Decreased nitrous oxide emissions associated with functional microbial genes under bio-organic fertilizer application in vegetable fields
2021-04-14YajunGENGYimingYUANYingchengMIAOJunzhangZHIMengyuanHUANGYiheZHANGHongWANGQirongSHENJianwenZOUandShuqingLI
Yajun GENGYiming YUANYingcheng MIAOJunzhang ZHIMengyuan HUANGYihe ZHANGHong WANGQirong SHENJianwen ZOU and Shuqing LI∗
1Jiangsu Key Laboratory of Low Carbon Agriculture and GHGs Mitigation, College of Resources and Environmental Sciences, Nanjing Agricultural University,Nanjing 210095(China)
2Jiangsu Key Lab and Engineering Center for Solid Organic Waste Utilization,Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization,Nanjing Agricultural University,Nanjing 210095(China)
3College of Resource and Environment,Anhui Science and Technology University,Chuzhou 233100(China)
ABSTRACT Bio-organic fertilizers enriched with plant growth-promoting microbes(PGPMs)have been widely used in crop fields to promote plant growth and maintain soil microbiome functions.However,their potential effects on N2O emissions are of increasing concern.In this study,an in situ measurement experiment was conducted to investigate the effect of organic fertilizer containing Trichoderma guizhouense(a plant growth-promoting fungus)on soil N2O emissions from a greenhouse vegetable field. The following four treatments were used: no fertilizer (control), chemical fertilizer (NPK), organic fertilizer derived from cattle manure(O),and organic fertilizer containing T.guizhouense(O+T,referring to bio-organic fertilizer).The abundances of soil N cycling-related functional genes(amoA)from ammonium-oxidizing bacteria(AOB)and archaea(AOA),as well as nirS,nirK,and nosZ,were simultaneously determined using quantitative PCR(qPCR).Compared to the NPK plot,seasonal total N2O emissions decreased by 11.7%and 18.7%in the O and O+T plots,respectively,which was attributed to lower NH+4 -N content and AOB amoA abundance in the O and O+T plots.The nosZ abundance was significantly greater in the O+T plot,whilst the AOB amoA abundance was significantly lower in the O+T plot than in the O plot.Relative to the organic fertilizer,bio-organic fertilizer application tended to decrease N2O emissions by 7.9%and enhanced vegetable yield,resulting in a significant decrease in yield-scaled N2O emissions.Overall,the results of this study suggested that,compared to organic and chemical fertilizers,bio-organic fertilizers containing PGPMs could benefit crop yield and mitigate N2O emissions in vegetable fields.
Key Words: chemical fertilizer,gene abundance,greenhouse vegetable,N cycle-related genes,plant growth-promoting microbe,Trichoderma guizhouense,yield-scaled N2O emission
INTRODUCTION
Nitrous oxide(N2O)is a potent greenhouse gas that has a 298-fold greater warming potential than carbon dioxide(CO2),and is involved in the depletion of stratospheric ozone(Ravishankaraet al., 2009). The concentration of N2O in the atmosphere reached 330µg L−1in 2017 and increased at a rate of 0.80µg L−1year−1(NOAA,2018).Agriculture contributes to nearly 80%of the global anthropogenic N2O emissions, making agriculture the largest anthropogenic source of N2O (UNEP, 2019). The N2O emissions from vegetable fields account for 9%of the total anthropogenic emissions(Rezaeiet al.,2015).
China is one of the largest vegetable producers in the world.The vegetable harvest area in China reached 23.9 million hectares in 2017, accounting for 41% of the world’s total vegetable harvest area(FAO,2018).In China,vegetable cultivation under simple plastic solar greenhouse conditions totaled 4.7 million hectares (Feiet al., 2018). The greenhouse vegetable cropping systems in China are typically characterized by intensive cropping rotation and frequent irrigation. In particular, fertilizers are applied at a rate of 1 000—2 000 kg N ha−1year−1for greenhouse vegetables,which is much more than for non-vegetable crops(Xionget al.,2006;Yaoet al.,2019).As a result,greenhouse vegetable cropping systems are highly vulnerable to N loss,with N2O emission as high as 31.1—60.5 kg N ha−1year−1(Yaoet al.,2019). Therefore, optimizing fertilizer management, such as by replacing chemical fertilizers with organic fertilizers,has been suggested as a method to reduce fertilizer-induced N2O emissions from greenhouse vegetable fields in China.
Fertilizer application can affect soil physicochemical properties and functional microbial genes that are involved in N2O production through nitrification and denitrification(Sunet al.,2015).Nitrification is the process of oxidizing ammonia to nitrite, with N2O being the byproduct of the first step in the cycle, which is performed by ammonium oxidizing bacteria(AOB)and archaea(AOA)(Tayloret al.,2012). Denitrification is the stepwise process of sequentially reducing NO−3/NO−2to N2. The four steps involved are sequentially catalyzed by nitrate reductase(encoded bynarG),nitrite reductase(encoded bynirK/nirS),nitric oxide reductase(encoded bynorB),and nitrous oxide reductase(encoded bynosZ).N2O is the final product or is an intermediate of the denitrification cascade(Canfieldet al.,2010).It is well-documented that these functional genes respond differently to chemical and organic fertilizers (Sunet al.,2015).For instance,meta-analysis has revealed that AOB populations have stronger responses to chemical N fertilizers relative to AOA populations(Careyet al.,2016),while organic fertilizers have positive impacts on soil functional microbial growth(Pereget al.,2018).
