Effects of the nitrification inhibitor nitrapyrin and tillage practices on yield-scaled nitrous oxide emission from a maize field in Iran
2021-04-14AzamBORZOUEIUloMANDERAlarTEEMUSKAlbertoSANZCOBENAMohammadZAMANDongGillKIMChristophLLERAliAskaryKELESTANIEParvanehSAYYADAMINEbrahimMOGHISEHKhadimDAWAR0andAnaGabrielaREZCASTILLO
Azam BORZOUEIUlo MANDERAlar TEEMUSKAlberto SANZ-COBENAMohammad ZAMANDong-Gill KIMChristoph MÜLLERAli Askary KELESTANIEParvaneh SAYYAD AMINEbrahim MOGHISEHKhadim DAWAR0 and Ana Gabriela PÉREZ-CASTILLO
1Agricultural Research School,Nuclear Science and Technology Research Institute,Karaj 31465-1498(Iran)
2Institute of Ecology and Earth Sciences,University of Tartu,Tartu 51014(Estonia)
3Higher Technical School of Engineering(ESTI),Technical University of Madrid,Madrid 28040(Spain)
4Soil and Water Management&Crop Nutrition,Joint Food and Agriculture Organization of the United Nations(FAO)/International Atomic Energy Agency(IAEA)Division of Nuclear Techniques in Food&Agriculture,Vienna A-1400(Austria)
5Wondo Genet College of Forestry and Natural Resources,Hawassa University,P.O.Box 128,Shashemene(Ethiopia)
6Department of Plant Ecology,University Giessen,Heinrich-Buff-Ring 26,Giessen D-35392(Germany)
7School of Biology and Environmental Science,University College Dublin,Belfield D04 V1W8(Ireland)
8Faculty of Agriculture and Natural Resources,University of Gorgan,Gorgan 3158777871(Iran)
9Department of Agronomy and Plant Breeding,College of Agriculture and Natural Resources,University of Tehran,Karaj 1417466191(Iran)
10Department of Soil and Environmental Sciences,Agricultural University Peshawar,Peshawar 25130(Pakistan)
11Center of Environmental Contamination Investigation,University of Costa Rica,Mountains of Oca 1150(Costa Rica)
ABSTRACT Nitrification inhibitors can effectively decrease nitrification rates and nitrous oxide(N2O)emission while increasing crop yield under certain conditions.However,there is no information available on the effects of nitrification inhibitors and tillage practices on N2O emissions from maize cropping in Iran.To study how tillage practices and nitrapyrin(a nitrification inhibitor)affect N2O emission,a split factorial experiment using a completely randomized block design with three replications was carried out in Northeast Iran,which has a cold semiarid climate.Two main plots were created with conventional tillage and minimum tillage levels,and two nitrogen(N)fertilizer(urea)management systems(with and without nitrapyrin application)were created as subplots.Tillage level did not have any significant effect on soil ammonium(NH+4 )and nitrate(NO−3 )concentrations,cumulative amount and yield-scaled N2O emission,and aboveground biomass of maize,whereas nitrapyrin application showed significant effect.Nitrapyrin application significantly reduced the cumulative amount of N2O emission by 41%and 32%in conventional tillage and minimum tillage practices,respectively.A reduction in soil NO−3 concentration by nitrapyrin was also observed.The average yield-scaled N2O emission was 13.6 g N2O-N kg−1 N uptake in both tillage systems without nitrapyrin application and was significantly reduced to 7.9 and 8.2 g N2O-N kg−1 N uptake upon the application of nitrapyrin in minimum tillage and conventional tillage practices,respectively.Additionally,nitrapyrin application increased maize biomass yield by 4%and 13%in the minimum tillage and conventional tillage systems,respectively.Our results indicate that nitrapyrin has a potential role in reducing N2O emission from agricultural systems where urea fertilizers are broadcasted,which is common in Iran due to the practice of traditional farming.
