In vitro and in vivo evaluations of Mg-Zn-Gd alloy membrane on guided bone regeneration for rabbit calvarial defect
2021-03-10JiwenSiHongzhouShenHongweiMioYunTinHuHungJunShiGungyinYunGuofngShen
Jiwen Si,Hongzhou Shen,Hongwei Mio,Yun Tin,Hu Hung,∗,Jun Shi,∗,Gungyin Yun,Guofng Shen
a Department of Oral and Craniomaxillofacial Surgery,Shanghai Ninth People’s Hospital,College of Stomatology,School of Medicine,Shanghai Jiao Tong University,Shanghai Key Laboratory of Stomatology,National Clinical Research Center for Oral Diseases,Shanghai,200011,China
bNational Engineering Research Center of Light Alloy Net Forming and Key State Laboratory of Metal Matrix Composites,School of Materials Science and
Engineering,Shanghai Jiao Tong University,Shanghai,200240 China
Received 27 May 2020;received in revised form 31 August 2020;accepted 25 September 2020
Available online 5 November 2020
Abstract To develop a biodegradable membrane with guided bone regeneration(GBR),a Mg-2.0Zn-1.0Gd alloy(wt.%,MZG)membrane with Ca-P coating was designed and fabricated in this study.The microstructure,hydrophilicity,in vitro degradation,cytotoxicity,antibacterial effect and in vivo regenerative performance for the membrane with and without Ca-P coating were evaluated.After coating,the membrane exhibited an enhance hydrophilicity and corrosion resistance,showed good in vitro cytocompatibility upon MC3T3E-1 cells,and exhibited excellent antibacterial effect against E.coli,Staphylococcus epidermis and Staphylococcus aureus,simultaneously.In vivo experiment using the rabbit calvarial defect model confirmed that Ca-P coated MZG membrane underwent progressive degradation without inflammatory reaction and significantly improved the new bone formation at both 1.5 and 3 months after the surgery.All the results strongly indicate that MZG with Ca-P coating have great potential for clinical application as GBR membranes.
Keywords:Magnesium alloy membrane;Calcium-phosphate coating;Antibacterial;Biodegradable;Guided bone regeneration.
1.Introduction
The increasing incidence of alveolar bone defects caused by the aging population as well as trauma,tumors and periodontal disease has posed an arduous challenge to dental restoration and periodontal treatment[1].Among the therapeutic approaches,guided bone regeneration(GBR)is considered as the most essential treatment technique to reconstruct alveolar bones,which involve the surgical placement of barrier membranes in order to create and maintain a secluded space to promote osseous regeneration[2,3].Owing to the excellent mechanical strength and space maintenance ability,the non-resorbable e-PTFE and titanium membranes were considered firstly for GBR technique.However,varies kinds of problems such as the high susceptibility to exposure and infection,the need for secondary surgical removal,potential damages to newly formed osseous tissue,increased medication duration and subsequent patient discomfort have always been a concern for clinicians and significantly limited their clinical application[2,4,5].Aiming at addressing these problems,a variety of biodegradable membranous materials have been developed and explored for GBR,such as the collagen,silk,poly(ε-caprolactone),poly(lactic-co-glycolic acid)and PEGylated poly(glycerol sebacate),et al.[5–10].While several inherent disadvantages have been noted with these membranes,including weak mechanical properties,variable degradation rates,undesirable byproducts during degradation and subsequent host immune reactivity[4,5].Given the unmet need in the clinical practice,a GBR membrane that can satisfy the balanced criteria of good biocompatibility,biodegradability,osteoinductivity,cell occlusion,mechanical stability,clinical manageability and space-maintaining ability is still highly desired[2–4].
Magnesium(Mg)alloys,due to their excellent biocompatibility,biodegradability and mechanical properties,have been extensively researched and developed as promising degradable orthopedic implants,coronary artery stents and bone tissue engineering materials in the very recent years[11–17].Unlike traditional non-degradable and degradable materials that are used today,Mg alloys exhibit complete biodegradability while maintaining superior biocompatibility and some positive osseous regenerative effects[17–20].The released Mg ions are known to be actively involved in the integrinmediated cellular adhesion process and significantly increase the attachment,proliferation,alkaline phosphatase activity and osteogenic genes expression of osteoblasts,while decreasing the osteoclast number temporarily in peri–implant bone remodeling[11,13–15,21].Moreover,Mg alloys possess suitable mechanical properties and their tensile strength and elastic modulus are closer to those of human bone,which can eliminate the stress shielding effect[17,18,22,23].Notably,previous in vivo studies have confirmed that Mg alloys as orthopedic screws,tissue stents and bone substitutes exhibited appropriate biocompatibility and bone regenerative performance in various animal models[13,15–17,22–26].Inspired by these outstanding results,it is of great potential that Mg alloys can be a promising alternative membrane for GBR procedure.
