Dual alloying improves the corrosion resistance of biodegradable Mg alloys prepared by selective laser melting
2021-03-10ChengeGoShengLiLongLiuShizhenBinYouwenYngShupingPengCijunShui
Chenge Go,Sheng Li,Long Liu,Shizhen Bin,Youwen Yng,Shuping Peng,Cijun Shui,,∗
aState Key Laboratory of High Performance Complex Manufacturing,College of Mechanical and Electrical Engineering,Central South University,Changsha410083,China
b Jiangxi University of Science and Technology,Ganzhou 341000,China
c Department of Oncology,Third Xiangya Hospital of Central South University,Changsha 410013,China
d NHC Key Laboratory of Carcinogenesis and The Key Laboratory of Carcinogenesis and Cancer Invasion of the Chinese Ministry of Education,Xiangya
Hospital,Central South University,Changsha,Hunan China
Received 8 October 2019;received in revised form 11 January 2020;accepted 3 March 2020
Available online 2 October 2020
Abstract Mg alloys have been regarded as revolutionary metallic biomaterials for biodegradable bone implants,but their applications are mainly blocked by the too rapid degradation in physiological environment.This study explores the dual alloying effects of Mn and/or Sn on the performance of Mg alloys prepared by selective laser melting.The observed microstructure indicated remarkable refinement of both the grains and intermetallic phases in the Mn-and/or Sn-containing alloys during the rapid solidification process.Moreover,approximately a half decrease in corrosion rate was observed for AZ61–0.4Mn-0.8Sn alloy with respect to AZ61 alloy.The improved corrosion behavior was primarily due to the enhanced protective effects of surface layers,in which Mn-and/or Sn-rich phases acted as a helpful barrier against medium penetration and thereby alleviated the current exchange with the matrix.In addition,the solute Mn and/or Sn positively shifted the corrosion potential,which also brought about a better corrosion resistance.Furthermore,the strength and hardness of the alloys were also effectively improved and comparable to those of cortical bone.This could be ascribed to the dissolved Mn and/or Sn atoms and the finely dispersed intermetallic phases,which might cause lattice distortion and precipitation hardening.Besides,the Mn-and/or Sn-containing alloys showed good cytocompatibility as indicated by the normal morphology and increased viability of MG-63 cells.These findings suggest that the developed AZ61-Mn-Sn alloy is a promising candidate for biodegradable bone implants.
Keywords:Dual alloying;Mg alloys;Selective laser melting;Corrosion resistance;Cytocompatibility.
1.Introduction
One of the challenges in bone regeneration is to develop suitable implants for assisting the restoration of defective bone and progressively degrade at the same time,as summarized by Zhang et al.[1-5].Recently,Mg alloys attract extensive attentions as potential bone implants owing to their full biodegradability and more aligned densities and modulus to natural bone in comparison with other metallic biomaterials,as mentioned by Agarwal et al.[6].Mg,as one of the basic elements,participates in many physiological functions in the human body.Moreover,lots of in vivo investigations confirm that Mg alloys possess favorable biocompatibility and can stimulate new bone formation through the released Mg ions,as reported by Gao et al.[7,8].Nevertheless,Thomas et al.[9]reported that Mg alloys corroded rapidly coupled with hydrogen evolution in a physiological environment,which easily led to excessive bubble aggregation and a dramatic reduction in mechanical integrity,and further to the failure of the implants before their functional retirement.Therefore,it has been a great concern to enhance the corrosion resistance of Mg alloys.
It is summarized that the undesirable corrosion resistance originates from the low potential of Mg,which makes Mg alloys active and easily corrodes[10–12].At present,element alloying has become an effective and flexible method to enhance their corrosion behavior.On the one hand,appropriate element alloying can inhibit the hydrogen evolution of Mg alloys and improve the anode’s Coulomb efficiency.On the other hand,surprising mechanical improvements have also been achieved by solid solution strengthening and age hardening.Extensive efforts have been devoted to developing alloying elements,and rare earth such as La,Ce,Y,Nd,Gd,and Sm,etc.,have gained the most attentions because of their effective enhancements in the corrosion resistance of Mg alloys based on studies of Zhao et al.[[13–15].However,Myrissa et al.[16,17]reported that some of the above elements may accumulate in animal organs,especially spleen,liver,lung and kidney,during the degradation of Mg alloys.Thus,in terms of biosafety,Mg alloys containing the basic elements of the human body should be concerned especially.
As bio-friendly elements,Sn and Mn have shown great potentials as alloying elements in Mg alloys[18,19].In physiology,Sn is an important element to human nutrition and health.Moreover,Wang et al.[20]showed that Sn can decrease the H2evolution rate owing to its high hydrogen overvoltage,which is beneficial for inhibiting the self-corrosion of Mg matrix.It may also be an efficient strengthening element in alloys due to the precipitate of Mg2Sn.As for Mn,a trace element for human body,involves in the synthesis and activation of many enzymes,as well as the metabolic cycle of carbohydrates and amino acids.Cho et al.[21]have also discovered that Mn could remove the harmful impurities such as heavy-metal elements and iron through forming other intermetallic phases.Meanwhile,the presence of Mn with higher electrode potential can increase the susceptibility of galvanic corrosion.Furthermore,Mn and Sn can alter the composition of surface films.These characteristics are beneficial for improving the corrosion resistance of the alloys.
Therefore,this study was concerned with the combined effects of alloying Sn and Mn on the enhancements of corrosion behaviors.AZ61(Mg-6Al-1Zn,wt.%)alloy was chosen as the matrix because of a combination of relatively good corrosion resistance and acceptable mechanical properties,and selective laser melting(SLM)was used for alloy preparation due to its superiority in modifying the microstructure of alloys compared with conventional methods.A comparative study of AZ61-x(x=Mn,Sn and Mn-Sn)alloys on microstructure,mechanical properties,corrosion behaviors and cytocompatibility,was performed with AZ61 alloy as a control.The dual effects of Mn and/or Sn on the corrosion behavior of AZ61 alloys were discussed.And a schematic diagram was also proposed to illustrate the underlying mechanisms.
2.Experimental methods
2.1.Materials and sample preparation
AZ61 alloy powder supplied by Tangshan Weihao Materials Co.,Ltd.,manganese(Mn)powder supplied by Shanghai Naiou Nano Science and Technology Co.,Ltd.and tin(Sn)powder supplied by Shanghai Naiou Nano Science and Technology Co.,Ltd.were served as-received.The morphology of the powders was studied by scanning electron microscopy(SEM)and its elemental compositions were studied by energy-dispersive spectroscopy(EDS).And the particle size distribution of the as-received powder was gauged using a laser particle size analyzer.The powders were mixed with proper proportions using a ball grinder at 200rpm for 4h under argon atmosphere.Three groups of mixed powder with 0.4wt.% Mn,0.8wt.% Sn and 0.4wt.% Mn-0.8wt.% Sn were obtained,respectively.
A SLM apparatus equipped with fiber laser was used to prepare a series of alloy samples,which were denoted as AZ61,AZ61–0.4Mn,AZ61–0.8Sn and AZ61–0.4Mn-0.8Sn,respectively.An argon atmosphere was maintained throughout the preparation process to prevent oxidation.The selective laser melted(SLMed)alloy samples were prepared with laser power(80 W),scanning speed(1000 mm/min),hatch spacing(0.1 mm)and layer thickness(0.08 mm),respectively.AZ61 alloy samples prepared by a conventional powder metallurgy method(PMed AZ61 alloy)and their performance were tested for the comparison purpose.Subsequently,the alloy samples were ground using abrasive papers up to 1500 grits and polished mechanically to a final mirror-like surface on cotton cloth with 0.5μm diamond grinding paste.