Key Words: conventional tillage,cumulative emission,minimum tillage,nitrogen use efficiency,N2O flux,soil inorganic nitrogen,urea
INTRODUCTION
Nitrogen (N) is an essential nutrient and a limiting factor for plant growth in most soils. The rising human population, especially in developing countries, has led to agricultural intensification with a high input of reactive N from chemical fertilizers(Zaman and Blennerhassett,2010).Nitrogen fertilizer results in low N use efficiency(NUE)and gaseous losses of nitrous oxide(N2O)and ammonia(NH3)if applied at the wrong time.Nitrous oxide is a potent and long-living greenhouse gas(GHG),increasing at a rate of 0.26%per year and contributing to 7%to the total GHGs(IPCC,2007;Sanz-Cobenaet al.,2012).Agricultural soils are reported to be the predominant source of atmospheric N2O(4.1 Tg N year1)(Fowleret al.,2009;IPCC,2013).Poor farm management practices,along with conducive soil and climatic conditions,are reported as the main determinants of N2O emission from agricultural soil(Stehfest and Bouwman,2006;Gagnonet al.,2011;Zamanet al.,2013).Among the management practices, high input of N fertilizer, low soil pH,low carbon(C)availability,and high moisture content(anaerobic condition)are the major factors contributing to the acceleration of N2O emission and,thus,the perturbation of terrestrial N cycling (Gallowayet al., 2008; Liuet al.,2012;Zamanet al.,2012).
Nitrification inhibitors,e.g.,dicyandiamide(DCD),nitrapyrin,and 3,4-dimethylpyrazole phosphate(DMPP),may delay the conversion of ammonium(NH+4)to nitrate(NO−3)by inhibiting the activities of nitroso-bacteria in soil(Prasad and Power,1995).This inhibition can result in the decrease of direct N2O emission by lowering the nitrification rate as well as the NO−3concentration for denitrification(Zhanget al.,2015).A considerable number of field studies have reported that nitrification inhibitors can reduce N2O emission from applying chemical fertilizers, farm effluents, and manure under a wide range of cropping and soil practices by more than 50%(Zamanet al.,2013;Zhanget al.,2015).In Iran,80% of total N2O and 5% of total carbon dioxide (CO2)are emitted through agricultural practices (IPCC, 2007);however,there is no investigation about farm management practices,especially nitrification inhibitor usage,in reducing N2O emissions from agricultural soils.We hypothesized that nitrification inhibitor application could lower N2O emission and increase maize yield by reducing nitrification rate and increasing NUE through enhanced NH+4uptake.
In conservation agriculture (CA), such as no-till or minimum tillage,the application of mulch has the highest potential to improve soil fertility by improving C storage and aggregate stability(Sixet al.,1999;Halvorsonet al.,2008;Liet al.,2016).Moreover,CA affects C and N distribution,retention of nutrients and water, and microbial activity(Beheydtet al.,2008).All these factors are likely to affect N2O emission from the soil.However,limited information is available on the influence of minimum tillage on N2O emission under the Iranian environmental conditions.The main objective of our study was to investigate the effects of minimum tillage and nitrification inhibitor(i.e.,nitrapyrin)on N2O emission,yield-scaled N2O,soil inorganic N,crop yield, and NUE in an irrigated maize field fertilized with urea.Additionally,the influences of environmental factors,such as soil water content and climate condition, on N2O emission dynamics were analyzed.
MATERIALS AND METHODS
Study site description
A field experiment was established on a farm(35◦49′19′′N,50◦44′2′′E)with a loamy texture at the Nuclear Agricultural Research School,Nuclear Science and Technology Research Institute,Karaj,Iran.Iran has a hot,dry climate characterized by long, hot, dry summers and short, cool winters.The mean long-term(more than 10 years)annual precipitation is 247 mm, while the mean air temperature is 14.4◦C (Karaj Meteorology Department, 2013). The selected site had been continuously cultivated with barley(Hordeum vulgarL.)for approximately five years.The soil type is loam, and the other key soil properties (0—20 cm)before treatments were applied are shown in Table I.

TABLE I Physical and chemical properties of soil(0—20 cm depth)at the study site of a farm at the Nuclear Agricultural Research School,Nuclear Science and Technology Research Institute,Karaj,Iran
Experimental design and treatments
Maize(Zea maysL.)seeds were sown on May 26,2015 at a density of 8 plants m−2(Guardiaet al.,2017).A total of 12 plots(5 m×4.2 m)were selected and arranged according to the treatments in a randomized complete block design by splitting approach,with three replications for each treatment.The main plots included two levels of tillage practice:minimum tillage(MT)and conventional tillage(CT).The subplots included two nitrification inhibitor treatments:urea fertilizer with no nitrapyrin application and with nitrapyrin application(NI).Therefore,four tillage practice-nitrification inhibitor combinations of MT,MT+NI,CT,and CT+NI were performed.The conventional tillage was implemented using mechanical tillage practices,such as moldboard plow,disk,and land leveler,for seed bed preparation.In minimum tillage,the moldboard plow was removed and only disk and land leveler operations were performed to prepare seed beds.Prior to seeding,phosphorous(P2O5)was broadcasted at 250 kg ha−1as superphosphate.Nitrogen fertilizer was applied at 170 kg N ha−1as urea in the MT and CT treatments twice during the growth season on June 26 and July 26,2015 as topdressing.In the MT+NI and CT+NI treatments,nitrapyrin was applied at the rate of 0.35%of the applied N(weight/weight);urea and nitrapyrin were dissolved in water,surface applied manually,and integrated into the cultivated layer using irrigation water.No rainfall occurred during the growth season;therefore,surface irrigation was applied at 12-d intervals.