However,the major concerns of active corrosion behavior as well as hydrogen gas formation significantly increased the difficulties of obtaining ideal Mg-based GBR membranes[27–29].Thus,appropriate Mg alloy for GBR application is still an open question,which requires coordinated corrosion rates,balanced mechanical and biological properties,proper clinical manageability,and a reproducible and economical fabrication technology.In our previous study,a novel magnesium alloy,Mg-2.0Zn-1.0Gd(wt.%,denoted as MZG hereafter)alloy has been fabricated with refined grain size,satisfactory mechanical properties and corrosion resistance[17].This new MZG alloy maybe suitable for GBR surgery.In this case,a biodegradable MZG membrane with calciumphosphate(Ca-P)coating was fabricated,in order to investigated unveil its applicability for GBR.The physiobiological properties including microstructure,hydrophilicity,degradation profile,cytotoxicity and antibacterial effect of this novel MZG membrane with and without Ca-P coated were investigated in vitro.Furthermore,the in vivo regenerative performance of the Ca-P coated MZG membrane were evaluated using a critical-sized rabbit calvarial defect model,and comparing with the calcium phosphate cement(CPC).
2.Materials and methods
2.1.Fabrication of the novel MZG membrane
The MZG alloy was produced by melting high purity Mg(99.99%),high purity Zn(99.995%),and Mg-30wt.%Gd master alloy as we previously described[17].The chemical composition of the alloy was determined by inductively coupled plasma(ICP-AES,iCAP6300,USA)analyzer and the result was Mg-2.33Zn-0.84Gd(the impurity content of Fe,Ni and Mn is 103ppm,5ppm and 51ppm respectively).The ingots used for extrusion were machined into billets with a dimension of 60mm in diameter and 60mm in height,and then solid solution treated at 470 °C for 16 h.All the billets were preheated at 250 °C for 1 hour and then extruded at this temperature with an extrusion ratio of 9:1.In addition,the extrusion speed was about 2mm/s.After that,the as-extruded rod was machined into billets with a dimension 20mm in diameter and 30mm in height,preheated at 300 °C for 1 hour and then extruded at this temperature with an extrusion ratio of 7:1.A thick plate with a dimension of 3mm in thickness was obtained,then the plate was rolled to 0.6mm in thickness after totally 6 pass rolling with a reduction of 20%-30% per pass,the rolling was conducted at 350 °C with 10min annealing between each pass.Naked MZG membrane samples were obtained by machining through cut and drill.Then samples were polished up to 6000 grid paper,ultrasonically cleaned in acetone and alcohol for 10min,respectively.For Ca-P coating treatment,the samples were first immersed in 40% HF for 8 h,then samples were washed with deionized water and alcohol,followed by being immersed in the mixed solution of NaNO3,Ca(H2PO4)2•H2O and H2O2for 24 h.Samples were washed with deionized water and alcohol respectively,and then dried in warm air.The schematic fabrication process of the novel Ca-P coated MZG membrane was presented in Fig.1.
2.2.Microstructure and hydrophilicity of the MZG membrane
The surface microstructure of MZG membrane with and without Ca-P coating were observed by scanning electron microscope(SEM)equipped with an energy dispersive Xray spectroscopy(EDS).Tensile test was carried out on a Zwick/Roell Z100 testing machine at room temperature with an initial strain rate of 1×10−3/s.The angle of contact experiment was conducted with a JC2000D1 angle-measuring device.Deionized water was used as the test liquid and at least 3 drops were measured for each test specimen.
2.3.In vitro degradation tests
The in vitro degradation behavior of MZG membrane with and without Ca-P coating were tested by hydrogen evolution method.Square samples for immersion test with a dimension of 10mm in diameter and 0.6mm in thickness were cut by an electric-sparking wire-cutting machine,grinded to 3000grit paper and then immersed in Hank’s solution for 10 days as we previously described[28].The ratio of solution volume to the specimen surface area is 60ml/cm2according to ASTM G31–72.The temperature was kept at 37±0.5 °C during experiment and the immersion solution was renewed every 24 h in order to keep a relatively stable pH value.Hydrogen volume was recorded every 12 h before renewing the immersion solution.At least three samples were tested at each state.The corrosion rate of the alloy was calculated by the following formula according to ASTM-G31–72[28]:

Fig.1.Schematic fabrication process of the novel MZG membrane.