Recently,several mitigation strategies have been proven effective at mitigating N2O emissions from vegetable fields through regulating microbial-related N2O production or reduction pathways.For example,biochar application can facilitate N2O consumption by increasing the population and transcription of thenosZgene(Xuet al.,2014);while nitrification inhibitors are able to constrain N2O production by inhibiting autotrophic ammonia-oxidizing bacteria(Chenet al.,2015).Although these synthetic methods could effectively mitigate N2O emissions,high costs and the potential for environmental contamination still limit their widespread application and development(Lamet al.,2017).Therefore,the development of ecofriendly approaches is anticipated to mitigate N2O emissions from vegetable fields.
Plant growth-promoting microbes(PGPMs)are a type of free-living microorganism in soils(Vacheronet al.,2013),which have been increasingly used to improve plant growth and N use efficiency in agriculture (Panget al., 2017;Fiorentinoet al., 2018). Furthermore, recent experiments have found that inoculation with PGPMs such asBacillus amyloliquefaciensorTrichoderma viridesignificantly reduced fertilizer N-induced N2O emissions from acid tea fields(Wuet al.,2018;Xuet al.,2018).Unfortunately,these selected plant-beneficial strains often suffer from low survival rates in soils(Saravananet al.,2003).Instead,commercial bio-organic fertilizers, which combine the advantages of beneficial microbial inoculants and organic substrate,were found to be more beneficial to plant growth (Panget al.,2017;Rodrigueset al.,2018).Although the widespread use of bio-organic fertilizers has been approved to promote vegetable growth and maintain diverse soil microbiome functions(Zhanget al.,2018),their potential roles in mitigating N2O emissions from greenhouse vegetable fields have yet to be addressed.Here,we conducted a field experiment to measure N2O fluxes from greenhouse vegetable cropping systems in Southeast China.First,we predicted that seasonal N2O emissions were lower in organic and bio-organic fertilizer plots than in chemical fertilizer plot. Second, we also revealed that,relative to chemical and organic fertilizers,bio-organic fertilizers containingT. guizhouensewould produce higher vegetable yields and lower yield-scaled N2O emissions.Finally, we proposed that the addition ofT. guizhouensewould decrease AOBamoAgene abundance but increasenosZgene abundance.The objective of this study was to gain an insight into the potential role of bio-organic fertilizers containingT.guizhouensein mitigating N2O emissions from vegetable cropping systems.We also attempted to explore the associated mechanisms by linking N2O emissions to key soil biogeochemical factors and functional microbial genes.
MATERIALS AND METHODS
Study site description
The field experiment was carried out at an experimental site of Nanjing Agricultural University, Nanjing, Jiangsu Province,China(31◦95′N,118◦83′E).The region possesses a subtropical monsoon climate,with cool winters and warm summers.The study was conducted from August to October 2015,during which total precipitation was 292.0 mm and an average air temperature was 22.9◦C.The field site was overwhelmingly dominated by polyethylene plastic cropping fields,in which there was no extra heating or lighting.The soil at the experimental site was classified as a yellow brown soil,consisting of 50.17%clay,31.15%sand,and 17.68%silt.The initial properties of the surface soil(0—15 cm depth)were as follows: initial organic C, 13.02 g kg−1; total N(TN),1.74 g kg−1;available P,10.09 mg kg−1;available K,227.5 mg kg−1;soil pH,5.63(1:2.5 soil to water ratio);soil bulk density,1.33 g cm−3.
Experimental design
In 2015, the field experiment was conducted in a polyethylene plastic film-covering greenhouse vegetable cropping system with a more than 5-year history of continuous vegetable cultivation.In a 180-m2grid of the greenhouse vegetable cropping system, four fertilizer treatments with three replicates were established using a randomized block design,with each plot covering 5.4 m2.The four fertilizer treatments were:i)control,unfertilized;ii)chemical fertilizer(NPK,180 kg N ha−1,135 kg P2O5ha−1,and 180 kg K2O ha−1;iii)organic fertilizer(O,180 kg N ha−1,135 kg P2O5ha−1,and 180 kg K2O ha−1);iv)organic fertilizer containingT.guizhouense(O+T),referring to bio-organic fertilizer(180 kg N ha−1,135 kg P2O5ha−1,and 180 kg K2O ha−1).The organic fertilizer was made up of cattle manure and mushroom residue in a mass ratio of 3:1,which contained 40.10%,1.64%,1.23%,and 1.29%of organic matter,TN,phosphorus(P2O5),and potassium(K2O),respectively.The bio-organic fertilizer was made by direct inoculation withTrichoderma guizhouenseNJAU 4742(Harzianumclade,108colony-forming units (CFU) g−1dry weight), which is an effective bio-antagonist ofFusarium oxysporumf.sp.cubense4in situin the vegetable crop rhizosphere(Zhang Jet al.,2016).The bio-organic fertilizer contained 44.49%,1.45%, 1.05%, and 1.45% of organic matter, TN, P2O5,and K2O,respectively.Greenhouse-grown seedlings of the“Jinyou 1”cucumber cultivar were transplanted into the field.All fertilizers were divided into two parts,with one half used as a basal fertilizer before seedlings were transplanted and the other half used as topdressing on the 35th day after transplanting.Following the local practices,basal fertilizers and topdressings were spread on the soil surface and then plowed into the soil,followed by irrigation.There were four irrigation events to meet crop growth demand over the entire growing season(Fig.1).