N2O gas sampling,analysis,and emission calculation
Gas samples of N2O were taken using opaque manual circular static chambers as described by Sanz-Cobenaet al.(2014).One chamber per plot was used,with a volume of 9 812.5 cm3(25-cm inner diameter and 20-cm height).The chambers were closed by placing them on PVC frames,which were inserted 15 cm into the soil at the beginning of the experiment with the aim of preventing soil disturbances(Sanz-cobenaet al.,2012).The N2O samples were collected from each chamber through a butyl septum installed on the upper part of the chamber.Gas samples(25 mL)were taken with a syringe 0, 15, and 30 min after the closure of the chambers in order to measure the evolution of the N2O concentration.The gas samples were stored in pre-evacuated 10 mL exetainers(Labco Co.,UK).
During the crop seasons,gas samples were collected once a week in the morning between 7:00—10:00.Thermometers were put inside three randomly selected chambers during the closure period of each measurement in order to correct the N2O fluxes for temperature. The gas samples were analyzed with a gas chromatograph(Shimadzu GC-2014,USA)operating at the column,injector,and detector temperatures of 55, 75, and 33◦C,respectively, in the laboratory of the Department of Geography of the University of Tartu,Estonia.
The average rate of change in gas concentration was calculated using linear regression equations,and N2O flux(F,mg m−2h−1)was calculated using the following equation:
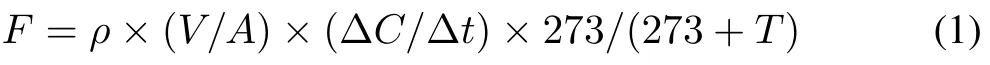
whereρis the density of N2O(mg m−3);Vis the volume of the chamber(m3);Ais the base area of the chamber(m2);∆C/∆tis the average rate of concentration change with time(m3m−3h−1); andTis the temperature in the chamber(◦C).For static chamber measurements,theR2threshold for accepting N2O fluxes was 0.80(P <0.1),except when the maximum difference in the concentration values was less than the gas-specific gas chromatographic detection limit(i.e.,<20 mg kg−1for CO2,<20µg kg−1for CH4,and<0µg kg−1for N2O)in which no filtering criterion was used(Järveojaet al.,2016).To meet quality criteria,10%of N2O fluxes were discarded from the subsequent data analysis.The cumulative amount of N2O emission(Ec,kg N2O-N ha−1)was calculated as follows:

whereFiandFi+1are theith and(i+1)th measured values,respectively,of N2O flux(µg N2O-N m−2h−1);tiandti+1are the days when theith and(i+1)th measurements of N2O flux were taken,respectively(d);andnis the total number of the measurements.
The yield-scaled N2O emission(Ey,g N2O-N kg−1N uptake) was calculated according to van Groenigenet al.(2010),considering aboveground N uptake(Nu,kg ha−1)(i.e.,by grain and straw)andEc:

Soil sampling and analysis
During the experimental period,soil samples from the upper layer(0—15 cm)were taken using a soil corer.Five soil samples were randomly collected from each plot and mixed in the laboratory.Soil samples were passed through a 2-mm sieve and then extracted with 2 mol L−1KCl (soil:water ratio of 1:5)for 1 h on a rotary shaker(Dinget al.,2011).The extracted solutions were filtered and stored at−18◦C until further analyses. According to Liet al.(2015), soil NH+4and NO−3concentrations were measured following the two-wavelength ultraviolet spectrometry and indophenol blue methods,respectively,using a Jenway 6305 ultraviolet spectrophotometer(Jenway Ltd.,UK).