2.4.In vitro cytotoxicity tests
The in vitro cytotoxicity was characterized by indirect contact method[17,18].Extraction medium of MZG membrane with and without Ca-P coating were prepared by using growth medium consisting of alpha-modified Eagle’s medium(α-MEM,Gibco,USA)supplemented with 10% fetal bovine serum and 1% penicillin &streptomycin(Gibco,USA),with the sample surface area to solution media ratio of 1.25 cm2/ml according to ISO 10,993–5.After 72 h incubation at 37 °C,the extraction media was diluted to 100%,50% and 10%concentration.Osteoblastic cells MC3T3-E1(Cell Bank,Chinese Academy of Sciences)were seeded in a 96 well plate at 1×104cells/ml,100μl for each well,and cultured in α-MEM,supplemented with 10% fetal bovine serum(FBS,Gibco,USA)and 1% penicillin &streptomycin at 37 °C for 24 h to allow cellular attachment.Then,the growth medium was replaced by 100μl extraction medium,while the control group was still culture in the growth medium.After 1-and 3-days culture,the medium was replaced by 100μl Cell Counting Kit-8(CCK-8,Beyotime Biotech,China)solution(a ratio of 1/10 inα-MEM).Relative cellular viability was calculated and three samples were tested in each group.After 3-days culture,the cells in extraction medium were gently rinsed with PBS and counterstained with DAPI(1:1000,Beyotime,China).The fluorescence images were taken separately to confirm the existence of cells using a fluorescence microscope(DP72,Olympus,Japan).
2.5.In vitro antibacterial effect of the MZG membrane
E.coli(E.coli,ATCC 25,922),Staphylococcus epidermidis(S.epidermidis,ATCC 35,984)and Staphylococcus aureus(S.aureus,ATCC 43,300)were employed to investigate the in vitro antibacterial effect of MZG membrane with and without Ca-P coating as we previously described[30].Each strain was cultured and expanded on sheep blood agar(SBA)plates at 37 °C for 24 h.A single colony of each strain was collected and cultured on the tryptic soy broth(TSB)plates overnight in a sharking incubator at 37 °C.Afterwards,the inocula of each strain were subjected to 10-fold gradient dilution into 1×106colony forming units(CFUs)/ml in TSB.3ml bacteria suspension(1×106CFUs/ml)was added into a 12-well plate containing sample and incubated at 37 °C for 1-and 3-days.3ml bacterial suspension were cultured without sample and taken as the control group.At each time point,the samples with the adhered bacteria in each group were transferred into a new 12-well plate,gently rinsed with PBS and stained with LIVE/DEAD BacLight bacteria viability kits(Invitrogen,China)according to the manufacturer’s instruction.The fluorescence images of the remaining adhered bacteria in each group were taken separately by confocal laser scanning microscopy(CLSM).At each time point,the planktonic bacteria in the culture medium in each group were collected,counted and were serially diluted 10-fold,plated in triplicate onto SBA and incubated at 37 °C for 24h by the spread plate method.Then,the numbers of CFUs of each group on the SBA were counted and the antibacterial rates of both Ca-P coated MZG and MZG samples for planktonic bacteria were calculated as we previously described[30]:Antibacterial rate(%)=(B−A)/(B)×100%
A:number of CFUs derived from the culture medium of both Ca-P coated MZG and MZG group;B:number of CFUs derived from the culture medium of control group.
2.6.In vivo bone regenerative performance of the MZG membrane
2.6.1.Animal model and surgical procedure
The animal management,surgical procedure and study protocol was approved by institutional animal research ethics committee and conformed to the recommended guidelines.In brief,48 male New Zealand white rabbits weighing 3–3.5kg and aged 16–20 weeks were used in this study.All animals were housed individually in a temperature-,light-,and humidity-controlled environment.Before the operation,each animal received general anesthesia with an intramuscular injection of the combination of ketamine(35mg/kg)and xylazine(5mg/kg).Then,the head of each animal was carefully shaved,and disinfected with betadine and alcohol.A 5cm longitudinal full-thickness dermal-periosteal incision was made along the midline of the cranium from the frontal bone to the occipital bone to expose the entire calvarium.After the skin and periosteum were retracted,two symmetrical,full-thickness critical-sized calvarial bone defects(10mm in diameter)were carefully created on both sides of the cranium midline with a bone trephine bur(Dremel,USA)under constant sterile saline irrigation.After adequate hemostasis,the bone defects were randomly assigned to the following 4 groups:empty control(Control group),restoration with calcium phosphate cement(CPC)bone grafts(CPC group),coverage with Ca-P coated MZG membrane(Ca-P-MZG group),restoration with CPC bone grafts and coverage with coated MZG membrane(Ca-P-MZG-CPC group).The soft tissues were then repositioned and sutured to achieve primary closure.After surgery,the animals were given antibiotics by intramuscular injection two times daily for two days.Animals in each group(n=6 at each time point)were sacrificed at 1.5 and 3 months after the surgery under deep anesthesia.After sacrifice of the animals,the calvarial bone was carefully excised,cleaned and fixed in 10% neutral buffered formalin in separate labeled vials for quantitative micro-CT analysis and histological analysis.