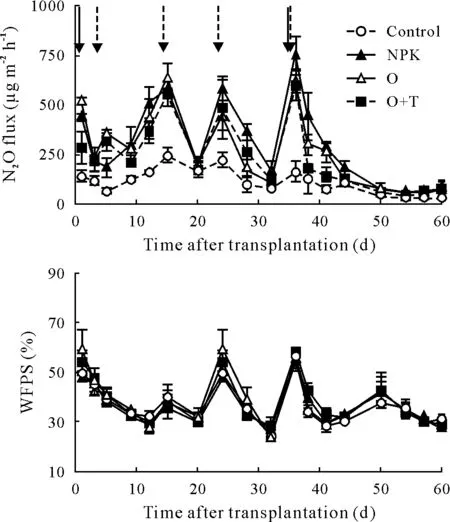
Fig.1 Seasonal dynamics of N2O fluxes and soil water-filled pore space(WFPS)under different fertilizer treatments over the cucumber growing season.Solid and dotted arrows represent fertilization and irrigation events,respectively. Vertical bars represent standard errors of means (n = 3).Control=unfertilized;NPK=chemical fertilizer;O=organic fertilizer;O+T=organic fertilizer containing Trichoderma guizhouense,referring to bio-organic fertilizer.
Measurement of N2O emissions
Soil N2O fluxes were measured using the static opaque chamber method, and the protocol details were described in our previous studies(Zouet al.,2005;Zhang Y Jet al.,2016).During the vegetable growing season,square PVC collars were installed(50 cm long×50 cm wide×15 cm high)in the center of each plot before the cucumber seedlings were transplanted.The upper sampling chamber(50 cm long×50 cm wide×50 cm high) was wrapped with a layer of sponge and aluminum foil to ensure dark conditions and to minimize air temperature changes inside the chamber.During gas collection,the top groove of the collar was filled with water to prevent air exchange,then the chamber was securely inserted into the collar.The air inside the chamber was homogenized with the help of a small fan fixed inside the chamber.Soil temperature and air temperature inside the chamber were simultaneously recorded during the time of sampling.Gas measurements were collected twice a week,but this was increased to every other day or every two days following fertilizer application.Gas samples were collected with an air pump every morning from 8:00 to 10:00, and samples were taken from the headspace chamber 0,5,10,15,and 20 min after the chambers were closed.
The N2O concentrations were analyzed by a gas chromatograph fitted with an electron capture detector (ECD;Agilent 7890A,USA)(Zouet al.,2005).The carrier gas was an argon-methane(1:19,volume:volume)mixture at a flow rate of 40 mL min−1.The temperatures of the column and ECD detector were 40 and 300◦C,respectively.
The N2O flux was calculated using the following equation:
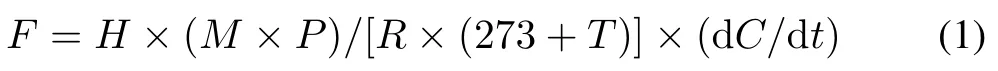
whereFis the N2O flux(µg m−2h−1),His the height of the static opaque chamber(m),Mis the molar mass of N2O(44 g mol−1),Pis the atmospheric pressure at the sampling site,which is regarded as the standard atmospheric pressure here(1.013×105Pa),Ris the universal gas constant(8.314 Pa m3mol−1K−1),Tis the temperature inside the static opaque chamber (◦C), and dC/dtis the rate at which the N2O concentration changes with time(µL L−1min−1).Total N2O emissions were sequentially accumulated from every two consecutive measurements.The N2O emission factors(EFs)of the fertilizers were calculated as the difference in total N2O emissions between the fertilized and unfertilized treatments divided by the amount of N applied(Zouet al.,2005).
Soil sampling
The soil samples(0—15 cm)were collected at 3,13,36,41, and 57 d after cucumber seedlings were transplanted.Each sample was passed through a 2-mm sieve and was then divided into three parts.One part was air dried and stored at room temperature for later analyses of chemical properties.The second part was stored at 4◦C to determine soil mineral N and dissolved organic carbon(DOC).The remaining part was stored at−80◦C for molecular analysis.