Soil water content (SWC, %) was determined gravimetrically at each time of gas analysis and presented as water-filled pore space(WFPS,%),which was calculated based on the relationship between gravimetric water contents and the soil bulk density(Db)and particle density(Dp)(Liuet al.,2017):
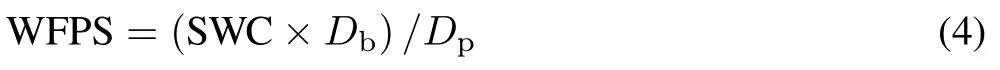
whereDbis 1.32 g cm−3in this experiment andDpis assumed to be 2.65 g cm−3.
Nitrogen use efficiency was calculated as partial factor productivity from applied N(PFPN,kg kg−1)according to Eq.5(Zhanget al.,2015):
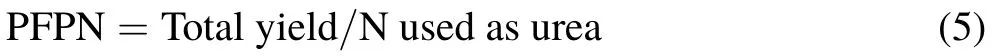
The PFPN index includes all factors affecting crop yield,such as indigenous N,N uptake efficiency,and the efficiency of N conversion to grain yield.
Soil temperature was monitored using a temperature probe inserted 10 cm into the soil.Mean hourly temperature data were stored on a data logger.
Determination of crop yield and aboveground N uptake
When the maize reached physiological maturity(September,2015),one sample for each plot,consisting of two lines(5-m long)of maize,was hand-harvested(Sanz-Cobenaet al.,2012;Guardiaet al.,2017).Ears and straw were separated and weighed in the field. Crop yield represents the production of biomass(e.g.,ear and straw).Ears and straw were dried for 4 d at 65◦C and weighed to obtain dry matter yields.Total N in different plant parts was measured on the basis of the Kjeldahl digestion(Lynch and Barbano,1999)and the aboveground N content was calculated based on the sum of N mass measured in grain and straw from each plot.
Statistical analysis
Data are presented as means±standard errors in all tables and figures.Two-way analysis of variance(ANOVA)was used to analyze the effects of nitrapyrin, tillage practices,and their interactions on soil mineral N,crop biomass,N2O emission,and yield-scaled N2O emission using SAS 9.1 (SAS Institute, 2004) software. Least significant difference values(P <0.05)were calculated only when the treatment effects were significant.Data distribution normality was assessed using the Anderson-Darling test.Pearson correlation coefficients were used to assess the significance of the interrelationships between N2O and other measured variables.
RESULTS
Soil temperature and WFPS
The growth season (June—September, 2015) was characterized by an average daily soil(10-cm depth)temperature ranging from 25◦C(July 20)to 34◦C(August 5)(Fig.1a).There was no significant difference (P >0.05) in WFPS between the two tillage practices(Fig.1b).Changes in WFPS were observed during the experimental period.Soil WFPS at all sampling dates was generally higher(3%—8%)in MT than in CT,but on August 10 and 19, WFPS values were higher(7%and 65%,respectively)in CT than in MT.
Soil NH+4 and NO−3 concentrations
A significant (P≤0.05) increase was observed in NH+4concentration as a result of nitrapyrin application in both tillage practices (Table II). In terms of tillage practice effects, there was no significant difference in soil NH+4concentrations. The highest difference in NH+4concentration between treatments with and without nitrapyrin application was observed two weeks after the second topdressing on August 10,2015 in both MT and CT practices(Fig. 2a). However, the difference in NH+4concentration between treatments with and without nitrapyrin application was higher in the CT practice than in the MT practice on August 10 and 19.
Tillage treatments did not demonstrate a significant effect on soil NO−3concentration (Table II). In CT system, the application of nitrapyrin significantly(P≤0.05)decreased the mean soil NO−3concentration.Mean NO−3concentration in the NI treatments displayed a rapidly decreasing trend two weeks after the second topdressing(August 10)for both tillage practices(Fig.2b).Moreover,the rate of this decrease was higher for the MT practice than for the CT practice.For the MT practice,there was no significant difference in soil NO−3concentration whether nitrapyrin was applied or not at the late growth stage of maize.

Fig.1 Temporal variations of soil temperature at 10-cm depth(a)and soil water-filled pore space (WFPS) (b) in the conventional tillage (CT) and minimum tillage(MT)treatments during the maize growth season in 2015 on a farm at the Nuclear Agricultural Research School,Nuclear Science and Technology Research Institute,Karaj,Iran.

