The corrosion behavior of Mg–Nd binary alloys in the harsh marine environment
2021-03-10QuantongJiangDongzhuLuNingWangXiutongWangJieZhangJizhouDuanBaorongHou
Quantong Jiang ,Dongzhu LuNing WangXiutong WangJie Zhang ,Jizhou DuanBaorong Hou
a CAS Key Laboratory of Marine Environmental Corrosion and Bio-fouling,Institute of Oceanology,Chinese Academy of Sciences,No.7 Nanhai Road,Qingdao 266071,China
b Open Studio for Marine Corrosion and Protection,Qingdao National Laboratory for Marine Science and Technology,No.1 Wenhai Road,Qingdao 266235,China
c Center for Ocean Mega-Science,Chinese Academy of Sciences,No.7 Nanhai Road,Qingdao 266071,China
Received 16 September 2019;received in revised form 17 December 2019;accepted 26 December 2019
Available online 7 August 2020
Abstract The corrosion behavior of Mg-Nd binary alloys in the harsh South China Sea environment was researched by scanning electron microscopy,energy-dispersive spectrometry and X-ray diffraction analysis.In order to explain the corrosion mechanism,corrosion resistance was analyzed by weight loss rate and electrochemical measurement in the laboratory.With a continuous enlargement of Nd-content,Mg12Nd phases increased and multiplied.The weight loss rate of Mg-0.5Nd alloy was 0.0436 mg•cm−2•y−1(0.0837 mm•y−1),whereas that of Mg-1.5Nd alloy was 0.0294 mg•cm−2•y−1(0.0517 mm•y−1)during the exposure corrosion in the South China Sea site.The mechanical strength of Mg-1.5Nd alloy was 148MPa before the exposure in the harsh marine environment,while the residual mechanical strength was merely about 94MPa after the exposure test.Both Mg-1.5Nd alloy and Mg-1.0Nd alloy occurred the brittle fracture,which resulted that the elongation was nearly equal to zero.The self-corrosion current density demonstrated that degradation rate of Mg-Nd binary alloys was as follows:Mg-0.5Nd>Mg-1.0Nd>Mg-1.5Nd.For the South China Sea corrosion site,a large amount of sea salts exited in the atmospheric environment.Due to the heavy rainfall and high humidity,sodium chloride in the atmospheric environment dissolved,more serious electrochemical corrosion occurred on the surface of Mg-Nd binary alloys.
Keywords:Magnesium alloy;Corrosion;South China sea environment.
1.Introduction
Magnesium alloys are the lightest metallic materials,which have many excellent properties,including high specific strength,high specific stiffness,favorable creep resistance,excellent damping performance and heat-conducting property[1–3].Due to the requirement of lightweight,magnesium alloys have been widely used in the transportation,weapons and aerospace fields,especially magnesium alloys contained rare earths(Mg-RE alloys)[4].With the rapid development of ocean exploration in the world,magnesium structural materials are gradually served in typical marine environment.Therefore,corrosion resistance of Mg-RE alloys become a prominent research issue in the typical marine atmospheric environment[5–7].
Neodymium is the common alloying element for magnesium alloy,which can improve the mechanical strength by refining grain ofα-Mg[8,9].With the addition of Nd to the magnesium alloys,creep resistance and fatigue performance also both have an effective improvement in the room temperature and high temperature conditions[10,11].Moreover,Neodymium element can improve the corrosion resistance of magnesium alloys[12–14].Therefore,the corrosion behavior of magnesium alloys contained Neodymium contents have been studied by many researchers in the recent years.Birbilis et al.[15]researched the corrosion resistance of Mg-La,Mg-Ce and Mg-Nd binary alloys,and found that additions of RE-contents resulted an enlargement of corrosion rate.Mirza et al.[16]studied the microstructure and performance of Mg-Nd-Zn-Zr alloy,and they found that the uniform and stable Mg12Nd precipitated phases partly improved corrosion resistance because of grain refinement mechanism.Williams et al.[17]studied the addition from 0.47 to 3.53wt.% of Nd element in the magnesium alloys,and they drew a conclusion that Nd-rich area occurred the cathodic activation,which led to a serious localized corrosion on the surface of Mg-Nd alloys.Zucchi et al.[18]used electrochemical tests to analyzed the corrosion behavior of WE43 magnesium alloy.They found that the Yttrium and Neodymium elements caused the improvement of Mg anode activity.Jiang et al.[19-21]explored the dual role of precipitated phases in Mg-Y-Nd ternary alloys system,which mainly depended on their distribution and type.The weight loss rate and electrochemical corrosion were also analyzed in Mg-Y-Nd ternary alloys system.Ma et al.[22]researched the precipitated phases and corrosion resistance of Mg-5Y-1.5Nd alloy after heat treatment,and obtained the conclusion that micro-galvanic reaction between cathodic and anodic had been changed by solution and age heat treatment.However,the research on corrosion behavior of Mg-Nd alloys in the typical marine atmospheric environment is rarely reported[23],especially in the harsh South China Sea,which is a specific high temperature,high humidity,high sea salts and high ultraviolet radiation environment.