2.6.2.Micro-CT analysis
But Big Lion kept all the choice bits for himself, and only gave away the little scraps30 that he did not care about eating; and the little hare grew very angry, and determined31 to have his revenge
Samples in each group at each time point were firstly proceeded for micro-CT analysis.Images were captured using micro-CT(Skyscan 1172,Bruker,Germany)with a voxel size of 9μm/pixel and reconstructed into 3D models from the resulting set of scans.The region of interest(ROI)was defined as a cylinder with 10mm in diameter and a height that could cover the entire thickness of the calvarial defect.The new formed bone volume(BV)and intertrabecular space(Tb.Sp)within the defect were calculated by using its auxiliary software.
2.6.3.Histological analysis
Three samples of the animals in each group at each time point were decalcified in 14% EDTA for 4 weeks,dehydrated in ascending grades of ethanol,embedded in paraffin,sectioned at the center of the original surgical defect(5μm thickness),stained with hematoxylin and eosin(HE)and submitted to microscopic examination.Images of three random sections from each sample were acquired and digitized using a light microscope(Axio Scope A1,Zeiss,Germany)with a digital camera(SPOT Flex,SPOT,USA).Image analysis was performed to evaluate the inflammatory infiltration and new bone formation.For evaluation of the inflammatory infiltration,the inflammatory tissue response scores(ITRS)were used to grade the inflammatory response of each section on a scale of 0–4,with 0 representing no inflammatory cells,1 representing a minimal number of inflammatory cells and 4 representing a very large number of inflammatory cells.For semi-quantification of new bone formation within the defect,area of newly formed bone within the defect and area of total defect were measured in the sections.Subsequently,the new bone formation was calculated as the dividing of newlyformed-bone area by the total-defect area and expressed as a percentage(percentage of bone fill,PBF).To further investigate the in vivo degradation behavior of the Ca-P coated MZG membrane,3 samples of the animals in each group at each time point were fixed in gradient ethanol for dehydration for 14 days and then embedded in polymethylmethacrylate.For histological examination,50μm thick sections were prepared and stained by hard tissue HE staining.
2.7.Statistical analysis
All data were expressed as mean±standard deviation and analyzed using one-way analysis of variance(ANOVA)followed by Turkey’s test.Statistical significance was represented as★(p<0.01).
3.Results and discussion
3.1.Microstructure,hydrophilicity and in vitro degradation of MZG membrane
Fig.2 shows the surface SEM images of the MZG membranes with and without coating.Secondary phases(white particles)distribution is comparatively uniform after extrusion and rolling due to the severe plastic deformation,as showed in Fig.2(A).Usually,homogeneously microstructure contributes to excellent mechanical properties and uniform degradation behavior.The mechanical properties of the studied Mg-2.0Zn-1.0Gd alloy is also shown in Fig.2(A),the yield strength(YS),ultimate strength(UTS)and elongation is 150MPa,235MPa,and 22.5% respectively,which should satisfy the requirements for application.To restrain the rapid degradation rate,a Ca-P coating was deposited on the surface of MZG membrane.The Ca-P coating exhibits a dense and uniform structure,as showed in Fig 2(B).The energy dispersive spectrometer(EDS)result of the coating was shown in the insert,which confirming the coating is Ca-P similar to previous reported[18,23].Contact angle experiment was conducted in order to measure the hydrophilicity and confirm the coating effect;the results were shown in Fig.3.The contact angle is about 116° between MZG sample and deionized water,while this value for Ca-P coated MZG sample is about 39° Hence,the contact angle between sample and deionized water decreased obviously after coating,which is beneficial to the initial attachment of the cells[31,32].