DNA extraction and quantitative PCR(qPCR)gene analysis
DNA samples were extracted from 0.25 g of soil using PowerSoilTMDNA isolation kits(Mo Bio,USA).The concentration and quantity of the DNA samples were determined using a Nanodrop spectrophotometer (Thermo Scientific,USA). The quantification ofamoAfrom AOA and AOB,andnirK,nirS,andnosZwas carried out in the StepOneTMreal-time PCR system(Applied Biosystems,Germany).Amplifications were performed in a reaction mixture with a total volume of 20µL,consisting of 10µL SYBR@Premix Ex Taq(Takara,China),0.4µL each primerTM(10µmol L−1),0.4 µL ROX reference dye II (50×), 6.8 µL sterile water,and 2µL template DNA.The DNA samples were diluted to 10 ng of DNAµL−1with sterile water prior to assays.The standard curves of all genes were created using triplicate 10-fold dilutions(10−1—10−7)of linear plasmid DNA.The amplification efficiencies for all genes ranged from 94.6%to 100.5%, withR2values ranging from 0.997 to 0.999.Each qPCR run also included corresponding standards and duplicate no-template controls.Melting curve analyses were used at the end of runs to confirm amplicon specificity.Details of the primer sets and qPCR cycling conditions for each primer set are summarized in Table I.
Auxiliary measurements
Soil volumetric water content was monitored with a portable rod probe(MPM-160,ICT International Pty Ltd.,Armidale,Australia)and was converted into water-filled pore space(WFPS)according to the following equation:WFPS=soil volumetric water content/(1—soil bulk density/2.65)×100%,assuming 2.65 Mg m−3as the soil particle density(Liuet al., 2013). Cucumber yields under different treatments were measured,and the yield-scaled N2O emissions were calculated as seasonal total N2O emissions divided by vegetable fresh yield(kg N2O-N t−1yield).
The soil physicochemical properties were determined according to the guidelines of the Chinese Soil Society(Lu,2000).Soil NH+4and NO−3were extracted from 2 mol L−1KCl solution(soil:water ratio of 1:10)after shaking for 1 h on a rotary shaker. Soil NH+4contents were determined by the indophenol blue method, and NO−3contents were measured by dual-wavelength(220 and 275 nm)ultraviolet spectroscopy(HITACHI,U-2900,Japan).
Total nitrogen was determined with an auto elemental analyzer(Vario EL III,Elementar,Germany).Soil pH and electrical conductivity (EC) were determined with a soil to water volume ratio of 1:2.5 (Lu, 2000). Soil dissolved organic carbon(DOC)was determined using a total organic C analyzer(Elementar,Laurel,Germany)after 5 g soil was extracted using 25 mL deionized water.
Statistical analysis
One-way analysis of variance(ANOVA)was used to test for significant differences(significance levelP=0.05)in seasonal total N2O emissions, vegetable yield, and yieldscaled N2O emissions. Pearson correlation analysis was conducted to examine the correlations between N2O fluxes and soil physicochemical parameters and between N2O fluxes and functional gene abundances.All functional gene copy numbers were log-transformed. A linear model with the personality of ordinary least squares (OLS) was used to fit the relationship of N2O fluxes with the copy number ratio ofnirSplusnirKtonosZ((nirK+nirS)/nosZratio).Statistical analyses were performed in SPSS Statistics 21(IBM Software,Chicago,USA).
RESULTS
N2O and yield-scaled N2O emissions
The seasonal dynamics of the N2O fluxes under the different fertilizer treatments showed similar patterns,except that the control remained at a relatively low level over the entire experimental period (Fig. 1). Four N2O flux peak events were detected at 1,15,24,and 36 d after seedlings were transplanted,generally following fertilizer application and/or water irrigation practices(Fig.1).Thereafter,N2O fluxes decreased at 41 d after transplanting and then stabilizedat lower release rates. The highest peak of N2O flux was found under the NPK treatment,with an average of 749.1±95.6µg m−2h−1.

TABLE I Primers used for quantitative PCR in this study
Over the experimental period,seasonal N2O fluxes averaged 121.1±14.6,296.7±7.7,261.9±7.6,and 241.3±6.2 µg m−2h−1for the control, NPK, O, and O+T treatments,respectively.Compared to the NPK treatment,seasonal total N2O emissions were significantly decreased by 11.7% and 18.7% under the O and O+T treatments,respectively (Table II,P <0.01). Relative to the organic fertilizer treatment,the NPK and O+T treatments enhanced vegetable yield by 4.0%and 8.4%,respectively(Table II).By relating N2O emissions to vegetable yields,the yield-scaled N2O emissions were the lowest under the O+T treatment across all treatments.The direct emission factors of fertilizer N for N2O were 0.89%,0.72%,and 0.61%under the NPK,O,and O+T treatments,respectively.
Soil physicochemical properties
Over the experimental period,soil moisture(WFPS)was primarily regulated by irrigation events,ranging from 25.9%to 56.1% (mean: 37.6%), with no significant differences among treatments(Fig.1).Soil NH+4-N content in the NPK treatment increased following the application of basal and topdressing fertilizers,and varied from 51.1 to 97.0 mg N kg−1(Fig.2).Compared to the NPK treatment,on average,soil NH+4-N content was 70.4%lower in the O treatment and was 64.7%lower in the O+T treatment over the experimental period(P <0.01)(Table SI,see Supplementary Material for Table SI).The soil NO−3-N content showed similar seasonal variation among the N-fertilized treatments,ranging from 50.5 to 142 mg N kg−1(Fig. 2). Relative to the NPK treatment,mean soil NO−3-N content decreased by 25%in the O plot and by 31%in the O+T plot over the experimental period (P <0.01). The NH+4and NO−3contents were relatively stable in the control and remained at low levels.The N fertilizer practices significantly increased TN content,but TN did not significantly differ among the NPK, O,and O+T treatments (Fig. 2). Compared to the control,mean TN contents were 8.0%, 6.3%, and 6.5% greater in the NPK,O,and O+T plots over the vegetable growing season,respectively(P <0.01)(Table SI,see Supplementary Material for Table SI).