TABLE II Effects of tillage practices and nitrification inhibitor(i.e., nitrapyrin)on cumulative N2O emission(Ec)and mean concentrations of soil NH+4 and NO−3 during the maize growth season in 2015 on a farm at the Nuclear Agricultural Research School,Nuclear Science and Technology Research Institute,Karaj,Iran
N2O emission
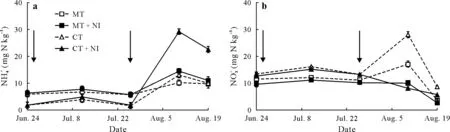
Fig.2 Soil NH+4 (a)and NO−3 (b)changes during the maize growth season in 2015 in the four treatments of an experiment conducted on a farm at the Nuclear Agricultural Research School,Nuclear Science and Technology Research Institute,Karaj,Iran.See Table II for descriptions of the treatments.Arrows denote the times of N(as urea)fertilization.Vertical bars indicate standard errors(n=12).
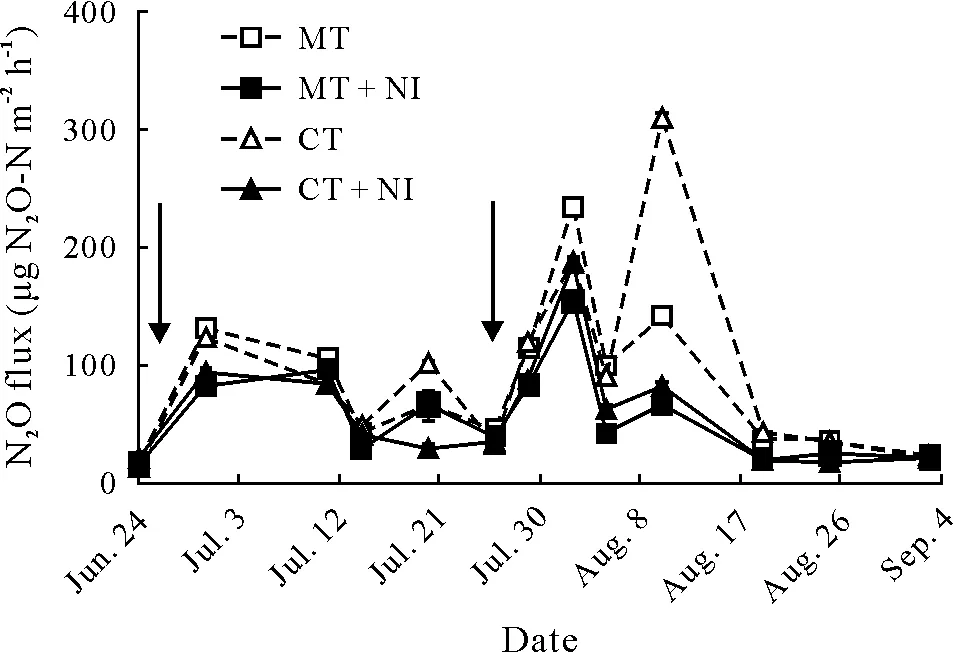
Fig.3 Changes of N2O flux during the maize growth season in 2015 in the four treatments of an experiment conducted on a farm at the Nuclear Agricultural Research School,Nuclear Science and Technology Research Institute,Karaj,Iran.See Table II for descriptions of the treatments.Arrows denote the times of N(as urea)fertilization.Vertical bars indicate standard errors(n=12).
The results indicated that for all sampling dates,N2O flux was lower(P≤0.05)in the NI treatments than in the treatments without nitrapyrin application(Fig.3).However,there was no significant difference in N2O flux between MT and CT practices.A peak of N2O flux was observed in the MT treatment on August 2, followed by a lower peak on August 10.In the MT+NI treatment,only one major peak appeared on August 2.The highest peaks of N2O flux for the MT practice were found after the second urea application and the subsequent irrigation,being 234 and 153µg N2O-N m−2h−1in the MT and MT+NI treatments,respectively.The application of nitrapyrin considerably reduced N2O flux by 34% on August 2 (Fig. 3). In the CT systems,N2O flux followed similar patterns to those in the MT systems. However, for the CT treatment, a sharp increase in N2O emission was observed on August 10, when the largest difference(P≤0.05)between the CT and CT+NI treatments was observed.This N2O emission peak coincided with a higher WFPS in the CT practice(Fig.1b).Nitrous oxide flux declined towards the late stage of maize growth in both tillage practices.