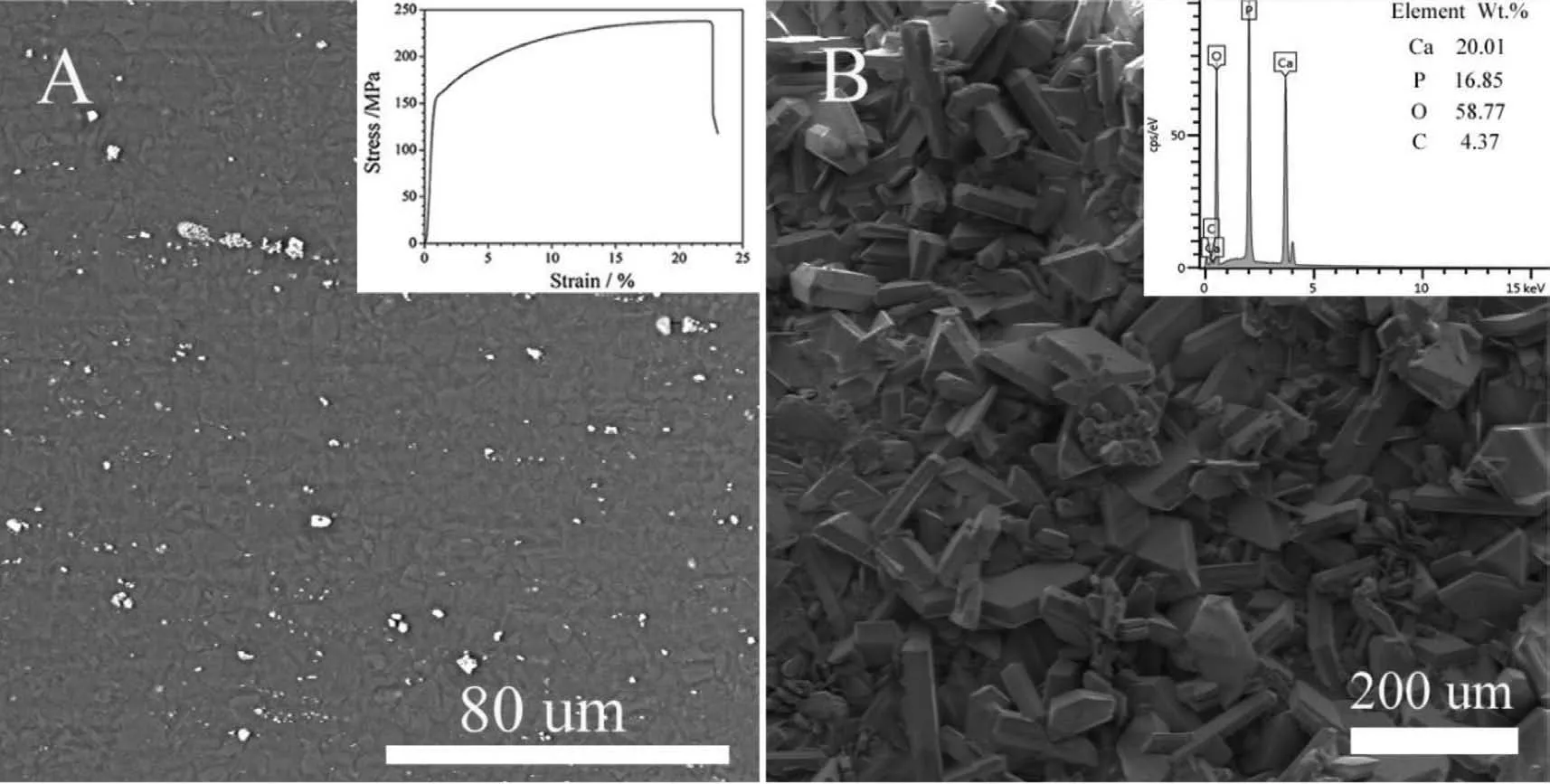
Fig.2.Surface SEM images of the(A)MZG and(B)Ca-P coated MZG membranes.The inset images are room temperature tensile curve of the studied alloy and SEM-EDS result of the Ca-P coating,respectively.

Fig.3.Contact angle experiment results.(A)MZG,(B)Ca-P coated MZG,(C)measured contact angle from(A)and(B).

Fig.4.In vitro degradation test of MZG membrane with and without coating.(A)Hydrogen evolution volume vs immersion time,(B)calculated corrosion rate according to(A).

Fig.5.In vitro cytotoxicity assessment of MZG and Ca-P coated MZG membrane.(A)relative cellular viability of both MZG and Ca-P coated MZG group at day1 and day3(The star★ represents statistical significance,p<0.01);(B)Fluorescence images of DAPI-stained cells of both MZG and Ca-P coated MZG group at day 3.The blue fluorescence refers to cell nuclear.
Since the critical issue for biomedical Mg alloys is to retard the corrosion rate,in this study,in vitro hydrogen evolution test was used for evaluating the dynamic degradation rate of the MZG membrane with and without coating.As showed in Fig 4,the Ca-P coated MZG group exhibits an in vitro degradation rate of 0.13mm/year,which is much lower than that of MZG group(0.26mm/year),indicating a good protection effect of the Ca-P coating.Hence,the degradation process was significantly inhibited due to the existence of Ca-P coating.This positive protective effect could also be confirmed in previous in vivo and in vitro studies,which showed that the Ca-P coating produced by chemical reaction method could significantly reduce the degradation rate of Mg alloy[18,23].