2.2.Microstructure characterization and mechanical properties
Optical microscopy(OM,PMG3,Olympus,Japan)was used for metallurgical observation.Prior to observation,the polished alloy samples were etched for 10s to reveal the grain morphology in an acetic picral solution which was composed of 4.2g of picric acid powder,10mL of acetic acid,70mL of ethanol and 10mL of distilled water.SEM/EDS analysis were performed to analyze the microstructure of the alloy samples and the element composition of the alloy samples were studied by the built-in EDS device.For the purpose of identifying the phase composition,the X-ray diffraction measurement was also carried out and its operating parameters were as follows:the running voltage and current of Cu X-ray tube to generate Cu Kαradiation were 30kV and 15mA,scanning speed of 8 °/min and scanning step size of 0.02° The hardness tests of the alloy samples were carried out by a Vickers microindenter.The applied load and dwell time used during the hardness test were 300 gf and 15s,respectively.For hardness measurements,ten different points were selected randomly on each sample.The compression tests were implemented using a universal testing machine.The crosshead speed used during the compression test was 0.2mm/min.For each group of samples during the compression test,at least six effective alloy samples from each condition were measured to guarantee the repeatability of the experiment result.Relative density measurement was performed to better understand the effects of porosity in the microstructure on the mechanical properties of the alloys.The relative density was calculated based on the following formula:[22,23]
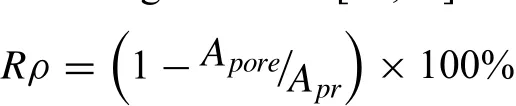
where Atotand Aporewere the total area and the area of pores in the metallurgical images,respectively.
2.3.Degradation properties
Electrochemical measurements were carried out on a Gamry Reference 600+electrochemical workstation(Gamry Instruments,USA)in a plexiglass beaker with 300mL simulated body fluid(SBF)solution at a temperature of 37 °C and a pH of 7.4.A standard three-electrode system was applied to perform the electrochemical tests.The alloy samples,which were encapsulated in resin leaving an exposed surface area of 1 cm2,was used as the working electrode.A platinum net was acted as the counter electrode and a saturated calomel electrode was acted as the reference electrode,respectively.The samples were immersed in SBF for 1800s to measure their stable open circuit potential(OCP).Then,the tafel potentiodynamic polarization tests were executed at a constant scan rate of 0.5mV/s started from−250mV below the OCP.Experiments repeated three times for each group were carried out to acquire the representative results.
Immersion tests of the alloy samples were performed in SBF with a surface area to solution volume ratio of 1.25 cm2/mL at 37 °C.The alloy samples put in the hydrogen collector were separately immersed in SBF and the volume of hydrogen was collected and recorded during the immersing process.After immersion for 7 days,the samples were taken out from the solution,then rinsed with distilled water three times and dried with the blower at room temperature.The surface morphology were characterized by SEM.The phase compositions of the degradation products were studied by XRD.The valence states of the elements in degradation products were characterized using X-ray photoelectron spectroscopy(XPS)which was used Al anode X-ray excitation source.The XPS data were acquired at 150W(15mA and 10kV)and subjected to deconvolution using the peak fit software.For the weight loss tests,the degradation products were eliminated by ultrasonic cleaning the samples for 10min in chromic acid solution which was composed of 200g/L chromic oxide and 10g/L silver nitrate.After eliminating degradation products,the samples were rinsed by distilled water and dried with the blower at room temperature.Original samples and samples eliminated degradation products were weighted and the degradation rates were calculated according to Gao et al.[24,25]:
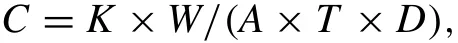
where C is the degradation rate(mm/year),K is a constant of 8.76×104,W is the weight difference of the sample before and after immersion in SBF(g),A is the area of the sample exposed to SBF(cm2),T is the immersion time in SBF(h)and D is the sample density(g/cm3).
2.4.Cytocompatibility
For cytocompatibility assays,the extracts of the alloys were primarily prepared as follows:after being sterilized with ultraviolet radiation,all the alloy samples were seperately immersed in Dulbecco’s modified Eagle’s medium(DMEM)with a surface area to solution volume ratio of 1.25 cm2/mL.Subsequently,the mixture was centrifuged for 5min at 3000rpm in order to experimentation for removal of particulate granules and the supernates were collected.Afterwards,10% fetal bovine serum was added into the alloy extracts and stored at 4 °C.The culture medium of blank control was DMEM with 10% fetal bovine serum.The inductively coupled plasma atomic emission spectrometry was used to quantitatively analyze the ion concentrations in the alloy extracts after immersing.
MG-63 cells,a human osteoblast-like cells,were applied to assess the cytocompatibility of the alloys.The cells were cultured at an appropriate number for proliferation(a density of 5×103cells per 100mL)in a flat-bottomed 96-well plate.In order to allow cell attachment,cells were cultured for 24h at 37 °C in a humidified atmosphere of 5% CO2.Then,the cell culture medium was removed and 100μL of the alloy extracts were added to the culture well,then cultured for 1,3 and 5 days.Finally,each well of the culture plate was added with cell counting kit-8 solution of 10μL and the cells were further incubated at 37 °C for an excess 2h.The absorbance of the stained cells at a wavelength of 450nm was immediately read employing a microplate reader.The relative growth rate(RGR)of the samples was calculated on the basis of the reports of Levy et al.[26]:
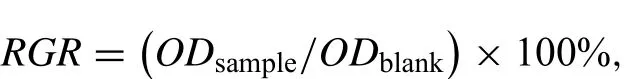
where ODsampleis the optical densities of cells in the sample group and ODblankis the optical densities of cells in the blank control group.
For cell morphology evaluation of MG-63 cell,cells were incubated in the alloy extracts for 5 days and then on slides for 24h.Afterwards,the culture medium was collected and the cells were rinsed with phosphate buffer saline(PBS)solution for three times.And then the rinsed cells were stained with co-staining kit(Calcein-AM and Ethidium homodimer to identify living and dead cells respectively)for 30min,followed by three times rinsing of the cells with PBS solution.Finally,the stained cells were observed through a fluorescence microscope and photographed using its built-in digital camera.
2.5.Statistical analysis
The experimental data of the test results were presented as means with their standard errors.To determine the statistical significance of the differences between groups,statistical analysis was conducted by one-way analysis of variance.All differences with P values less than 0.05 were accepted as a statically significant difference.