Relative to the NPK plots,organic N application consistently increased soil dissolved organic carbon(DOC)and pH values in the O and O+T plots(Fig.2).Over the vegetable growing season,DOC content increased from 87.3—119.5 mg kg−1in the NPK plots to 103.8—212.3 mg kg−1in the O and O+T plots.Similarly,pH values increased from 4.89—5.45 in the NPK plot to 5.52—6.61 in the O and O+T plots.Compared to the NPK treatment,DOC content and pH were 70.4%and 16.9%greater in the O plot,respectively,and were 64.7% and 15.9% greater in the O+T plots, respectively,over the experimental period(P <0.01)(Table SI,see Supplementary Material for Table SI).Electrical conductivity(EC)ranged from 0.35 to 1.63 mS cm−1in the N-fertilized treatments and was highest in the NPK treatment(Fig.2).
Abundances of AOA amoA,AOB amoA,nirK,nirS,and nosZ genes
The abundances of nitrification and denitrification functional genes were determined by qPCR(Fig.3). The logtransformed copy numbers of AOA and AOBamoAgenes ranged from 6.12 to 6.48 and from 7.23 to 7.97 copies g−1dry matter(DM),respectively,across all treatments(Fig.3).The dynamic patterns of AOAamoAgene abundance were similar across all treatments in the cucumber growing season,and there were no significant differences in the AOAamoAgene abundance among all treatments(Table SII,see Supplementary Material for Table SII).In contrast,the AOBamoAgene abundance was more sensitive to chemical fertilizer application. Compared to the O and O+T treatments,chemical fertilizer application significantly increased AOBamoAgene abundance(Fig.3,Table SII,see Supplementary Material for Table SII).
The log-transformednirK,nirS, andnosZgene copy numbers ranged from 5.94 to 6.27,from 6.10 to 6.75,and from 6.70 to 7.21 copies g−1DM,respectively(Fig.3).ThenirKandnosZabundances in the O and O+T treatments were significantly higher than in the control and NPK treatments(Table SII,see Supplementary Material for Table SII).Compared to the O treatment,in addition,the inoculation ofT.guizhouensein the O+T treatment resulted in 38%and 22%increases innirSandnosZgene abundances,respectively(Table SII,see Supplementary Material for Table SII).The(nirS+nirK)/nosZratio was slightly higher in the NPK treatment than in the O and O+T treatments during the N2O peak flux periods(Fig.3).

TABLE II Seasonal total N2O emissions,vegetable yield,yield-scaled N2O emissions,and the direct emission factors(EF)under various fertilizer treatments

Fig.2 Dynamics of soil NH+4 -N,NO−3 -N,total N(TN),dissolved organic C(DOC),pH,and electrical conductivity(EC)under different fertilizer treatments over the cucumber growing season.Vertical bars represent standard errors of means(n=3).Control=unfertilized;NPK=chemical fertilizer;O=organic fertilizer;O+T=organic fertilizer containing Trichoderma guizhouense,referring to bio-organic fertilizer.
N2O emissions in relation to soil properties and functional gene abundances
Seasonal N2O fluxes were significantly positively correlated with soil NH+4-N content,WFPS,and EC(Table III).In addition,N2O fluxes were also positively correlated with AOBamoAgene abundance(Table III).The gene abundance of AOBamoAwas positively associated with NH+4-N and NO−3-N contents and with EC(Table III).The copy numbers of the denitrifying functional genes(nirK,nirS,andnosZ)were all positively associated with soil pH and DOC.Although no significant correlations were found between N2O flux and the abundances of the denitrifying genes,the(nirK+nirS)/nosZratio was positively correlated with N2O flux(Fig.4,P <0.01).
DISCUSSION
Greenhouse vegetable cropping systems have been recognized as an important source of agricultural N2O emissions in China,mainly due to the considerable N fertilizer input (Riyaet al., 2012; Yaoet al., 2015). In the present study,four N2O flux peaks were observed,generally following fertilizer N application and/or water addition events(Fig. 1). Indeed, numerous studies have revealed that N fertilizer application and water addition events induce significant N2O emissions(Yaoet al.,2015;ZhangY Jet al.,2016).This is probably because rewetting dry soil stimulates soil microbial activity and thereby increases N2O emissions(Williams and Xia,2009).