Pearson correlation analysis results showed that daily N2O flux was significantly correlated with soil temperature and NO−3concentration in the NI treatments (P≤0.05)(Table III).Although N2O flux was found to be affected by soil WFPS,the correlation was non-significant.
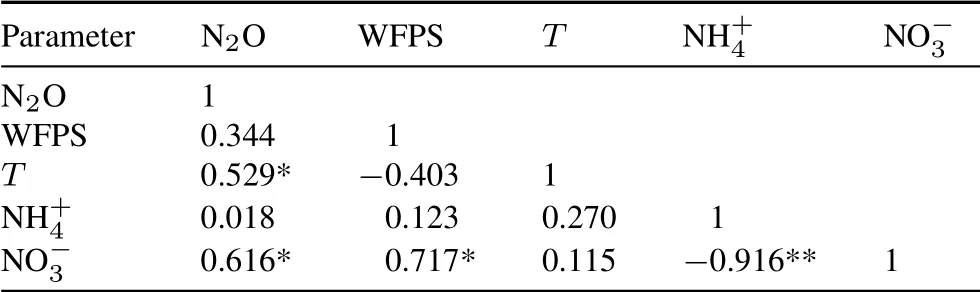
TABLE III Pearson correlation coefficients between N2O flux and soil water-filled pore space(WFPS),temperature(T)at 10-cm depth,and NH+4 and NO−3 concentrations during the maize growth season on a farm at the Nuclear Agricultural Research School,Nuclear Science and Technology Research Institute,Karaj,Iran(n=12)
According to Table II,tillage practices had no significant effect on cumulative N2O emission, but cumulative N2O emission from plots receiving nitrapyrin was significantly lower(P≤0.05)than that from plots not receiving nitrapyrin.Cumulative N2O emission in plots under MT and CT was reduced by 32%and 41%,respectively,after the application of nitrapyrin.
Maize biomass and PFPN
Maize biomass was increased by 47%in the MT+NI treatment compared with that in the MT treatment,and by 9%in the CT+NI treatment compared with that in the CT treatment(Table IV).However,the difference between MT+NI and MT treatments was not significant,indicating that maize biomass was not significantly influenced by nitrapyrin application in the MT system.Due to the increase of crop yield, the average PFPN was 8.6% higher by nitrapyrin application.The mean PFPN value increased in both tillagepractices by adding nitrapyrin to the plots,but this increase was only significant(P≤0.05)for the CT treatments.The highest PFPN was observed in the CT+NI treatment.

TABLE IV Effects of tillage practices and nitrification inhibitor(i.e., nitrapyrin)on maize biomass(ear plus straw), partial factor productivity from applied nitrogen (PFPN), and yield-scaled N2O emission (Ey) on a farm at the Nuclear Agricultural Research School,Nuclear Science and Technology Research Institute,Karaj,Iran
Yield-scaled N2O emission
Yield-scaled N2O emission decreased significantly(P≤0.05)by 38%in the MT+NI treatment compare to that in the MT treatment,while emission decreased significantly(P≤0.05)by 47%in the CT+NI treatment compared to that in the CT treatment(Table IV).In contrast,tillage treatments had no significant influence on yield-scaled N2O emission;nevertheless,yield-scaled N2O emission was higher in the CT treatments than in the corresponding MT treatments.
DISCUSSION
Impacts of nitrification inhibitor and tillage practices on soil mineral N and N2O emission
Nitrapyrin application significantly reduced N2O emission in this study (Table II). Several studies have demonstrated the effectiveness of nitrification inhibitors,such as nitrapyrin,for mitigating N2O losses(Parkin and Hatfield,2010; Guardiaet al., 2017). Our results confirm that this inhibitor can significantly inhibit nitrification,resulting in lower NO−3concentrations and higher NH+4concentrations after N fertilization(Fig.2),thus abating N2O losses from both nitrification (directly) and denitrification (indirectly,by decreasing the availability of substrate for denitrifiers)(Guardiaet al.,2017).
Nitrapyrin decomposition was perceived to increase at high soil water contents and soil temperatures.Since both nitrapyrin degradation in the soil and the nitrification process increase with increasing soil temperatures, the variability in weather conditions is an important environmental factor affecting the effectiveness of nitrapyrin(Zamanet al.,2012).In the present study,we observed that most of N2O in the MT practice was emitted 6 d after fertilization during the maize growth season.On the other hand,the daily average surface temperature of the soil increased from 25◦C on July 20 to 34◦C on August 5. The elevated N2O emission in all the treatments,observed on August 2,could have been due to the increasing soil temperature,which decreased the effectiveness of nitrapyrin.