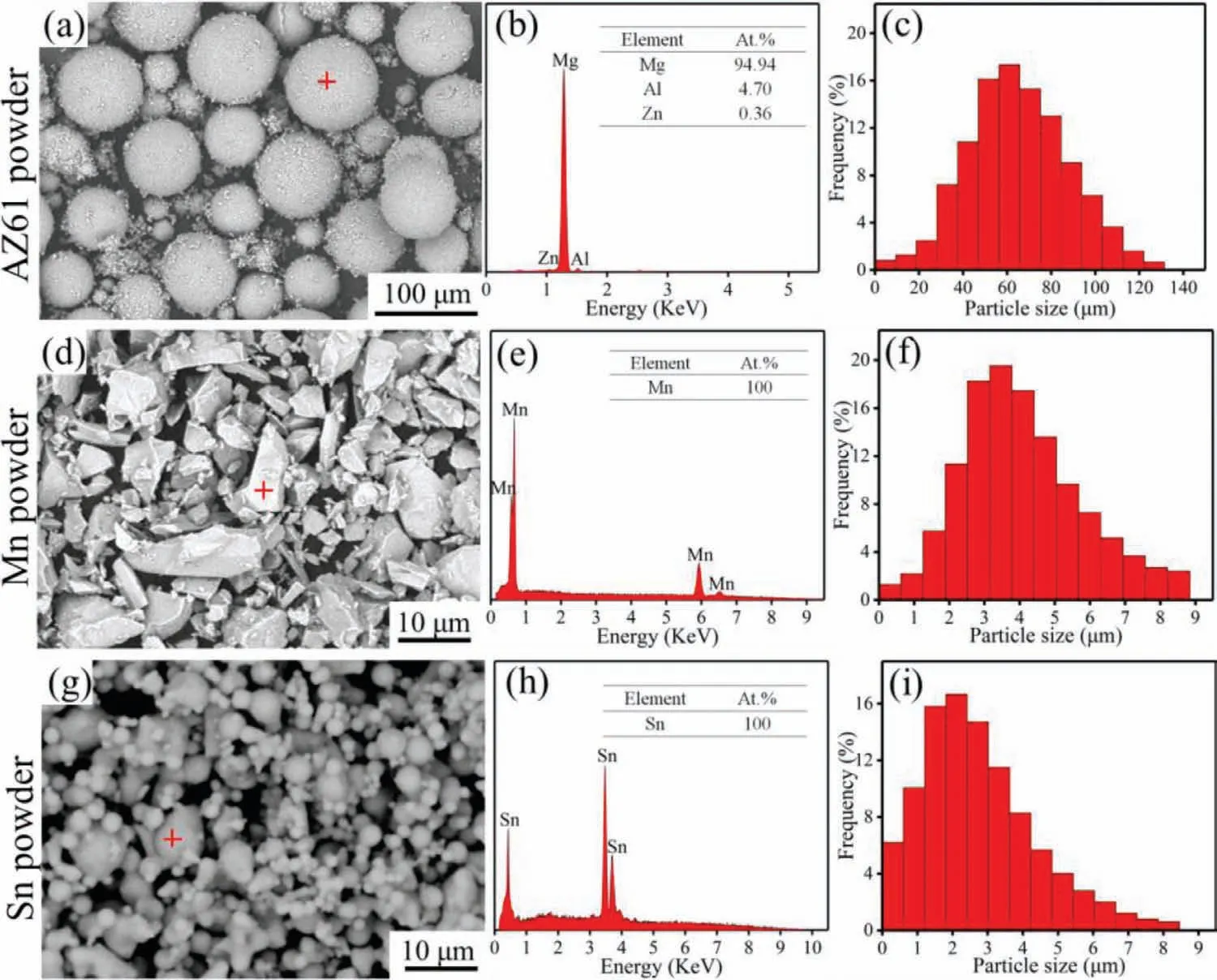
Fig.1.The morphology,composition and particle size distribution of the as-received AZ61,Mn and Sn powders.
3.Results
The characteristics of the used raw powders in the present study were shown in Fig.1.SEM images showed that both the AZ61 and Sn powders consisted of spherical particles but the latter showed the presence of much finer particles.In comparison,the Mn powder was composed of irregular polyhedral particles with sizes similar to that of the Sn powder.EDS analysis was carried out on the typical particles(indicated by red crosses)to investigate the element composition.It can be seen that the spectrum of AZ61 particle consisted of Mg,Al and Zn peaks,indicating a high purity of the powder.Similar results were also observed in the spectra of Mn and Sn powders.The size distribution of the powders was quantitatively measured.As can be seen,a large variation in powder size was observed for AZ61 powder with an average value of~34μm,while the particle sizes of Mn and Sn powders were both<9μm with average values of~5±0.3μm and~3±0.2μm,respectively.
The typical microstructure of PMed AZ61 alloy and SLMed AZ61-x alloys in OM mode was displayed in Fig.2(a1-e1).The results showed that PMed AZ61 alloy was composed of irregular particles with small pores among the particles.For SLMed alloys,it was obvious that AZ61-x alloys had nearly equiaxed grain structure and the grain size showed the tendency to decrease with the addition of Mn and/or Sn.When compared with AZ61–0.4Mn and AZ61–0.8Sn alloys,AZ61–0.4Mn-0.8Sn alloy exhibited a more refined grain structure with very small grain sizes<5μm.The reason was that the solute elements,i.e.Mn and Sn,accumulated in the liquid ahead of the solidification front.Based on the constitutional supercooling-driven nucleation theory from Qian et al.[27],the abundance of solute elements around solid/liquid interface would lead to component supercooling.This could promote the nucleation and meanwhile impede the growth of grains,thus showing refinement effects on AZ61-x alloys.
To determine the distribution and morphology of the second phases,SEM and EDS analysis were further carried out on PMed AZ61 alloy and SLMed AZ61-x alloys.In PMed AZ61 alloy,large particles were found to bond together with Mg17Al12phase(Point 1)distributing throughout the alloy(Fig.2a2-a3).It can be seen that all the SLMed alloys consisted ofα-Mg grains and intermetallic compounds.In AZ61 alloy(Fig.2b2-b3),typical light-gray Mg17Al12phase with an island-shape morphology(Point 2)was observed distributing at grain boundaries.With the addition of 0.4wt.% Mn,the size of Mg17Al12phase was significantly reduced(Fig.2c2).Moreover,new bright granular phases formed at grain boundaries(Point 3).Quantitative analysis by EDS showed that the main components of the new granular phase were Mn,Al and Mg(Fig.2c3).As for AZ61–0.8Sn alloy(Fig.2d2),the addition of Sn also led to the remarkable reduction of Mg17Al12phase and the formation of bright granular phases(Point 4)at grain boundaries.The corresponding EDS results revealed that the fine and discrete particles were Sn-rich phases.Moreover,EDS map(Fig.2d3)presented the uniform distribution of Sn element,suggesting that Sn existed in terms of either solid solution in the matrix or intermetallic particles along the grain boundaries.The SEM micrograph of AZ61–0.4Mn-0.8Sn alloy(Fig.2e2)also showed highlighted interphases precipitating at grain boundaries,which contained high concentrations of Mn and Sn as revealed by related EDS maps(Fig.2e3-e4).In addition,it was difficult to morphologically distinguish the generated Mn-rich and Sn-rich phases due to their similar fine globular-shape.
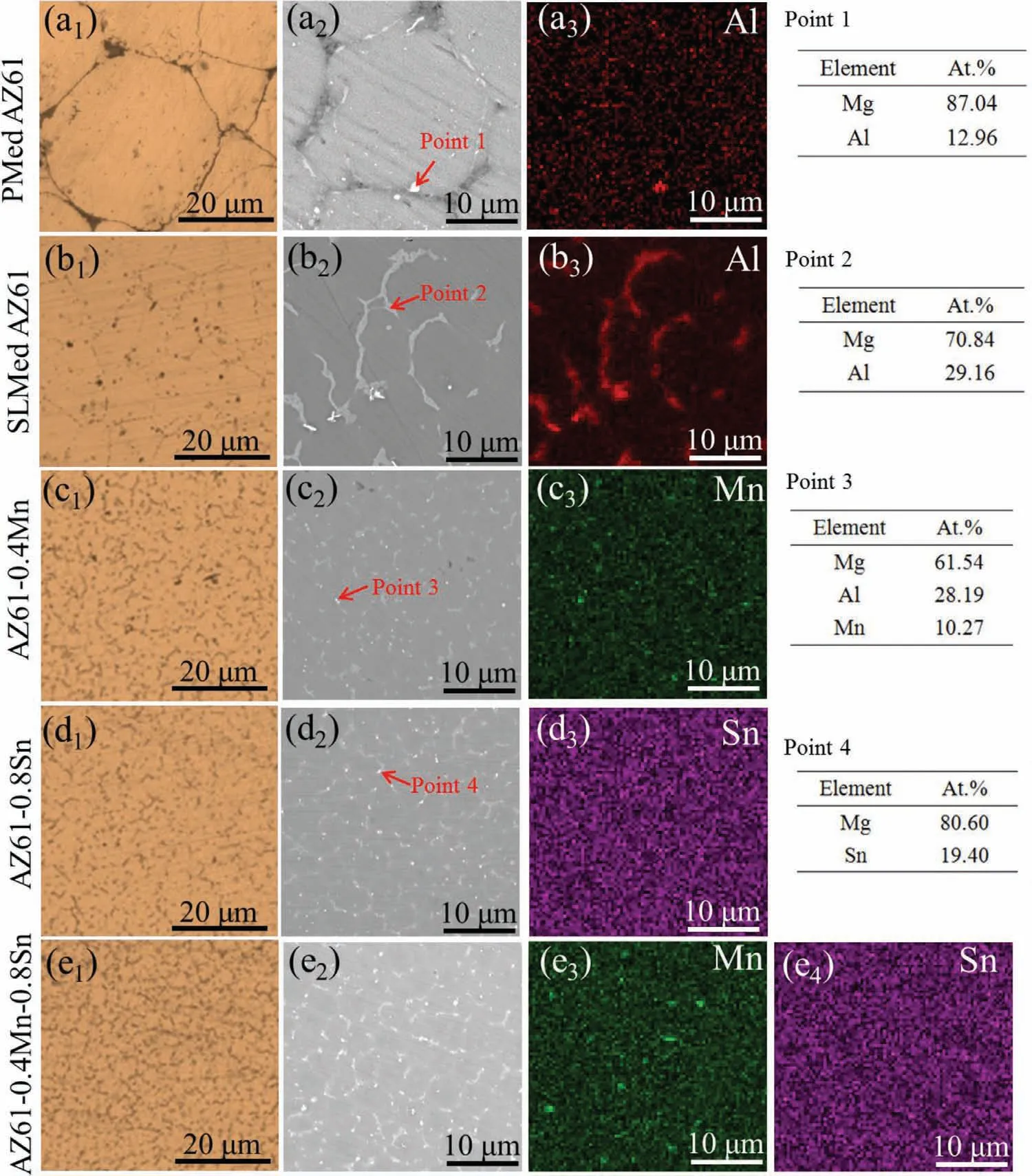
Fig.2.Typical microstructure of AZ61-x alloys in OM and SEM/EDS mode.