In this work,the corrosion behavior of Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys in the harsh South China Sea environment was researched.The corrosion resistance was analyzed by weight loss rate and electrochemical measurement in the laboratory.The microstructure was investigated in order to explain the corrosion behavior and corrosion characteristics.The requirement of materials corrosion testing in the marine environment was also analyzed from the aspects of the ocean exploited and military applications.
2.Experimental
2.1.Material preparation
A steel crucible was used to smelted the Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys,which were heated outside by an electrical resistance furnace.The magnesium alloys were protected by proportional argon and tetrafluoroethane gas during the process of smelting.The commercial purity Mg(>99.95%)and purity Nd(99.9%)were used to prepare Mg-Nd binary alloys.The upper smelting temperature should not exceed 850 °C.The casting temperature of Mg-Nd binary alloys was about 720�750 °C.The nominal composition of the binary alloys was Mg-0.5Nd,Mg-1.0Nd andMg-1.5Nd,respectively.All of the exposure test samples in the harsh South China Sea environment had dimensions of 5cm×5cm×0.5cm.All of the microstructural analysis and electrochemical corrosion samples in the laboratory environment had dimensions of 1cm×1cm×1cm.
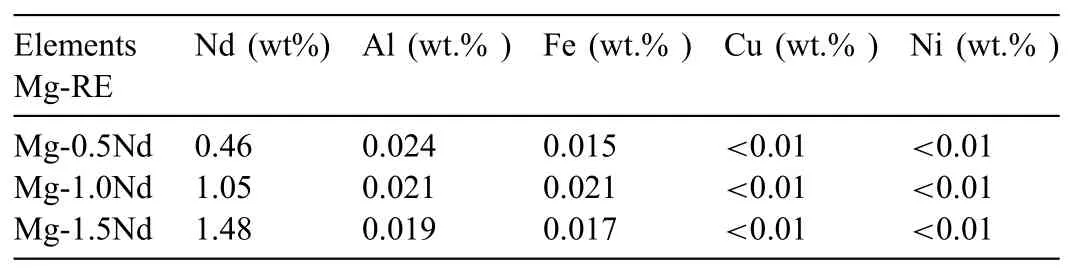
Table 1The actual compositions of Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys.
2.2.Material characterization
The actual compositions of Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys were analyzed via inductively coupled plasma atomic emission spectrometry.The detection results were shown in Table 1.
To conduct microstructural analysis,samples were prepared by polishing and etching in a weak solution of nitric acid and alcohol.The ethanol was used to clean the surfaces of samples.The microstructure,precipitated phases and surface morphology after corrosion were all measured by SEM(JSF-6700F)and EDS(INCA).The characteristic peaks of Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys were tested by XRD(D/Max 2550),whereas the test step was 4°/min.
2.3.Exposure tests in South China Sea environment
The exposure corrosion tests were carried out in the South China Sea corrosion test stations,which belong to Institute of Oceanology,Chinese Academy of Sciences.In this station,the tropical marine climate conditions result the high temperature(average 28�30 °C per year,the land surface temperature can reach to 60 °C in summer),high humidity(more than 2800mm rain per year),high sea salts and high ultraviolet radiation.The main atmospheric particles are Cl−and Na+.The location of the corrosion test station in the South China Sea was shown in Fig.1(a).
The Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys for the exposure tests were 5×5×0.5cm in size.All the samples were polished smoothly by the abrasive papers from 800# to 5000#.And then acetone and de-ionized water were successively used to rinse thoroughly the surfaces of all the samples.The weight of different samples was tested via the analytical balance.Original weight(W0),original thickness and surface area(S)were all recorded before the exposure corrosion.Exposure corrosion rack was used to attach the samples.All the samples mounted at a 45° angle horizontal to the ground.The corroded surface should face the south direction.Four replicate samples for each Mg-Nd alloy were mounted,and exposure corrosion results were stable and credible.All the Mg-Nd alloy samples were exposed in the South China Sea corrosion test stations for 2 years in Fig.1(b).

Fig.1.The location of the corrosion test station(a)and exposure corrosion tests of Mg-Nd binary alloys samples(b)in the South China Sea.

Fig.2.Tested five different locations on the surfaces of magnesium alloys.