3.2.In vitro cytotoxicity and antibacterial effect of MZG membrane

Fig.6.In vitro antibacterial effect assessment of MZG and Ca-P coated MZG membrane.(A)Fluorescence images of adhered E.coli,S.epidermidis and S.aureus strains on the surface of culture-plate(Control),MZG and Ca-P coated MZG samples after 1-and 3-days culture(scale bar:100μm);(B)Antibacterial rates of both MZG and Ca-P coated MZG samples for planktonic E.coli,S.epidermidis and S.aureus strains in the medium after 1-and 3-days culture.
As shown in Fig.5(A),the relative cellular viability of MZG membrane with and without coating group maintains stable after 1-and 3-days culture with 50% and 10% extracts,while cellular viability of MZG group exhibits a significant drop after 1-and 3-days culture with 100% extracts due to the high pH value and osmolality[17,28].Moreover,a significant decrease of DAPI-stained cells in MZG group after 3-days culture with 100% extracts can be observed in Fig.5(B),indicating that Ca-P coating significantly reduced the in vitro cytotoxicity by reducing the metal ion content.Our result was in accordance with previous studies,that various biomimetic coatings can effectively improve the initial biocompatibility and degradation behavior of Mg alloy by reducing exposure to the corrosive environment[12,19,20,24,26,33].
One of the main factors that related to unsuccessful bone regeneration treatment is the adherence and proliferation of bacteria on GBR membrane,which may lead to postoperative infection and implants exposure[34–36].As showed in Fig.6(A),the adhered bacteria on the surface of cultureplate(Control),MZG and Ca-P coated MZG samples after 1-and 3-days culture were successfully stained with the LIVE/DEAD bacteria viability kit.The green fluorescence referred to viable bacteria with intact cell membrane,while the red fluorescence referred to nonviable bacteria with damaged membranes.Specifically,at day 1 and day 3,an intense green fluorescence can be observed in the control group,indicating severe bacteria adhesion and significant biofilm formation.An apparently weak green fluorescence mixed with red fluorescence is observed on the surfaces of MZG and Ca-P coated MZG samples at day 1,indicating less amount of bacteria adhesion with a high proportion of dead bacteria and no biofilm formation.An intense red fluorescence mixed with sparsely distributed green fluorescence is observed on the surfaces of MZG and Ca-P coated MZG samples at day 3,indicating barely bacteria adhesion and progressive bacterial death upon the material.Moreover,both MZG and Ca-P coated MZG samples can also effectively suppress planktonic bacteria in the medium.As shown in Fig.6(B),the antibacterial rates of the MZG samples are 65.56±4.46% and 87.19±3.57% at day 1 and 3 for E.coli,62.76±6.26% and 90.4±5.3% for S.epidermidis,71.6±3.01% and 93.56±3.17% for S.aureus,while the antibacterial rates of the Ca-P coated MZG samples are 63.69±5.78% and 85.87±1.38% at day 1 and 3 for E.coli,56.82±5.92% and 81.88±3.83% for S.epidermidis,65.25±5.38% and 81.07±3.89% for S.aureus,respectively.Taken all together,both MZG and Ca-P coated MZG samples exhibited superior antibacterial capability against E.coli,S.epidermidis and S.aureus strains.This results is supported by our and other’s previous studies,which showed that Mg and Zn alloys exhibited significant antibacterial effect on adhering bacteria as well as planktonic bacteria[29,30,37–39].

Fig.7.Micro-CT overview and parameters quantification of the critical-sized rabbit calvarial defect.(A)3D reconstruction and coronal scan image of the new bone formation in the calvarial defect at 1.5-and 3-month after the surgery(white arrow represents residual Ca-P coated MZG membrane at 1.5-month after the surgery);(B)micro-CT parameters quantification of BV and Tb.Sp in the calvarial defect at 1.5-and 3-month after the surgery(The star★represents statistical significance,p<0.01).