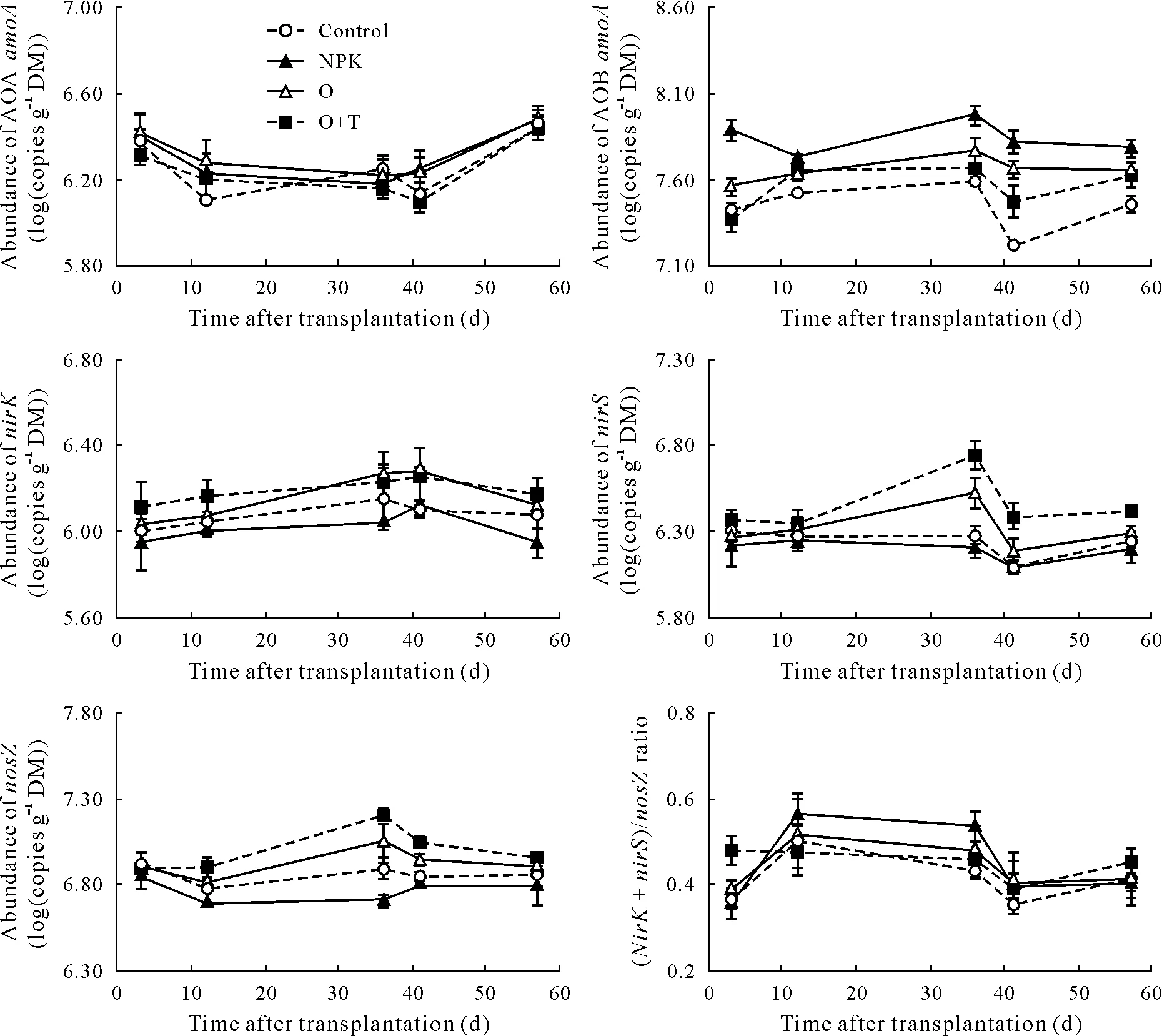
Fig.3 Dynamics of abundances of amoA from ammonium-oxidizing bacteria(AOA)and archaea(AOB)and nirK,nirS,and nosZ,and the copy number ratio of nirS plus nirK to nosZ((nirS+nirK)/nosZ ratio)under different treatments over the cucumber growing season.Vertical bars represent standard errors of means(n=3).Control=unfertilized;NPK=chemical fertilizer;O=organic fertilizer;O+T=organic fertilizer containing Trichoderma guizhouense,referring to bio-organic fertilizer;DM=dry matter.
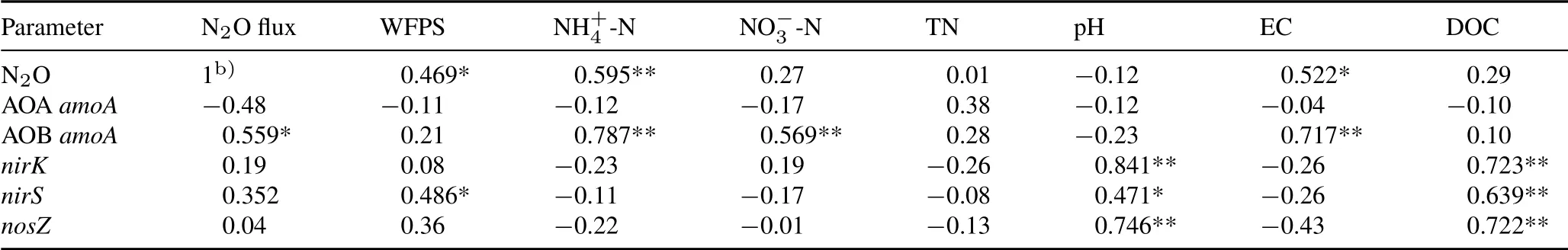
TABLE III Correlation coefficients(r)for relationships between N2O fluxes,functional gene abundances,and soil propertiesa)
Consistent with our first prediction, organic or bioorganic fertilizer significantly decreased seasonal N2O emissions by 11.7%—18.7%,relative to chemical fertilizer(Table II,P <0.01).Presumably,slow mineral N release from organic fertilizer limits the N available for denitrification and nitrification(Gutseret al.,2005).This is supported by the evidence that NH+4-N content was lower in the O and O+T treatments than in the NPK treatment(Fig.2).In this study,N2O flux was positively correlated with NH+4-N content,which is in agreement with previous study(Cuiet al.,2016).In addition, organic fertilizer application significantly increased DOC content(Fig.2),which generally enhanced soil respiration and consumed oxygen,and thereby formed anaerobic conditions favorable for denitrification(Krameret al.,2006).Similarly to our results,previous studies have shown that organic fertilizer application decreases N2O emissions,as the final product of denitrification is N2rather than N2O(Krameret al.,2006;Taoet al.,2018),although the opposite finding has also been reported(Hayakawaet al.,2009;Weiet al.,2014).Nevertheless,the effect of fertilizers on N2O emissions depends on the soil properties and fertilizer characteristics(Huanget al.,2004;Meijideet al.,2007).