After the exposure corrosion tests,corrosion depths on the surfaces of different samples were measured by the thickness gauge(Instrument type:GTS8102).The thickness gage needs to be calibrated using the standard Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys without corrosion,respectively.As shown in Fig.2,each corrosion samples tested five different locations on the surfaces of magnesium alloys.All the testing surfaces selected the sides facing the sunshine,which corroded more seriously than the reverse sides because of the rain and ultraviolet radiation.
The Mg-Nd binary alloys immersed into the chromic acid,and then heated the solution to a boiling state to remove the corrosion products.Next,deionized water and ethyl alcohol were used to flush the surfaces of Mg-Nd binary alloys[24–26].Finally,analytical balance was employed to measure the final weight(W1)of the drying samples.The weight loss Mg-Nd binary alloys after exposure corrosion obtained from the difference between W0and W1.The corrosion morphology was observed by SEM,whereas the mechanical strength was tested by a universal tensile test machine.
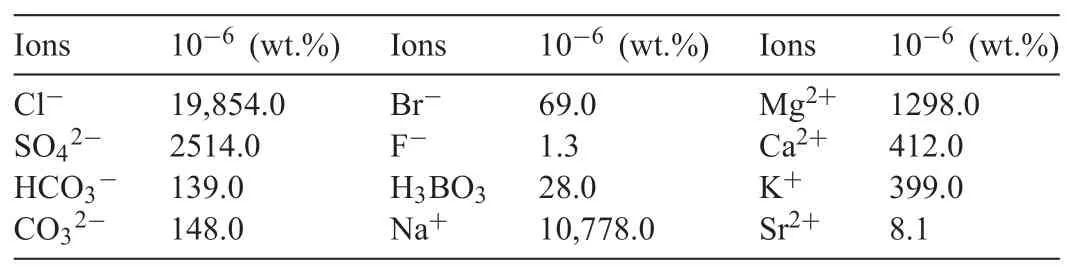
Table 2Main compositions of seawater in the present work.
2.4.Electrochemical corrosion tests in the laboratory environment
Princeton P4000+workstation was employed to test electrochemical corrosion data,including OCP,potentiodynamic polarization and EIS.The electrolytes for the electrochemical tests was the seawater obtained from South China Sea by a Plastic bucket.The samples were exposed in seawater to simulate the influence in typical South China Sea marine environment.The main compositions of seawater in the present work were shown in Table 2.
The samples for electrochemical testing consisted of 1cm×1cm×1cm cubes.Prior to electrochemical testing,the electrodes were encapsulated in epoxy resin,leaving a 1cm×1cm surface exposed.The samples were ground using a 5000-grit SiC emery paper,degreased by ethyl alcohol,and then dried by cold flowing air.The used electrolyte was 3.5% NaCl(consisting of A.R.(Analytical Reagent)NaCl and deionized water)at 25±1 °C.The open circuit potential(OCP),the potentiodynamic polarization curve,and electrochemical impedance spectra were obtained by a PARSTAT 4000A electrochemical workstation under a threeelectrode configuration with 450mL of electrolyte(Fig.3).A platinum foil and a saturated calomel electrode(SCE)acted as the counter and reference electrodes,respectively.All the potentials were given with respect to the SCE.The potentiodynamic polarization curves were obtained at a scanning rate of 0.5mV/s after the cell was held at the OCP for 1000s.Electrochemical impedance spectroscopy(EIS)at the OCP was conducted for 10min.For the EIS,the frequency ranged from 100,000Hz to 0.1Hz,with 5mV of amplitude of sinusoidal potential signals with respect to the OCP.Electrochemical measurements were performed in three replicates to guarantee data accuracy.
