cellular interface occurred in this composite powder. At the initial stage of solidification, the undercooling of the droplet is high enough to maintain the cellular interface growth. Due to the release of latent heat, the undercooling decreases with the progress of solidification, and the velocity of the advancing solidification front decreases correspondingly. Then, at a later stage of solidification, the advancing solidification front becomes unstable, which results in a dendritic structure. Similarly, the coexistence of cellular and planar structures observed in the composite powder ofD=14 μm (Fig.3(e)) indicates the occurrence of a critical transition velocity from the cellular-to-planar interface;i e, an absolute stability velocity is achieved.
Compared with those of pure Cu powders, the sizes of the composite powders corresponding to cellular or planar interface growth are much smaller. This effect is due to the effect of the preexisting TiB2particles.First, even though the liquid phase in the composite suspension can be assumed to be pure Cu after the in situ reaction is complete, the absolute stability velocity in the composite powder may be higher than that in pure Cu droplets because the presence of particles in liquids can cause instability in the interface[26]. Second,because the ceramic particles can serve as heterogeneous nucleation sites, the composite droplets exhibit less undercooling before nucleation than the pure Cu counterparts with the same size. Due to the combined effect of these two factors, the critical transitions of the interface morphologies in composite droplets occur at a higher cooling rate corresponding to a smaller droplet size compared with those of pure Cu droplets.
4.3 Interaction between TiB2 particles and advancing solidification front
Because the preexisting particles can be pushed,engulfed or entrapped by the advancing solidification front, the variation of the TiB2particle distribution state shown in Fig.5 results from the interaction between the particles and the advancing solidification front. There are many factors that affect the behavior of particles at the advancing solidification front[27]. In the 2wt% TiB2/Cu composite powders, the most influential factors are the powder size and the TiB2particle size.
In the Cu-2wt% TiB2composite suspension, TiB2particles with varying sizes were formed after the in situ reaction was completed. For a certain particle size,a critical velocity for the advancing solidification front below which particles are pushed and above which particles are engulfed is often used in the literature to quantify the particle pushing/engulfment transition. An empirical correlation has shown that the critical velocity is inversely proportional toRn, whereRis the particle radius and the value of the exponentnis between 0.5 and 2 according to the properties of the system under consideration[28]. For large composite droplets, the velocity of the advancing solidification front is lower than the critical velocity of most particles. The particles were pushed by the advancing solidification front and then entrapped in the interdendritic regions of well-developed dendrites (see Fig.5(a)). When the composite droplet size decreases, the increased velocity of the advancing solidification front surpasses the critical velocity of some large TiB2particles. Therefore, some of the TiB2particles were engulfed in the growing grains,and some smaller TiB2particles were still pushed by the advancing solidification front to the interdendritic regions. This effect leads to the microstructures shown in Fig.5(b).
5 Conclusions
Here, 2wt% TiB2/Cu composite powders with high sphericity were fabricated in situ by the vacuum reactive gas atomization technique. Compared with pure Cu droplets, the critical transitions of the interface morphologies (i e, the dendritic-to-cellular and cellular-to-planar interfaces) in the composite droplets occur at a smaller droplet size corresponding to a higher cooling rate because the existence of TiB2particles can cause instability in the advancing solidification front and heterogeneous nucleation. With decreasing powder size, the extent of TiB2particle interdendritic segregation decreases as the result of enhanced engulfment of TiB2particles by the advancing solidification front.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Experimental Study on Viscosity Characteristics of Expanding Polymer Grout
- Thermal-responsive Photonic Crystals based on Physically Cross-linked Inverse Opal Nanocomposite Hydrogels
- Evalution of Thermal Oxidative Degradation of Trimethylolpropane Trioleate by TG/DTA/DSC
- Transformation Characteristics and Microstructure of Rail under Low Stress during Continuous Cooling
- Formation Mechanism and Existing Form of Sb in Heat Resistance Mg-Gd-Y-Sb Alloy
- Rapid Dendrite Growth in Solidification of Highly Undercooled Alloys