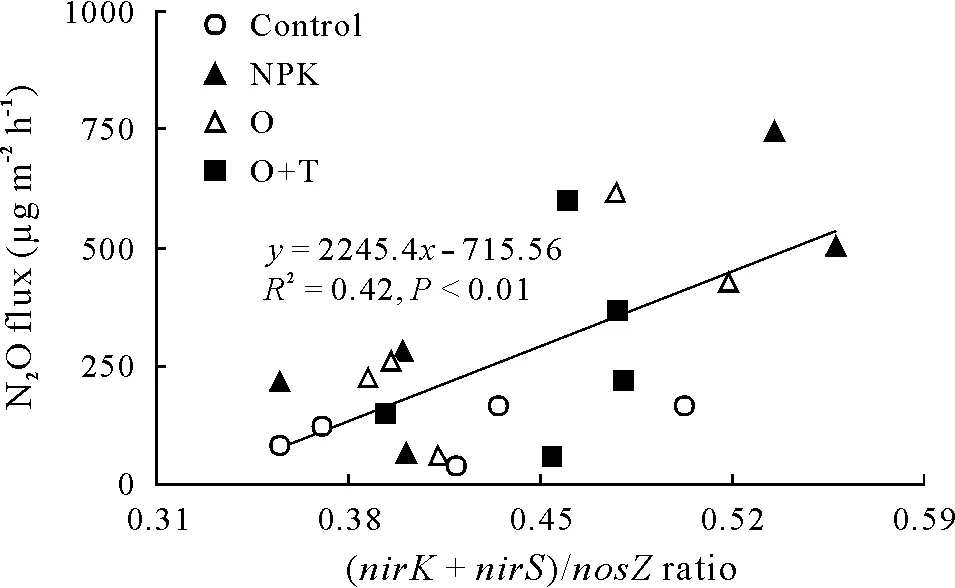
Fig. 4 Dependence of N2O fluxes on the copy number ratio of nirS plus nirK to nosZ ((nirK + nirS)/nosZ ratio) under different fertilizer treatments over the cucumber growing season. Control = unfertilized;NPK=chemical fertilizer;O=organic fertilizer;O+T=organic fertilizer containing Trichoderma guizhouense,referring to bio-organic fertilizer.
In support of our second prediction, vegetable yieldscaled N2O emissions were significantly lower in the O+T treatment than in the O and NPK treatments,while there was no significant difference between the O and NPK treatments(Table II).The plant growth benefits of bio-organic fertilizer containingTrichodermaspp. have been well documented in previous studies(Panget al.,2017;Zhanget al.,2018),and are due to modifications to soil nutrient availability,soil microbial diversity,and root growth(Harmanet al.,2004;Fiorentinoet al., 2018). Therefore, bio-organic fertilizers may have win-win effects on vegetable yield enhancement and N2O mitigation.
In this study,the abundances of the functional microbial AOA and AOBamoAgenes differed in response to fertilizer application(Fig.3).The application of both chemical and two organic fertilizers increased AOBamoAabundance,while these fertilizers had no significant effect on AOAamoAabundance in soils(Fig.3).The abundance of AOBamoAwas consistently higher than that of AOAamoAover the experimental season.The dynamic patterns of the abundances of the AOB and AOAamoAgenes in response to nutrient input were consistent with recent findings that AOB dominates in high nutrient soils while AOA is well-adapted to low nutrient environments(Diet al.,2010;Tayloret al.,2012).Similarly, Ouyanget al. (2016) also observed that AOB population was significantly modified by ammonium fertilizers,but AOA population was unaffected by ammonium or organic fertilizers in an agricultural soil.Our study indicated that AOB responded more strongly to the chemical fertilizer than to organic or bio-organic fertilizers.Previous studies have also confirmed that AOB abundance is significantly increased under high ammonium levels(Tayloret al.,2012;Hinket al.,2018).Therefore,the microbial AOBamoAgene could play an important role in soil N2O production through nitrification when a chemical fertilizer is applied(Hayakawaet al.,2009).