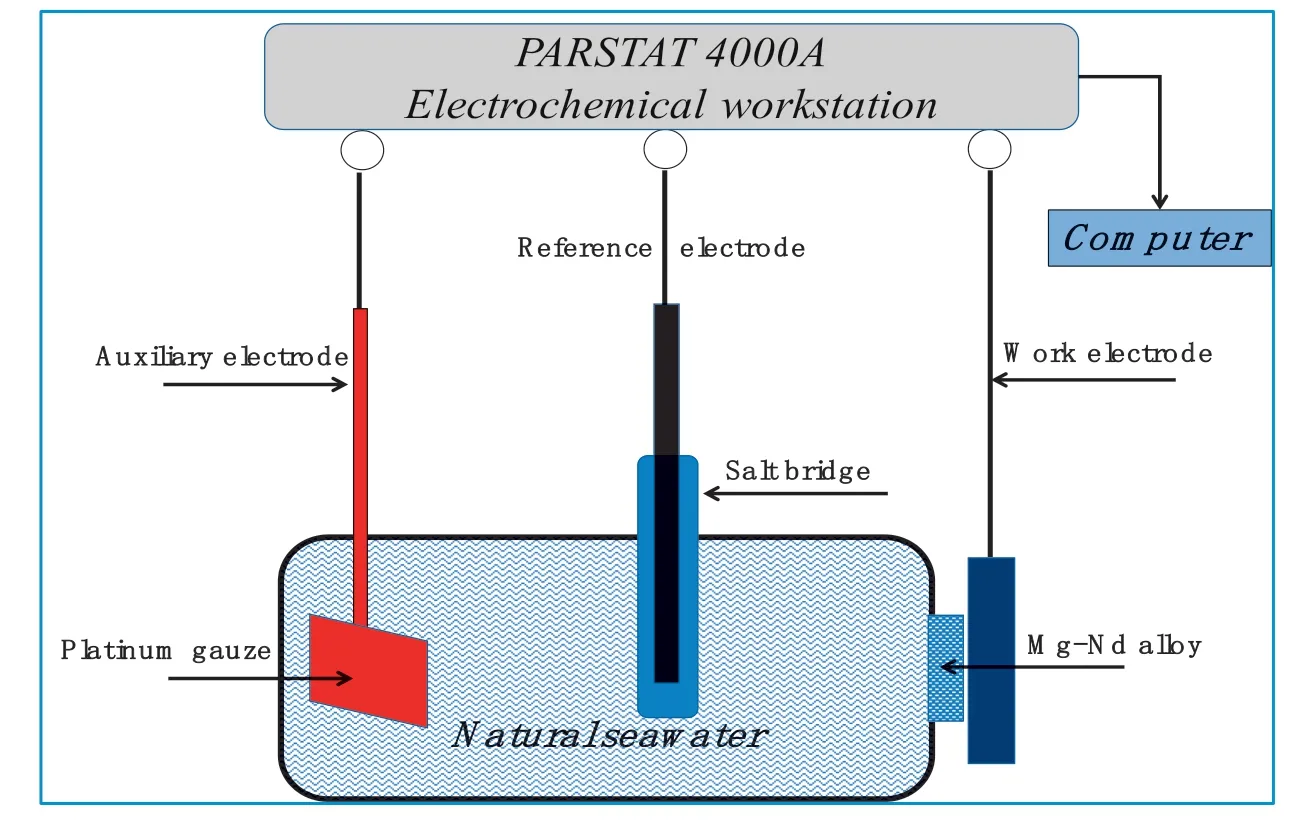
Fig.3.Schematic of the electrochemical testing of magnesium alloys in Natural seawater.
3.Results and discussion
3.1.Microstructure of Mg-Nd binary alloys
The microstructure analysis of Mg-Nd binary alloys is shown in Fig.4.From the diagram,the microstructures of Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys are all composed ofα-Mg matrix and precipitated phases.A large number of precipitated phases are uniformly distributed in the interior of the matrix and along the grain boundaries.Generally,precipitated phases in the magnesium alloys often act as the cathode of microelectrochemical coupling reaction,accelerating the corrosion ofα-Mg matrix[27-28].With the alloying element increased gradually,more and more Nd-content reacted withα-Mg matrix,grain boundaries also became more and more apparently.The precipitated phases were distributed more uniformly at the same time.Meanwhile,the number of precipitated phases also increased tremendously.
Fig.5(a),Fig.5(b)and Fig.5(c)shows SEM photographs of different alloys,and the results of EDS elemental analysis follow in the Table.From the SEM amplifying photographs,precipitated phases can be seen distributed in the interior of the matrix and along the grain boundaries.The growth rate of different crystal surfaces determines the shape of the precipitated phases,which is the kinetic preferential orientation in the magnesium alloys.The SEM images clearly showed the white patterned precipitated phase along the grain boundary(marked by“1,2 and 3′′),of which the latter had a higher Mg and a lower Nd content.The EDS analysis concluded that the composition of phases are about 93.33%Mg+6.67% Nd(Fig.4a),93.84% Mg+6.16% Nd(Fig.4b),93.37% Mg+6.63% Nd(Fig.4c),respectively.According to Mg/Nd atomic ratios and locations of patterned precipitated phases,and based on the Mg-Nd binary phase diagram,the conclusion can be drawn that these phases are Mg12Nd precipitated phases[9,29-30].