To further identify the phases in the AZ61-x alloys,XRD analysis was carried out and the patterns were shown in Fig.3(a).In PMed AZ61 alloy,only one set of well-defined peaks existed forα-Mg and no peaks for second phases were detected.As indicated,the XRD pattern of AZ61 alloy revealed two sets of well-defined peaks,one forα-Mg and one for Mg17Al12phase.After alloying Mn,a relatively small,but noticeable,peak corresponding to Al8Mn5phase appeared at approximately 42.8° And a reflection of Mg2Sn phase with a low intensity appeared in AZ61–0.8Sn alloy.This indicated that the bright granular intermetallic phases in the SEM micrographs of AZ61–0.4Mn and AZ61–0.8Sn alloys were Al8Mn5and Mg2Sn precipitates,respectively,which was in accord with the EDS results.The XRD pattern of AZ61–0.4Mn-0.8Sn alloy displayed combined diffraction peaks corresponding toα-Mg matrix,Mg17Al12,Al8Mn5and Mg2Sn phases.Moreover,it could be observed that the relative peak intensity of Mg17Al12phase decreased after the addition of Mn and/or Sn,suggesting the decreased volume fraction.The suppressing effects of Mn and/or Sn on the formation of Mg17Al12phase were consistent with the SEM observations.
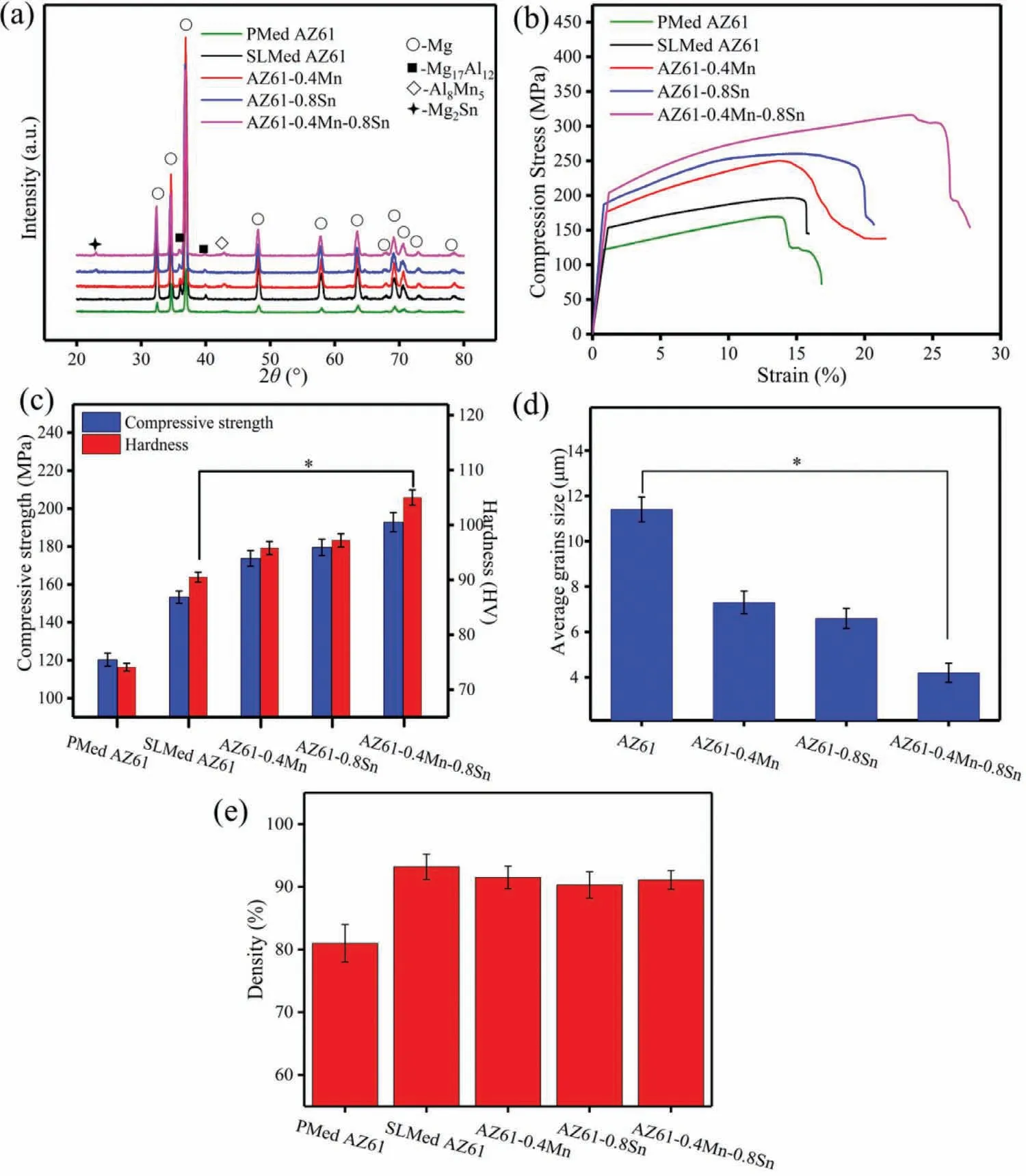
Fig.3.XRD patterns,stress-strain curves,mechanical properties,average grain sizes and relative densities of the AZ61-x alloys.∗P<0.05.