3.3.In vivo bone regenerative performance of MZG membrane
By using the critical-sized rabbit calvarial defect model,the effect of MZG membrane on the in vivo bone healing process was studied.During the experiment,all animals remained in good health and achieved uneventful healing.No prominent signs of inflammation,allergic reactions or other complications around the surgical site were observed postoperatively in all groups throughout the experimental periods.As shown in Fig.7(A),the three-dimensional micro-CT examination provided a complete overview of the bone defect healing process in all groups.In details,the Control group showed the slowest and minimal bone formation and remained large bone defect at 3-month after the surgery,while a significantly higher amount of new bone formation in all the three experimental groups was observed at both 1.5 and 3 months after the surgery.Moreover,both Ca-P-MZG-CPC and CPC groups yielded prominent new bone formation compared with the other two groups.As showed in Fig.7(B),micro-CT parameters quantification further demonstrated a significantly higher BV and lower Tb.Sp in Ca-P-MZG-CPC and CPC groups compared with the rest of the groups at both 1.5 and 3 months after the surgery,indicating a denser and more mature mineralized matrix deposited in the defect.Notably,the Ca-P-MZG group displayed a higher BV compared to the control group at both 1.5 and 3 months after the surgery,while the Ca-P-MZG-CPC group displayed the highest BV value compared to all the other groups at 3 months after the surgery,which further indicated the superior osteoinductivity of the Ca-P coated MZG membranes and the most beneficial effect on bone regeneration of the combined application of the Ca-P coated MZG membrane and CPC grafting material.
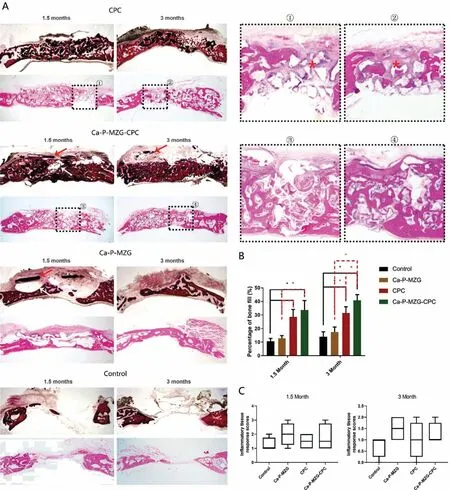
Fig.8.Hard-tissue and decalcified tissue HE staining of the critical-sized rabbit calvarial defect and histomorphometric quantitative analysis.(A)Hard-tissue and decalcified tissue HE staining of the new bone formation in the calvarial defect at 1.5-and 3-month after the surgery(white arrow represents gas formation released from the Ca-P coated MZG membrane;red arrow represents residual Ca-P coated MZG membrane;frames 1○-4○represent detail views of decalcified tissue HE staining of sections in Ca-P-MZG-CPC and CPC groups;red∗represents interrupted new bone formation and occupation of fibrous tissues within the defect in CPC groups);(B)Histomorphometric quantitative analysis of new bone formation in the calvarial defect at 1.5-and 3-month after the surgery(The star★represents statistical significance,p<0.01);(C)ITRS scores at 1.5-and 3-month after the surgery.
To confirm the results of micro-CT analysis,hard-tissue and decalcified tissue HE staining of the sections in each group was observed under the light microscope.As shown in Fig.8(A),a clear sign of progressive new bone deposition in the defect was shown in each group,while a significantly more bone formation was displayed in all experimental groups than in control groups at both 1.5 and 3 months after the surgery.In details,at 1.5 months after the surgery,newly formed immature mineralized matrix,lined by a continuous layer of osteoblasts,was observed in all the experimental groups.In both Ca-P-MZG-CPC and CPC groups some island-like bone formation was observed within interspace of the CPC material,while new bone was only formed at the base of the defect in Ca-P-MZG group.At 3 months after the surgery,further bone regeneration and a more intense of new bone formation were observed in all the experimental groups.Notably,a larger and continuous mature bone structure featured with a cell-enriched area resembling bone marrow was only observed in the Ca-P-MZG-CPC groups,while the CPC groups showed interrupted new bone formation and occupation of fibrous tissues within defect(Fig.8A,detail view 1○2○3○4○).In agreement with the results of micro-CT parameters quantification,histomorphometric quantitative comparison of the percentages of new bone filled in the defect showed that the Ca-P-MZG-CPC and CPC groups achieved statistically the largest amount of new bone formation at both 1.5 and 3 months after the surgery compared with the other two groups(Fig.8B).Notably,the Ca-P-MZG-CPC group displayed the highest amount of new bone formation compared to all the other groups at 3 months after the surgery.In addition,the healing response to the implanted materials was assessed by the ITRS scale(Fig.8C).All the implanted materials were tolerated well by the surrounding soft tissues,with no evidence of necrosis or severe inflammatory infiltration.No significant difference was observed among the groups at 1.5 and 3 months after the surgery,indicating superior biocompatibility of both Ca-P coated MZG membranes and CPC grafting materials.