The abundances of the denitrifying genes(nirK,nirS,andnosZ) were consistently lower in the NPK treatment than in the O and O+T treatments in this study(Fig.3).As an important indicator for predicating N2O emissions,the(nirS+nirK)/nosZratio was greater in the NPK treatment than in the organic fertilizer treatments during the N2O peak flux period (Fig. 3). Organic fertilizers have been shown to stimulate heterotroph growth in the short term due to higher C availability(Tattiet al., 2013). Similarly,Hallinet al. (2009) and Pereget al. (2018) reported that long-term application of organic amendments significantly increasednirK,nirS,andnosZgene abundances compared to chemical fertilizers,supporting the hypothesis that organic carbon is one of the most important factors in terms of explaining the abundances of denitrifier genes(Cuiet al.,2016;Taoet al.,2018).Soil pH is considered to be another important factor affecting denitrifier gene abundance(Saleh-Lakhaet al.,2009;Čuhelet al.,2010).Pearson correlation analysis showed that the abundances of denitrifier genes were significantly correlated with soil DOC and pH (Table III,P <0.01).Field experiments have also indicated that higher copy numbers of all denitrifier genes appeared in neutral pH soil(Čuhelet al.,2010).In the present study,no significant correlations were found between N2O flux and the abundance of any single denitrified gene, while the ratio of (nirK+nirS)/nosZwas positively correlated with N2O flux(Fig.4,P <0.01). Therefore, the net production of N2O was simultaneously catalyzed by N2O-derived nitrite reductase and N2O-consumed nitrous oxide reductase,the denitrifiers functional as a group could be a good proxy for N2O emission(Moraleset al.,2010).
A slight decrease in N2O emissions in the O+T plot relative to O plot may be associated with lower AOBamoAabundance due to nitrification and/or highernosZ gene abundance through denitrification.Consistent with our prediction,the bio-organic fertilizer containingT.guizhouensesignificantly decreased AOBamoAabundance relative to organic fertilizer,but increasednosZgene abundance(Table SII,see Supplementary Material for Table SII).Similarly,Wuet al.(2018)found that inoculation with the PGPMBacillus amyloliquefaciensdecreased AOB abundance,thereby inhibiting nitrification and decreasing N2O emissions. On the other hand,in this study,the addition ofT.guizhouenseincreasednosZgene abundance compared to the application of organic fertilizer alone,which may also have contributed to the decline in N2O emissions.Recently,beneficial microorganisms have been increasingly used as microbial inoculants to promote plant growth and even to reduce environmental risks (Rodrigueset al., 2018; Liuet al., 2019). Calvoetal. (2016) found that inoculating soils planted with corn with plant growth-promotingBacillusstrains decreased N2O emissions in soils when some kinds of mineral nitrogen fertilizers were applied.Xuet al.(2018)reported that the use of urea amended withTrichoderma viridereduced N2O emissions from tea plantation soil.These studies on PGPMs have confirmed their potential roles in reducing N2O emissions,but the extent of this reduction depends on the type of N fertilizer and on the species or strain of PGPM.Therefore,additional research should focus on the effects of combinations of PGPMs and different N substrates on soil N2O emissions.Moreover,isotope tracer technology should be used to explore the interplay between PGPM strains and the N cycle-related microbial community.
CONCLUSIONS
Organic and bio-organic (containingT. guizhouense)fertilizers significantly decreased N2O emissions compared to a conventional chemical fertilizer in greenhouse vegetable cucumber cropping fields.The decrease in N2O emissions was strongly associated with lower soil NH+4-N contents and higher soil pH and DOC contents in organic or bio-organic fertilizer treatments. Moreover, the bio-organic fertilizer containingT. guizhouensetended to decrease N2O emissions relative to the organic fertilizer applied alone,which could be due to lower AOBamoAabundances inhibiting N2O production through nitrification and/or highernosZabundances facilitating the reduction of N2O to N2through denitrification. The significant linear positive correlation between the (nirK+nirS)/nosZratio and N2O flux over the cucumber growing season highlighted that the(nirK+nirS)/nosZratio could be a good proxy for N2O emissions.In addition, the bio-organic fertilizer enhanced vegetable yields and reduced yield-scaled N2O emissions,suggesting that PGPM inoculation in organic fertilizers could be used as a win-win strategy to enhance vegetable yields and reduce N2O emissions in vegetable cropping systems.
ACKNOWLEDGMENT
This work was supported by the National Key Research and Development Project of China(No.2017YFD0800200),the National Natural Science Foundation of China (Nos.41877093 and 41771323),the Fundamental Research Funds for the Central Universities of China (No. KYZ201621),and the Ministry of Education 111 Project of China (No.B12009).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Yield-scaled nitrous oxide emissions from nitrogen-fertilized croplands in China:A meta-analysis of contrasting mitigation scenarios
- Optimizing the use of open chambers to measure ammonia volatilization in field plots amended with urea
- A simple and easy method to measure ammonia volatilization:Accuracy under field conditions
- Nitrification inhibitor nitrapyrin does not affect yield-scaled nitrous oxide emissions in a tropical grassland
- Nutrient cycling and greenhouse gas emissions from soil amended with biochar-manure mixtures
- Nitrapyrin effectiveness in reducing nitrous oxide emissions decreases at low doses of urea in an Andosol