The stress curves for SLMed AZ61 alloy with PMed counterpart as control were plotted in Fig.3(b).It could be seen that the stress for all the samples significantly increased and then gradually increased to a maximum value.Finally,the stress rapidly decreased,which indicated the occurrence of fracture.Moreover,it could be seen from Fig.3(c)that PMed AZ61 alloy showed relatively low compressive strength and hardness of 120.3±3.4MPa and 74.1±0.7 HV,respectively.Compared with the PMed AZ61 alloy,SLMed AZ61 alloy possessed higher compressive strength and hardness of 153.3±3.2MPa and 90.5±0.9 HV,respectively.The alloying of 0.4wt.% Mn into AZ61 alloy caused increases in the compressive strength to 173.7±4.1MPa and the hardness to 95.8±1.2 HV.In comparison,the compressive strength was improved to 179.5±4.3MPa and the hardness reached about 97.2±1.2 HV when 0.8wt.% Sn was introduced to AZ61 alloy.Thus,alloying Sn might induce a stronger strengthening effect than alloying Mn.In the case of AZ61–0.4Mn-0.8Sn alloy,the compressive strength and hardness were further enhanced to 192.8±5.1MPa and 105±1.4 HV,respectively,suggesting~26% and~16% improvements compared to those of AZ61 alloy.These improvements could be ascribed to grain refinement,solution strengthening and second phase strengthening.As aforementioned,the metallographic microstructure of AZ61-x alloys was obviously refined with a substantial reduction in grain size from 11.4±0.55μm to 4.2±0.42μm after alloying Mn and Sn(Fig.3d),which conduced to the enhancement of mechanical properties.Moreover,the Al8Mn5and/or Mg2Sn phases at the grain boundaries was also responsible for the mechanical improvements since these precipitate phases could act as barriers to grain boundary sliding and thereby consume a lot of fracture energy in the matrix.In addition,the solid solution of Mn and/or Sn atoms in the alloy crystal lattice might also lead to an increase in the mechanical properties.The mechanical properties were also affected by the defects,especially pores in the microstructure.Applied mechanical stress would result in the formation of cracks out of these pores,and fractures would happen when the cracks grew in the samples.It could be found from Fig.3(e)that the fabricated samples by SLM possessed higher relative density(93.2±2%,91.5±1.8%,90.3±2.1%,91.1±1.5% for SLMed AZ61,AZ61–0.4Mn,AZ61–0.8Sn,AZ61–0.4Mn-0.8Sn alloys,respectively)than the counterpart by powder metallurgy(81.1±3% for PMed AZ61 alloy),which demonstrated that the SLM process can be used to fabricate relatively dense alloys.Moreover,there were no significant differences among the relative densities of SLMed samples.
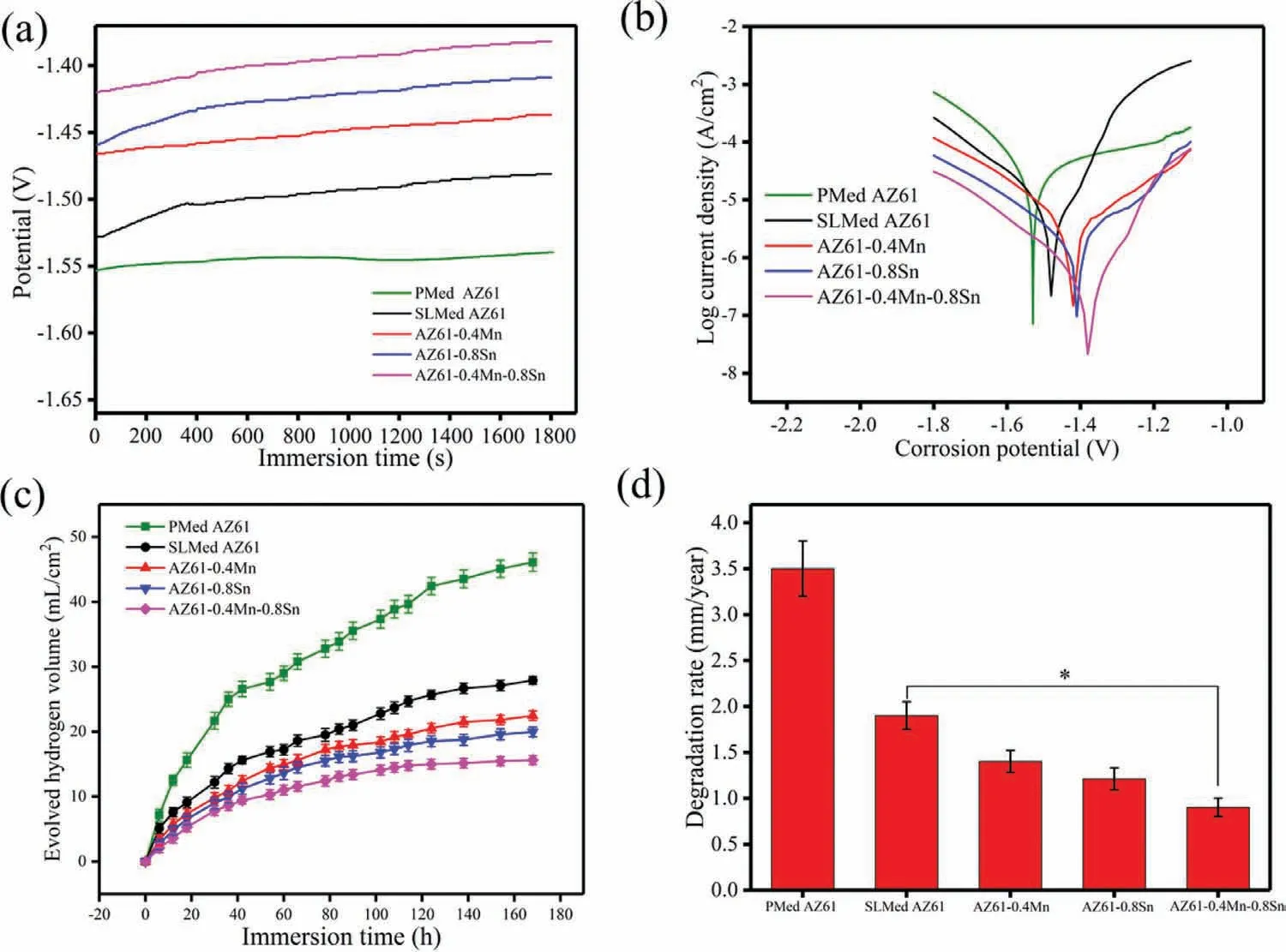
Fig.4.The OCP,polarization curves,hydrogen evolution and degradation rates of the AZ61-x alloys resulting from electrochemical tests and immersion tests.∗P<0.05.
Potentiodynamic polarization tests in SBF were performed to investigate the electrochemical behaviors of the AZ61-x alloys.The measured OCP and polarization curves were shown in Fig.4.PMed AZ61 alloy possessed the lowest OCP,indicating the highest thermodynamic tendency to corrode in the medium.For SLMed alloys,it can be observed that the OCP of all the alloys gradually shifted towards positive and achieved a final dynamic balance(Fig.4a).This might be ascribed to the Mg(OH)2film which inhibited the direct contacts of the matrix with SBF.During the first 400s immersion,the OCP of AZ61 alloy shifted at a high rate followed by a noticeable drop,which might originate from the local breakdown of the Mg(OH)2film.In comparison,the OCP of the Mn-and/or Sn-containing alloys showed a continuous increase with immersion time,suggesting a better passivation and the thickening of the surface film.Moreover,the stabilized potentials of Mn-and/or Sn-containing alloys appeared to be nobler than that of AZ61 alloy,also indicating the promoted passivation.The polarization curves of the AZ61-x alloys measured around their respective stable OCP were shown in Fig.4(b).The anodic branch revealed similar polarization characteristics of the growth and formation of the passive surface film,followed by the occurrence of transpassivation.Moreover,the addition of Mn and/or Sn ennobled the Ecorrand decreased the Icorrof AZ61-x alloys,thus leading to improved corrosion resistance in the order of AZ61–0.4Mn-0.8Sn>AZ61–0.8Sn>AZ61–0.4Mn>SLMed AZ61>PMed AZ61.Meanwhile,Liang et al.[28]stated that the abrupt decrease in corrosion potential was usually defined as breakdown potential(Eb)and a more positive Ebindicated less tendency for localized corrosion.It can be seen that after alloying Mn and/or Sn,the Ebshifted towards more positive values with wider passivation region,indicating the formation of more protective and stable surface film.