XRD microanalysis was used to characterize different phases in the binary Mg-Nd alloys.Each type of alloys tested three replicate samples to render the experimental results in certain.The results shown in Fig.6 indicated thatα-Mg was the major phase in the Mg-xNd alloys.Moreover,characteristic peak intensity of Mg12Nd precipitations were also detected.Actually,previous studies showed that Mg12Nd phase was the common phases existed in the grain boundary in Mg-Nd alloy system[31-32].As shown in Fig.6,when the alloying element Nd increased,the characteristic peak intensity of α-Mg contained(1 0 0),(0 0 2)and(1 0 1)crystallographic planes obviously decreased.However,the characteristic peak intensity of Mg12Nd contained(2 2 2),(1 0 3)and(3 3 4)crystallographic planes showed the opposite trend.This phenomenon indicated that the amount of Mg12Nd precipitated phases increased.The results proved that the XRD microanalysis(Fig.5)was consistent with the microstructures of SEM(Figs.3 and 4).The difference among the characteristic peak intensity signified that the amount of the precipitated phases were variant for the Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys,which indicated that the driving forces of micro-electrochemical corrosion between precipitated phases andα-Mg matrix were different.
3.2.Surface morphologies and weight loss rate
In this study,△W is marked as the value of the weight loss rate of Mg-Nd alloys after 2 years exposed corrsion,which unit of measurement is equalled to g•cm−2•y−1.△W can be calculated by the expression:△W=(W0-W1)/ST.In this expression,W0(g)and W1(g)means the original weight and the final weight,respectively.W0of the Mg-Nd binary alloys was measured before the exposed corrosion,whereas the W1was measured after all the corrosion products were removed from the surface of the alloys.S(cm2)is equalled to the sum of the six surface areas of each sample.T(y)is behalf of the period of exposed corrosion,which is equalled to 2 years.Actually,the weight loss rate in this study is an average value on the total surface areas of each sample after 2 years exposed corrosion.The results were shown in Table 3.

Fig.4.Microstructure analysis of SEM:(a,b)Mg-0.5Nd;(c,d)Mg-1.0Nd;and(e,f)Mg-1.5Nd.

Table 3The weight loss rate of Mg-Nd alloys after 2 years exposed corrosion.

Fig.5.The SEM photographs and EDS elemental analysis of different alloys:(a)Mg-0.5Nd;(b)Mg-1.0Nd;(c)Mg-1.5Nd.
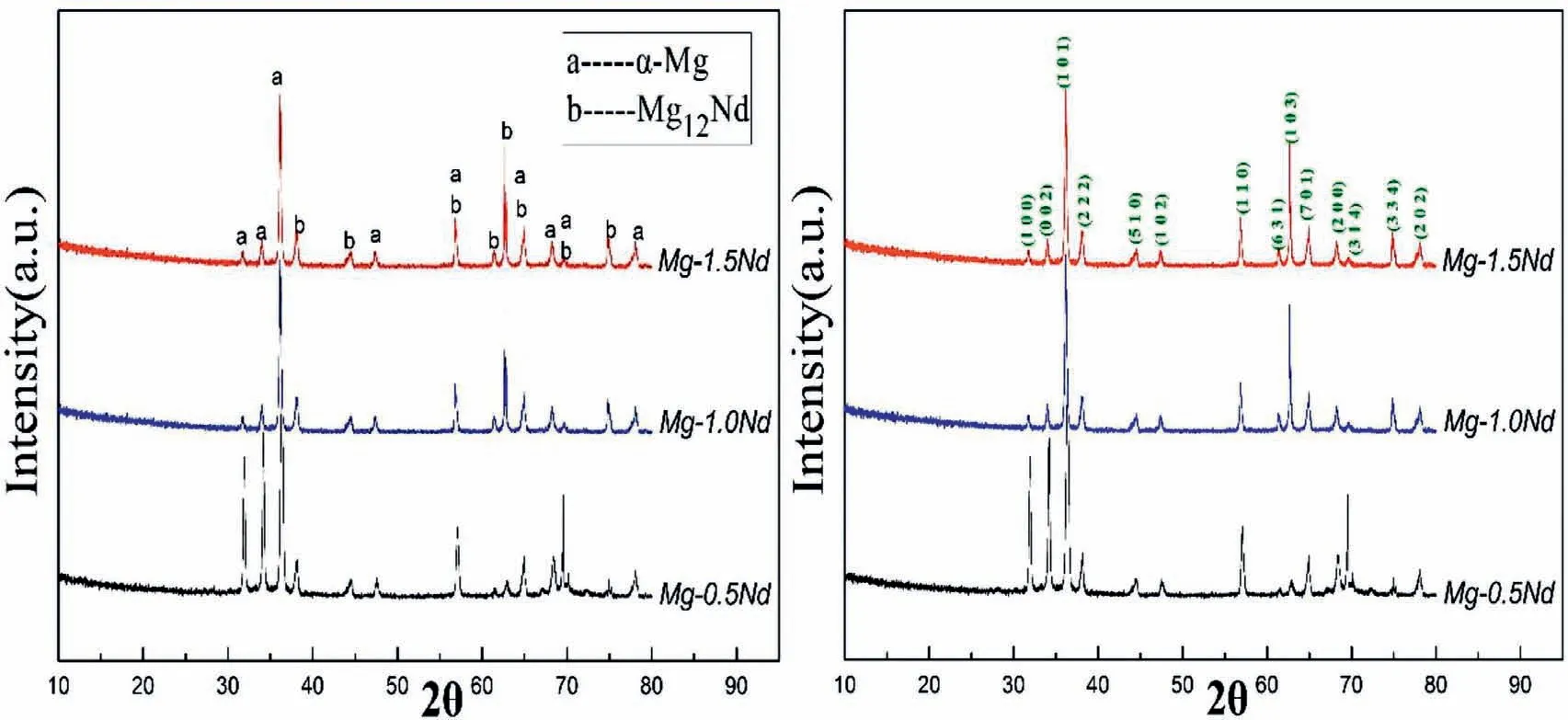
Fig.6.XRD microanalysis of Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys.