The in vivo results indicated that Ca-P coated MZG membrane combined with CPC materials significantly improved both the new bone formation and bone remodeling process.Moreover,the application of Ca-P coated MZG membrane alone significantly increased the volume of the regenerated bone comparing to the blank control,indicating a superior osteoinductivity of the novel Ca-P coated MZG membrane.Notably,this phenomenon was also reported by Kitayama et al.,Humber et al.,Gabriel et al.,and Park et al.[40–43].All these studies suggested that the presence of the Ca-P coated MZG membrane in the grafted or even ungrafted bone defects may exert the cell-occlusive function and significantly promote the bone healing process,resulting in higher amount of new bone formation,advanced bone remodeling and low proportions of immature tissue.This bone regenerative effect may also come from the crucial role of Mg ions on bone health and bone healing.According to previous studies,the Mg ions were shown to promote the in vivo bone formation and in vitro osteogenic differentiation of periosteum-derived stem cells,bone marrow-derived stem cells and MC3T3-E1 cells et al.The Mg ions are known to enhance integrin-mediated cellular adhesion via ligand binding to integrin and promote bone regeneration through the activation of the canonical Wnt signaling pathway[11,13–17,21–26].Moreover,the regenerative effect of Mg ions may also involve its beneficial role on osteoclast activity and bone remodeling[44–46].
However,the concerns of active corrosion behavior,hydrogen gas formation and alkalization and potential cytotoxicity of Mg-based alloys,which may cause local tissue inflammation,rapid loss of mechanical integrity or even hindrance of bone regeneration,are the major drawbacks for further clinical application[18,25,27–29,46].With regards to corrosion protection and biocompatibility improvement of Mg alloys,the most utilized approaches are divided into addition of rare earth alloying elements and formation of protective coating to isolate the Mg matrix from corrosive environment[12,16,17,19,20,23].In this study,both solutions have been used.Briefly,Mg has been alloyed with Gd and Zn,while a Ca-P coating was deposited on the surface of MZG membrane to improve its biocompatibility,mechanical strength and corrosion resistance[17,18,23,28].Also,gas bubbles generated from the degradation of MZG membrane were still observed in both Ca-P-MZG and Ca-P-MZG-CPC groups at 1.5 months after the surgery(Fig.8A).In the Ca-P-MZG group,the newly formed bone was even partially separated from the Ca-P coated MZG membrane by gas bubbles.However,no gas bubbles were observed at 3 months after the surgery,while more new bones were formed in the defect.Notably,the micro-CT and histological examination both confirmed the proper degradation behavior of the Ca-P coated MZG membrane,which was in harmony with the new bone formation of the rabbit calvarial defect.Therefore,our results effectively confirmed the positive impact of Ca-P coated MZG membrane on the in vivo bone defect healing process.
4.Conclusion
The mechanical,physiochemical,biodegradable,biocompatible and antibacterial properties of MZG membrane with and without Ca-P coating were evaluated in this study.Then,MZG membrane with Ca-P coating was selected for in vivo tests and compared to the CPC.The following results can be drawn:
(1)The studied MZG membrane with and without Ca-P coating possess excellent mechanical properties and good cytocompatibility,exhibit promising prospect for application in GBR membrane.
(2)In vitro degradable rate for MZG membrane is about 0.26mm/year.Ca-P coating used in this study could decrease the rate effectively.Nevertheless,unsatisfying early gas formation have been observed in vivo study.
(3)Comparison of the performances of the membranes used in this study,restoration with CPC bone grafts combined with coverage with coated MZG membrane(Ca-P-MZG-CPC group)is best.
Declaration of Competing Interest
The authors declare no competing financial interest.
Acknowledgements
This work was supported by National Natural Science Foundation of China(No.81600827,No.U1804251,No.81600827 and No.51971134),the National Key R&D program of China(No.2016YFC1102103),the Science and Technology Commission of Shanghai(18441908000),and Shanghai Jiao Tong University Biomedical Engineering Research Fund(YG2019ZDA02).Dr.Jiawen Si wants to thank his wife Qifan Hu and daughter Jinnuo Si for their support,care and love over the past years,and say“thank god for sending you to me on angel’s wings”.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Tailoring the degradation rate of magnesium through biomedical nano-porous titanate coatings
- Modified embedded-atom method interatomic potentials for Mg–Al–Ca and Mg–Al–Zn ternary systems
- Dual alloying improves the corrosion resistance of biodegradable Mg alloys prepared by selective laser melting
- The corrosion behavior of Mg–Nd binary alloys in the harsh marine environment
- In vitro corrosion resistance,antibacterial activity and cytocompatibility of a layer-by-layer assembled DNA coating on magnesium alloy
- Relieving segregation in twin-roll cast Mg–8Al–2Sn–1Zn alloys via controlled rolling