The degradation characteristics of the AZ61-x alloys were further investigated by immersion tests.The hydrogen evolution curves within 168h for the alloys were displayed in Fig.4(c).As indicated,the hydrogen evolution volume all increased with the immersion time and can be divided into two stages.One was the initial stage of rapid evolution of hydrogen and the other was the later stage of relatively stable evolution of hydrogen.It could be ascribed to the degradation product layer on the alloy surface,which inhibited the current exchange and hydrogen evolution.Moreover,PMed alloy possessed the highest degradation rate duo to the presence of pores making the corrosive fluid more vulnerable to intrusion.The hydrogen evolution rate of AZ61–0.4Mn-0.8Sn alloy was slower than those of the other three alloys.This was in accordance with the electrochemical measurement results.The calculated degradation rates also showed similar trend among the alloys(Fig.4d),demonstrating improved corrosion resistance for the Mn-and/or Sn-containing AZ61 alloys.
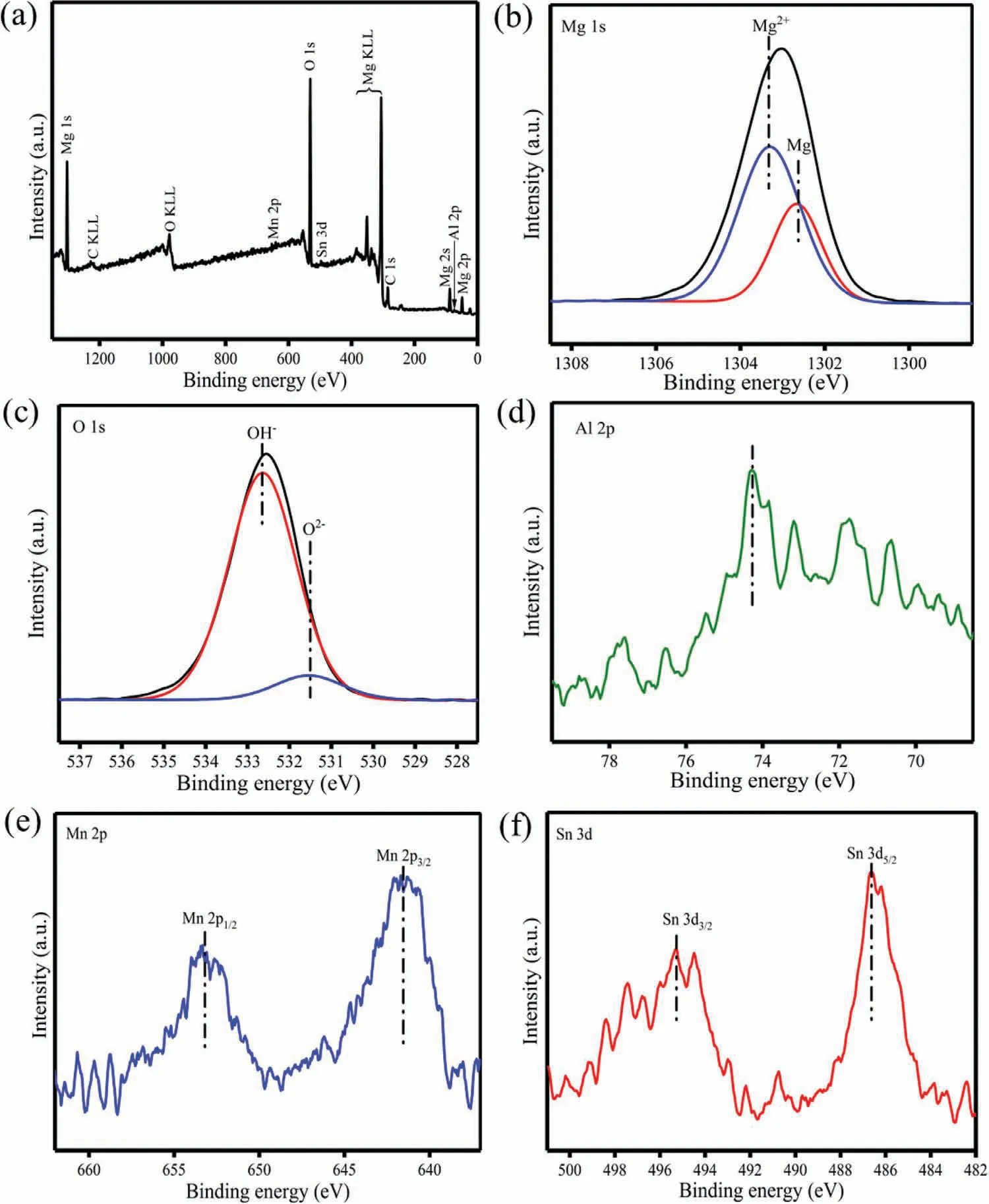
Fig.5.XPS binding energy spectra of(a)overall survey,(b)Mg 1s,(c)O 1s,(d)Al 2p,(e)Mn 2p and(f)Sn 3d in the corrosion product layer on AZ61–0.4Mn-0.8Sn alloy after immersion test.
In order to reveal the reason for the improved corrosion resistance,the corrosion product layer on AZ61–0.4Mn-0.8Sn alloy after immersion test was characterized by XPS and the chemical states of the elements in the corrosion product layer were identified by the binding energy spectra of Mg 1s,O 1s,Al 2p,Mn 2p and Sn 3d(Fig.5).As indicated,Mg(OH)2was primarily confirmed in the corrosion layer by the peak of Mg 1s at 1303.3eV and that of O 1s at 532.6eV[29].The binding energy signals corresponding to MgO also appeared at 531.5eV in the O 1s spectrum and at 1302.6eV in the Mg 1s spectrum[30].Moreover,notable 2p1/2and 2p3/2peaks of Mn 2p were detected at 653.2 and 641.5eV,respectively,which were related to the formation of Mn oxides/hydroxides[31].The Sn 3d spectrum was dominated by the peaks at 487.1 and 495.5eV,which corresponded to the oxide state of Sn4+[32].It thus can be concluded that the product layer mainly consisted of MgO,Mg(OH)2,Sn-and Mn-oxides/hydroxides,which may become a more effective barrier to restrain Cl¯penetration,thereby improving the stability of the product layer.
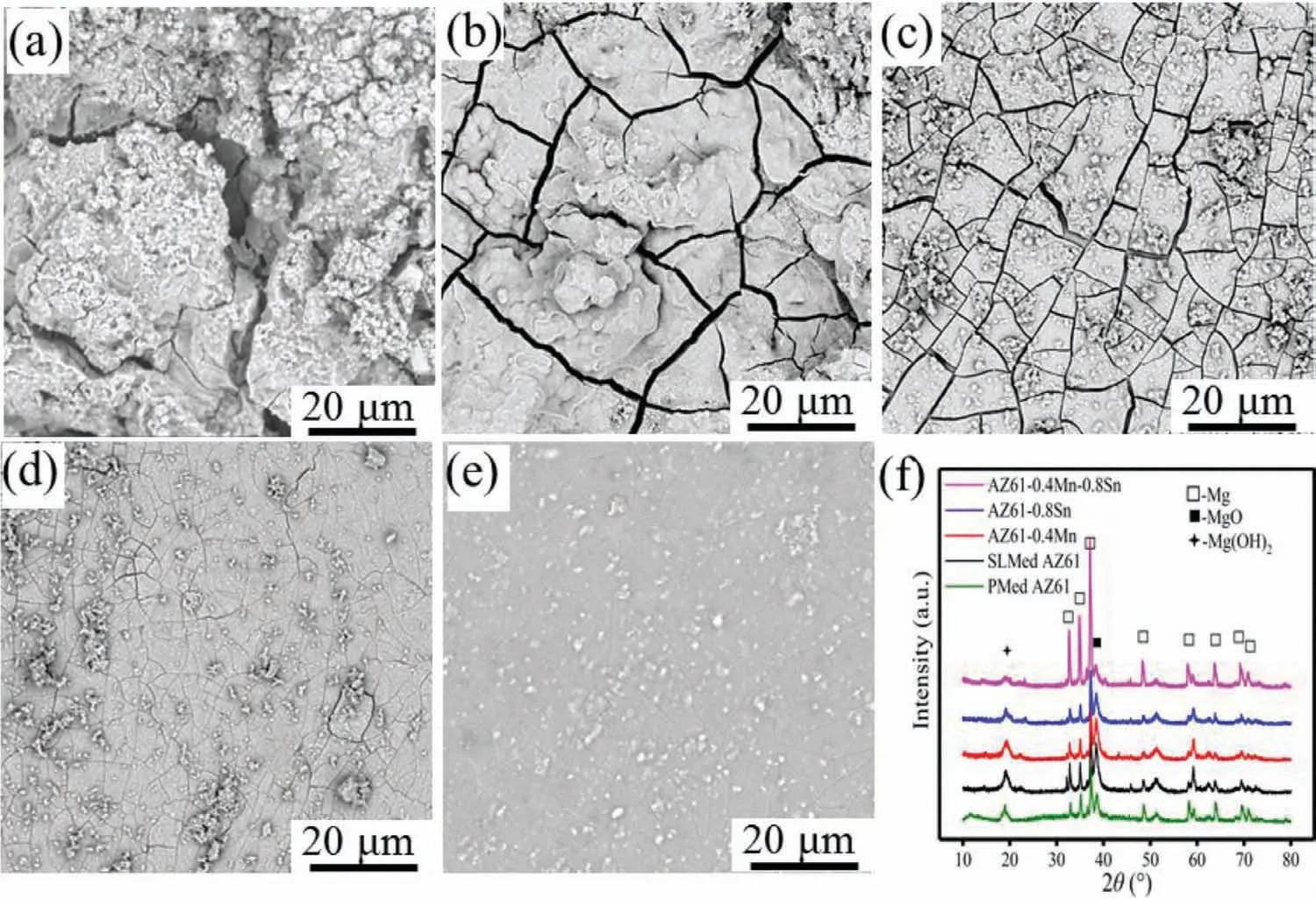
Fig.6.SEM morphologies of(a)PMed AZ61,(b)SLMed AZ61,(c)AZ61–0.4Mn,(d)AZ61–0.8Sn and(e)AZ61–0.4Mn-0.8Sn alloys after immersion for 7 days.(f)XRD patterns of the corrosion products.