Dinget al. (2007) reported that nitrification and denitrification occurred mainly at WFPS values of 45%—60%and 75%,respectively.In our study,the highest percentage of WFPS (58%) and NO−3concentration were found on August 10;furthermore,the second peak of N2O emission occurred in the MT treatment at that time, indicating that N2O emission might mainly have originated from nitrification. Such a pattern was not observed in the MT + NI treatment,probably due to a strong inhibition of nitrification by nitrapyrin. These results correspond to those reported by Zhanget al.(2015)who showed that high soil inorganic N concentrations in treatments with nitrification inhibitor application did not display higher N2O emission compared to treatments without nitrification inhibitor application.This confirms that the processes for the conversion of NH+4to NO−3were inhibited by nitrapyrin;therefore,the products and byproductsof nitrification and denitrification,including N2O,were reduced.
Soil temperature,WFPS,and mineral N concentration are the key factors affecting N2O emission (Beheydtet al., 2008; Zamanet al., 2012). Our study confirmed that changes in mineral N concentration and temperature are responsible for the changes in N2O emission during the maize growth season.Furthermore,we found that the soil NH+4concentration increased in the presence of nitrapyrin.Dinget al. (2015) reported a direct relationship of N2O emission with the amount of available mineral N in the soil and that application of inhibitors effectively regulated soil NO−3and NH+4concentrations. It has been reported that nitrification inhibitors inhibit nitrification by suppressing the activity of ammonia-oxidizing bacteria or relevant enzymes(Zhanget al., 2015). Retaining N in the NH+4form by using nitrifciation inhibitors can effectively alleviate NO−3accumulation and leaching loss in addition to reducing N2O emission(Zhanget al.,2015).
Furthermore, the efficiency of nitrification inhibitors in mitigating N2O emission and NO−3leaching is reported to vary with the application rate, time and method, field management practices(irrigation type,tillage practice,and application method of NH+4-based fertilizers),climate(precipitation and temperature),and soil properties(moisture,pH,texture,organic C,and mineral N)(Dinget al.,2011,2015).Maet al.(2013)reported that two types of nitrification inhibitor(nitrapyrin and DCD)were more effective in reducing N2O emission in a no-till area than in a conventionally tilled area.But in this study,it should be mentioned that nitrapyrin was more effective in reducing N2O emission for the conventional tillage practice than for the minimum tillage practice.Liuet al.(2005)found that the environment of soils under no tillage was very different from that of soils under conventional tillage.Soils under no tillage are generally moist,and exhibit more organic C and better conditions for N2O production.Halvorsonet al.(2008)showed that soil water content tends to be the highest in no tillage practice compared to the other cropping practices such as conventional tillage.Moreover,a change from conventional till to no-till practices typically lead to decreased soil mineral N and soil temperature at the 0—7.5 cm surface soil layer,with a lesser change observed below this layer(Halvorsonet al.,2008).Such conditions,however,are considered to inhibit N2O emission from no-tillage soils.Our results demonstrate that N2O fluxes and cumulative N2O emission among the two tillage practices during the maize growth season did not differ significantly.Thierfelderet al.(2017)reported no significant difference in N2O emission between no-tillage and conventional tillage after three decades of research,although the emission tended to be lower under no-tillage than under conventional tillage (Thierfelderet al., 2017).Similarly,van Kesselet al.(2013)observed no differences in N2O emission between conventional tillage and reduced tillage systems.However,when disaggregated by climate in experiments carried out over 10 years,N2O emission was 27% lower under reduced tillage than under conventional tillage in drier climates(Thierfelderet al.,2017).It seems that more detailed studies on the impact of tillage operations on N2O emission for specific soil types and climates will be required to make better estimates of how tillage practices affect N2O emission at specific locations.