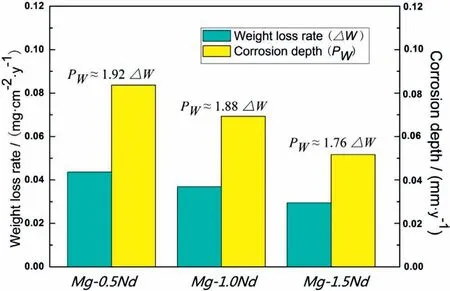
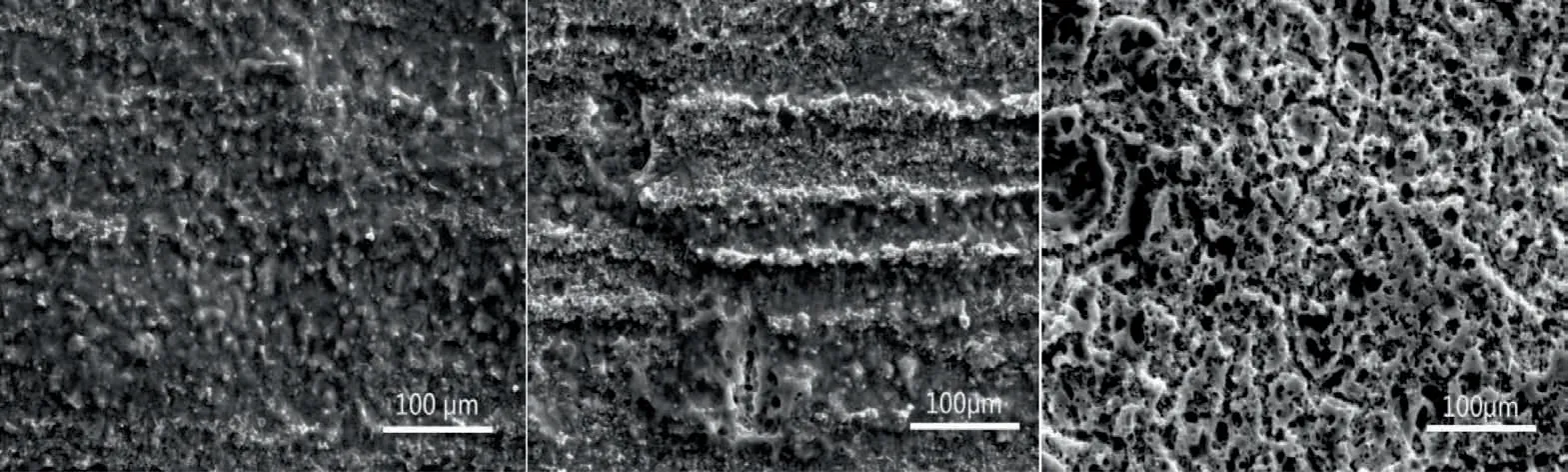
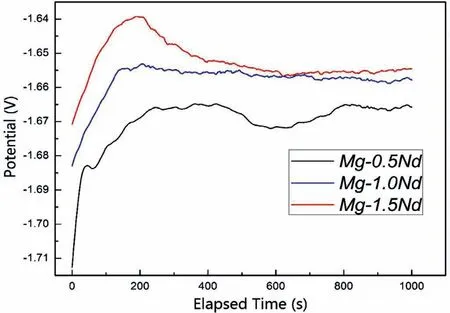
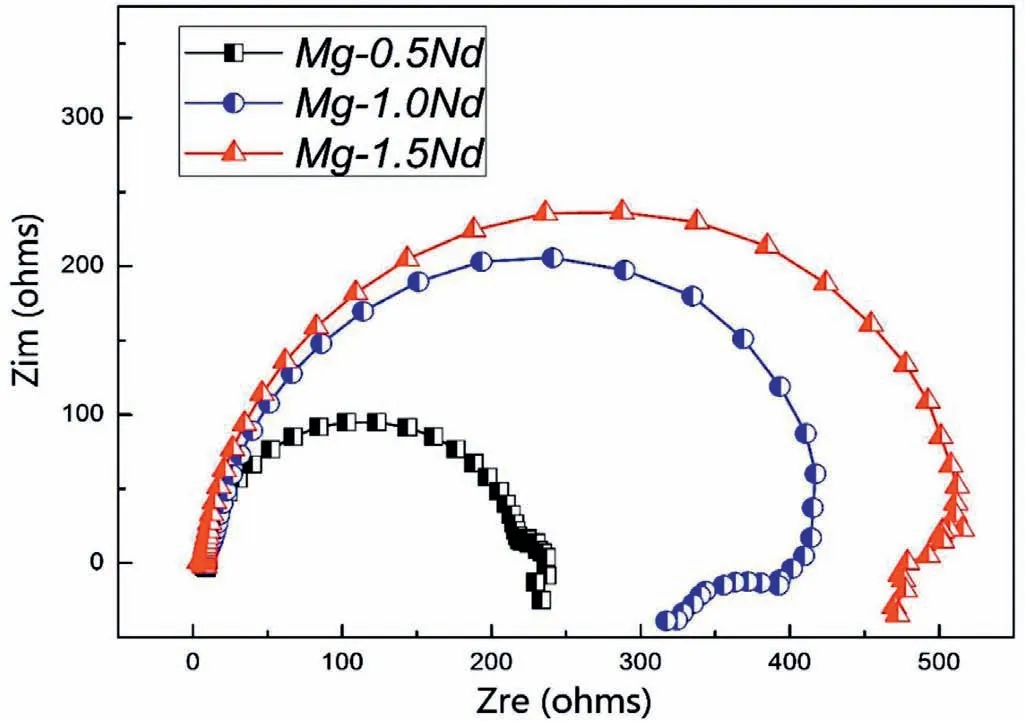
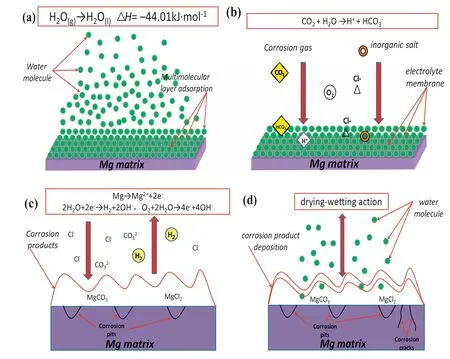
As shown in Table 4,Mg-1.5Nd alloy had the lowest average corrosion rates 0.0294 g•cm−2•y−1.This phenomenon may be attributed to the amount and distribution of the precipitated phases in the microstructure of Mg-1.5Nd alloy.Actually,the precipitated phases in the magnesium alloys often have a more positive potential than theα-Mg matrix,and then they act as the cathode of the micro-electrochemical reaction in the microstructure.However,uniformly distributed precipitated phases formed a barrier to decrease the weight loss rate in the magnesium alloys in many cases[33-35].This case is consistent with the present study.As a conclusion,the uniformly distributed precipitated phases in the Mg-1.5Nd alloy formed a barrier to decrease the weight loss rate.In the present study,the order of weight loss rate is as follows:Mg-1.5Nd Moreover,the corrosion depth PW(mm•y−1)of Mg-Nd alloys after 2 years exposed corrosion was measured via the thickness gage,and the results were shown in Table 4,as follows.The corrosion depth of Mg-0.5Nd sample showed a maximum value at 0.0837 mm•y−1,whereas that of Mg-1.5Nd samples showed a minimum value at 0.0693 mm•y−1.The corrosion depth of Mg-1.0Nd samples was intermediate at 0.0517 mm•y−1.This phenomenon signified that the uniformly distributed Mg12Nd phases formed in the Mg-Nd alloys determined the corrosion depth. According to previous research[36-37],the corrosion depth PW(mm•y−1)can be calculated by the formula:PW≈ 2.10△W.In this study,the quantitative relationship between the weight loss rates and corrosion depths was also calculated.However,the results shown in Fig.7 indicated that the quantitative relationshipbetween the weight loss rates and corrosion depths for exposure tests in marine atmospheric environment was different from that of immersion tests in the laboratory.The quantitative relationship of Mg-0.5Nd samples,Mg-1.0Nd samples and Mg-1.5Nd samples was respectively PW≈1.92△W,PW≈1.88△W and PW≈1.76△W.This phenomenon suggested that quantitative relationship between the weight loss rates and corrosion depths may be calculated according to the types of magnesium alloys,corrosion method and the experimental environment. Table 4The corrosion depth of Mg-Nd alloys after 2 years exposed corrosion. Fig.7.The quantitative relationship