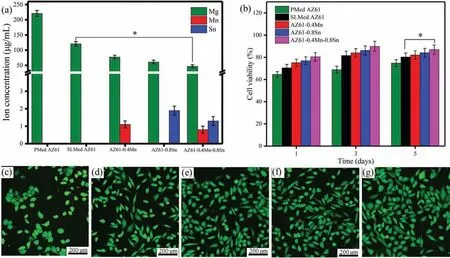
Fig.7.(a)Ion concentrations of Mg,Mn and Sn in the extracts of AZ61-x alloys,(b)The of MG-63 cell viabilities after 1,3 and 5 days’culture,and the resulting fluorescence morphologies of MG-63 cells after 5 days’culture in(c)PMed AZ61,(d)SLMed AZ61,(e)AZ61–0.4Mn,(f)AZ61–0.8Sn and(g)AZ61–0.4Mn-0.8Sn alloy extracts.∗P<0.05.
The SEM morphologies of the AZ61-x alloys after immersion were depicted in Fig.6.After immersion,corrosion products were formed to rebuild different morphologies and the alloy surfaces presented a cracked appearance due to the dehydration of the corrosion layer after drying[33].For PMed AZ61 alloy in Fig.6(a),there were large amounts of degradation products accumulating on the alloy surface.The delamination for the degradation product film was observed on PMed AZ61 alloy.It was seen from Fig.6(b)that SLMed AZ61 alloy also suffered severe corrosion attack with deep and wide cracks as well as many large agglomerated clusters,indicating the presence of thick corrosion product layers.The existence of cracks allowed easier medium invasion into the matrix,thereby accelerating corrosion.By contrast,AZ61–0.4Mn and AZ61–0.8Sn alloys showed more compact and thinner products with smaller cracks,as shown in Figs.6(cd).As for AZ61–0.4Mn-0.8Sn alloy,a relatively intact surface with slight cracks could be observed in Fig.6(e),indicating enhanced surface protection.The phase compositions of the corrosion products were further characterized by XRD in Fig.6(f).The peaks corresponding to MgO and Mg(OH)2were present accompanied by the peaks ofα-Mg in all the alloys.It was interesting to note that the peak intensities of MgO and Mg(OH)2became lower in the alloys with Mn and/or Sn,especially AZ61–0.4Mn-0.8Sn alloy,as compared with AZ61 alloy,suggesting the reduced amounts of corrosion products.Conversely,the peak intensities corresponding to α-Mg in AZ61–0.4Mn-0.8Sn alloy were obviously the highest.This suggested that the corrosion was relatively slighter in comparison with the other three alloys.These results depicted that Mn and Sn retarded the corrosion propagation and endowed AZ61–0.4Mn-0.8Sn alloy with excellent corrosion resistance.
Generally,the degradation of alloys was related to ion release into the physiological environment and a rapid degradation might produce an overburden of metallic ions for the human body[34].In the present work,the released ion concentrations of Mg,Mn and Sn in the AZ61-x alloy extracts for cell culture were measured by ICP-MS and shown in Fig.7(a).The results showed that PMed AZ61 alloy extract possessed a large amount of Mg ion duo to its rapid degradation.In comparison,the Mg ion concentration was significantly reduced in SLMed AZ61 alloy extract.The much lower amounts of Mg ion in the Mn-and/or Sn-containing alloy extracts implied relative better corrosion resistance in the cell culture environment.This was in consistent with the degradation results.Furthermore,the Mg ion concentration in the extract of AZ61–0.4Mn-0.8Sn alloy(46.8±5.0μg/ml)was approximately three times lower than that of AZ61 alloy(120.5±8.0μg/ml)after 72h immersion.In addition,the ion concentrations of Sn and Mn in the extracts were less than 1.9±0.3 and 1.1±0.2μg/ml,respectively.The cytocompatibility of AZ61-x alloys was assessed by examining the viability and morphologies of MG-63 cells.The cell viabilities after 1,3 and 5 days’culture were expressed as the percentage of negative control group(Fig.7b).For PMed AZ61 alloy,the cell viability was only 64.6±2.5% at day 1,68.8±3.3% at day 3 and 74.8±3.1% at day 5.It could be found that the AZ61-x alloys shared a similar trend of cell viability evolution from day 1 till day 5 and the cell viabilities were all in the range of 75%~90%,except for AZ61 alloy at day 1,suggesting very slight cytotoxicity according to ISO 10,993–5:2009[35].Furthermore,MG-63 cells showed a slightly higher viability(P<0.05)in the extract of AZ61–0.4Mn-0.8Sn alloy than that of AZ61 alloy at day 5.The corresponding fluorescence morphologies of MG-63 cells within different extracts were shown in Fig.7(c-g).The fluorescence morphologies of most MG-63 cells within PMed AZ61 alloy extract showed spherical appearance,which could be ascribed to the high osmotic pressure and alkalinity caused by the rapid degradation.In comparison,the cells exhibited healthy cobblestone or polygonal morphologies in all the SLMed alloy extracts,indicating better cytocompatibility.
4.Discussion
Compared with PMed AZ61 alloy,SLMed AZ61 alloy possessed better comprehensive performance(higher degradation resistance,mechanical properties and cytocompatibility).It could be attributed to that the rapid melting and solidification characteristics of SLM,which was a kind of re-melting technology so that the samples with higher density and smaller grains could be prepared.The microstructure analysis after SLM demonstrated that Mn and/or Sn addition altered the microstructural factors of the AZ61-x alloys,including the grain size and the volume of Mg17Al12intermetallic phase.Mn and/or Sn acted as the effective restricting solutes for grain growth which were beneficial for the grain refinement of Mg alloys.The main reason was that Mn and/or Sn could segregate ahead of the S/L interface due to their limited solubility in Mg,which suppressed the growth of the solid phase.Besides,the solute segregation can also improve the degree of constitutional undercooling,thereby increasing the number of nucleation sites by decreasing the critical nucleus size in the melt.Similar refinements have also been reported in the microstructure of as-casting Mg-Zn-Ca-Mn alloy[36]and as-extruded Mg-Sn alloy[37],in which the small size and uniform distribution of intermetallic phases were found to inhibit the growth of recrystallized grains.