Impacts of nitrification inhibitor and tillage practices on yield and yield-scaled N2O emission
The result showed that the application of nitrapyrin produced the highest biomass(15.2 t ha−1)under CT treatment(Table IV).The increased yield due to inhibitor application resulted in improved PFPN in conventional tillage,which confirmed the findings of Maet al.(2013)and Zhanget al.(2015).The average increase in biomass due to the addition of the nitrapyrin was 8.5%in both conventional and minimum till plots(Table IV).Zhanget al.(2015)reported that nitrapyrin significantly increased vegetable yield by 12.6%,which was probably due to the benefits of nitrapyrin for the growth and N assimilation of the crops.The results of this study showed that the average PFPN was 8.6%higher in the treatments with nitrapyrin application(Table IV).This result confirmed other studies that documented higher maize yields associated with the use of nitrapyrin(Parkin and Hatfield,2010;Liuet al.,2013).Liuet al.(2013)demonstrated that the application of DCD and DMPP in a wheat-maize cropping system increased the annual crop yield by 8.5%—9.1%(1.1—1.2 t ha−1year−1) and 8.6%—9.7% (2.8—3.2 t ha−1year−1),respectively.They also implied that nitrification inhibitors are very effective in reducing N2O emission induced by NH+4-based fertilizers.The use of nitrification inhibitors generally tends to increase soil NH+4concentration, crop yield,biomass,plant N uptake,and NUE as well as decrease soil NO−3concentration and NO−3leaching.Therefore,nitrification inhibitors can play an important role in enhancing yield and NUE in addition to reducing N2O emission from the wheat-maize cropping system(Liuet al.,2013).
Analyzing N2O emission on a yield basis provides valuable information for estimating the environmental impacts of intensive agricultural production practices (Qinet al.,2012). van Groenigenet al. (2010) described the aboveground yield-scaled N2O emission to be in the range of 5—15 g N2O-N kg−1N uptake when N application was in the optimal agronomical range or below.Our yield-scaled emission ranged 8—15 g N2O-N kg−1N uptake in different treatments (Table IV). The results of our study indicated nitrapyrin application increased the average maize biomass yield by 8%and reduced yield-scaled N2O-N emission by 41%, which agreed well with previous results (Maet al.,2013; Liet al., 2015). Liet al. (2015) assessed the influences of nitrification inhibitor (nitrapyrin) and biochar incorporation on yield-scaled N2O emission in an intensively managed vegetable field from 2012 to 2014 in southeastern China.They reported that nitrapyrin significantly decreased yield-scaled N2O emissions(42%)during the experimental period. Treatments with nitrapyrin application resulted in the highest N uptake(116.2—125 kg ha−1)and the lowest yield-scaled N2O-N emission(7.44—8.17 g N2O-N kg−1N uptake)for both tillage practices.Nitrapyrin application was more effective in mitigating yield-scaled N2O-N emission in the conventional tillage(47%)relative to the minimum tillage(34%).Since NI treatments exhibited the lowest yieldscaled N2O-N emission under both tillage practices, the application of nitrapyrin has the most potential for increasing crop biomass and mitigating N2O emission in maize fields in semi-arid regions.This effect should be further examined in various agro-ecosystems.
CONCLUSIONS
This study exhibited significantly lower N2O emission from treatments with nitrapyrin application during the maize growth season.However,there was no significant interaction between nitrapyrin application and tillage in N2O loss over the entire year.Nitrapyrin application reduced cumulative N2O emission by 41%and 32%and increased maize biomass by 13%and 4%in conventional tillage and minimum tillage practices, respectively. Our results showed that nitrapyrin application in the two tillage practices reduced N2O emission(expressed as yield-scaled N2O emission)and improved maize yield.We only evaluated direct N2O emission from the soil.The influence of nitrapyrin on reduction of fertilizer N lossviaN leaching and NH3volatilization and the subsequent impact on indirect N2O emission need to be further investigated under field conditions in future studies.
ACKNOWLEDGEMENT
This work was funded by the International Atomic Energy Agency,Vienna,through the coordinated research project Minimizing Farming Impacts on Climate Change by Enhancing Carbon and Nitrogen Capture and Storage in Agro-Ecosystems (No. 18595) of Soil and Water Management and Crop Nutrition Section, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture,Department of Nuclear Sciences and Applications,Vienna,Austria.
杂志排行
Pedosphere的其它文章
- Yield-scaled nitrous oxide emissions from nitrogen-fertilized croplands in China:A meta-analysis of contrasting mitigation scenarios
- Optimizing the use of open chambers to measure ammonia volatilization in field plots amended with urea
- A simple and easy method to measure ammonia volatilization:Accuracy under field conditions
- Nitrification inhibitor nitrapyrin does not affect yield-scaled nitrous oxide emissions in a tropical grassland
- Decreased nitrous oxide emissions associated with functional microbial genes under bio-organic fertilizer application in vegetable fields
- Nutrient cycling and greenhouse gas emissions from soil amended with biochar-manure mixtures