between the weight loss rates and corrosion depths of Mg-Nd alloys samples. The corrosion morphology of Mg-xNd alloys samples covered with corrosion products in the South China Sea corrosion site was characterized by optical camera(Fig.8).Visible to the naked eyes,the side facing the sky of all the samples are covered with corrosion products after exposure tests.However,the difference on the surface corrosion morphologies of Mg-Nd binary alloys was also clearly.Fig.8(b)reveals that the surface of Mg-0.5Nd alloy covered with corrosion products was like the fold shape.But the gathering points of corrosion products deposited on the surface of Mg-1.0Nd sample,which was shown in Fig.8(c).Mg-1.5Nd alloy was corroded the slightest,as shown in Fig.8(d),which had a more compact corrosion particles formed on the surface.The exposure corrosion tests were performed in the South China Sea corrosion test site,which belong the tropical marine climate conditions.Temperature of the corrosion test site is average 28�30 °C each year,moreover,the land surface temperature can reach to 60 °C in summer,which is an important factor for corrosion rate.On the other hand,the high humidity and precipitation rainfall capacity resulted that corrosion products could not remain on the surface for a long period,which exposed the magnesium matrix,accelerating the dissolution of theα-Mg.Finally,high sea salts(Cl−and Na+)and high ultraviolet radiation led a serious corrosion of magnesium alloys in the South China Sea corrosion test site. After the exposure in the South China Sea,the corrosion products that formed on the surface of samples were removed by pickling in the chromic acid solution.The surface morphologies without corrosion products are displayed in Fig.8,showing that the corrosion attack was initiated in theα-Mg phase.Upon further exposure,the corrosion dents grew irregularly in area until the a-Mg phase on the surface was almost completely corroded.Only few Mg24Y5phases remained on the surface after exposure(Fig.9a).The corroded pits without corrosion products appear to have been formed by a larger area.For samples of Mg-1.0Nd alloy,many localized corrosion areas were visible on the surface(Fig.9b).With prolonged exposure times,the number of the pits increased and gradually appeared as a honey-comb distribution on the surface of Mg-1.5Nd alloy(Fig.9c). Fig.8.Corrosion morphology of samples with corrosion products:(a)all the samples,(b)Mg-0.5Nd alloy,(c)Mg-1.0Nd alloy