The enhanced mechanical properties were mainly ascribed to the refinement of grains,solid solution and dispersed intermetallic particles.Firstly,Mn and Sn atoms partially dissolved into the Mg alloys and induced severe lattice distortion,which can hinder dislocation movement by pinning effect,as pointed out by Li et al.[38].Secondly,the refined and homogenized microstructure arising from Mn and/or Sn addition could also be a reason for the mechanical improvement base on Hall-Petch relationships described by Tan et al.[39].In addition,it is known that the dislocation movement can be hindered by grain boundaries and dislocation tangle.In the present study,the refined microstructure led to more grain boundaries.This could thus effectively impede the deformation and slip of the basal plane under loading.Thirdly,the presence of fine and hard intermetallic particles might also act as strong pinning sites for impeding the dislocation movement.Consequently,the SLMed AZ61–0.4Mn-0.8Sn alloy exhibited enhanced compressive strength and hardness,which were comparable to those of cortical bone(130–180MPa and 95–119 HV,respectively[40]),thereby illustrating its potential application as orthopedic implants.
Both of the immersion and electrochemical tests demonstrated that the addition of Mn and/or Sn could enhance the corrosion resistance of Mg alloys,especially for AZ61–0.4Mn-0.8Sn alloy.To clarify the underlying mechanisms,a schematic diagram of the corrosion process was proposed,as shown in Fig.8.In general,corrosion initiates preferentially from the grain boundaries due to the galvanic couples between the Mg17Al12phase and Mg grains.As the corrosion proceeds,the outside grains of AZ61 alloy may peel from the matrix and a loose Mg(OH)2film rapidly forms on the matrix.The corrosive ions can easily penetrate the loose layer,thereby accelerating the dissolution of Mg matrix or even inducing pitting corrosion.In comparison,the better corrosion resistance of AZ61 alloy with Mn and/or Sn is originated from the relatively refined and homogenized microstructure,which leads to the introduction of more grain boundaries as corrosion barriers.The finer Mg17Al12phase can also slow down the corrosion process due to the reduced area ratio of anode to cathode.Moreover,the corrosion potentials of AZ61 alloy with Mn and/or Sn have been shifted to more positive values compared with that of AZ61 alloy because of the nobler nature of Mn and Sn(standard potentials of−1.18V and+0.15V,respectively)than Mg(standard potential of−2.37V).It has also been reported that Sn is able to restrain the cathodic H2evolution reaction owing to the higher hydrogen overpotential while Mn can act as an impurity-removing agent by forming compounds with the impurities in the alloy,hence contributing to the improved corrosion resistance.In addition,Mg dissolves faster when in contact with the nobler Mn and/or Sn and results in the accumulation of Mnand/or Sn-enriched oxides/hydroxides within the Mg(OH)2layer.Zhen et al.[41]reported that these oxides/hydroxides were thermodynamically stable in much broader pH ranges than Mg(OH)2and can thus stabilize the Mg(OH)2layer and hinder the diffusion of corrosion effectively.Another reason for the high corrosion resistance may be ascribed to the interactions of Mn and/or Sn atoms with harmful Cl−anions,which are expected to slow down the deeper penetration of Cl−into the layer and thereby enhance the surface passivity.In addition to the above positive aspects,it should also be pointed out that Mn and Sn also formed Al8Mn5and Mg2Sn phases,which may introduce more micro-galvanic couples in the alloys.Nevertheless,this is likely to induce slight corrosion due to the small potential differences of the fine Al8Mn5and Mg2Sn phases with respect to Mg matrix.Therefore,the introduction of Mn and/or Sn presents positive dual effects on the corrosion resistance of Mg alloy,despite the galvanic effects between Mg and Al8Mn5/Mg2Sn.
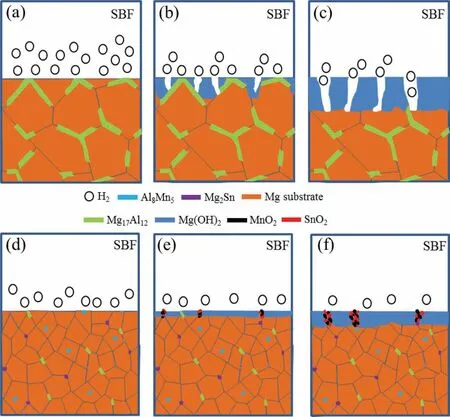
Fig.8.The corrosion Schematic illustrations of(a-c)AZ61 and(d-f)AZ61–0.4Mn-0.8Sn alloys.
Cell culture is a widely used in vitro method to investigate the cell/biomaterial interactions.And the cell responses are related to the ionic concentrations in the extracts[42].In the present work,the ICP-MS results indicated significantly reduced concentrations of Mg ion in the Mn-and/or Sncontaining alloy extracts.The results of CCK-8 tests showed that the cells cultured with the Mn-and/or Sn-containing alloy extracts exhibited relatively better proliferation than that in AZ61 alloy extract.Moreover,all the SLMed alloy extracts showed acceptable cytotoxicity of Grades 0–1[43].The positive cytocompatibility of the SLMed AZ61-x alloys was further confirmed by the healthy elongated and spread morphologies of the cells.Therefore,it can be concluded that the extracts possessed tolerable elemental levels for MG-63 cells,suggesting the promising nature of the SLMed AZ61-Mn-Sn alloys for biomedical applications[44,45].
5.Conclusions
This work was set out to study the dual alloying effects of Mn and/or Sn on the degradation behavior of Mg alloys prepared by SLM.The microstructure,mechanical properties,corrosion behavior and cytocompatibility of the prepared AZ61-x alloys were studied to explore their potential applications as bone implants.
(1)The introduction of Mn and/or Sn was found to refine both the grain structure of the Mg matrix and the intermetallic phases.
(2)The Mn-and/or Sn-containing alloys showed positive shifts in corrosion potential,slower H2evolution rates and lower degradation rates as compared with AZ61 alloy.
(3)The high corrosion resistance was due to both the enhanced protective capability of the surface layer and refined microstructure induced by the dual alloying of Mn and/or Sn.
(4)The AZ61–0.4Mn-0.8Sn alloy presented improved compressive strength(192.8±5.1MPa)and hardness(105±1.4 HV),which were comparable to those of cortical bone.
(5)The SLMed AZ61-x alloys displayed favorable biocompatibility in terms of fluorescence morphologies and cell viability.
Acknowledgments
This study was supported by the following funds:(1)The Natural Science Foundation of China(51705540,51935014,51905553,81871494,81871498);(2)Hunan Provincial Natural Science Foundation of China(2018JJ3671,2019JJ50774,2019JJ50588);(3)JiangXi Provincial Natural Science Foundation of China(20192ACB20005);(4)Guangdong Province Higher Vocational Colleges & Schools Pearl River Scholar Funded Scheme(2018);(5)The Open Sharing Fund for the Large-scale Instruments and Equipments of Central South University;(6)The Project of Hunan Provincial Science and Technology Plan(2017RS3008).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Tailoring the degradation rate of magnesium through biomedical nano-porous titanate coatings
- Modified embedded-atom method interatomic potentials for Mg–Al–Ca and Mg–Al–Zn ternary systems
- The corrosion behavior of Mg–Nd binary alloys in the harsh marine environment
- In vitro and in vivo evaluations of Mg-Zn-Gd alloy membrane on guided bone regeneration for rabbit calvarial defect
- In vitro corrosion resistance,antibacterial activity and cytocompatibility of a layer-by-layer assembled DNA coating on magnesium alloy
- Relieving segregation in twin-roll cast Mg–8Al–2Sn–1Zn alloys via controlled rolling