and(d)Mg-1.5Nd alloy. Fig.9.Microscopic corrosion morphology of samples without corrosion products:(a)Mg-0.5Nd alloy,(b)Mg-1.0Nd alloy,(c)Mg-1.5Nd alloy. Fig.10 shows electrochemical open circuit potential of Mg-xNd alloys measured in the seawater obtained from the South China Sea.The OCP should be relatively stable before all the measurements,which guaranteed that the uniform corrosion product film formed on the surface of Mg-Nd magnesium alloys[38,39].The value of the OCP fluctuated with the extension of times.This was due to the formation and peeling of corrosion products on the surface of Mg-Nd magnesium alloys.Immersion in the natural seawater for 1000s,the value of the OCP had a shift to the positive direction.This phenomenon may be due to the stable protective oxidation film,which made the partial corrosion incubation period of samples longer.The value of Mg-1.5Nd alloy was more positive,indicating that the activation period of local corrosion was shorter,which may be caused by more precipitated phases in grain boundary.Moreover,with the extension of immersion time,the OCP of Mg-Nd alloys tended to be stable,and the location corrosion and corrosion product deposition were almost in a stable state[40].As can be seen in Fig.10,the electrochemical activity sequence of samples was as follows:Mg-0.5Nd>Mg-1.0Nd>Mg-1.5Nd.The corrosion resistance of Mg-1.5Nd alloy was better than other alloys.This conclusion was mainly due to the amount and distribution of the precipitated phases[41–42].According to previous studies,precipitated phases often played dual roles.If the precipitated phases were scarce and scattered,they could be served as an effective cathode in the corrosion reaction.Otherwise,if the precipitated phases were numerous and evenly distributed,they could be served as a better barrier to prevent the corrosion reaction ofα-Mg matrix[43]. Fig.10.Open circuit potential of different samples measured in the seawater. Fig.11.Tafel slopes of Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys measured in the natural seawater. The Tafel slopes of different samples measured in the natural seawater was shown in Fig.11.As shown in Fig.11,the Tafel curves of Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd samples were similar,which proved that the corrosion reactions were identical.The cathode branch of Tafel curves indicates the occurrence of hydrogen evolution,whereas the anode branch represents the dissolution of magnesium matrix[44].In the corrosion of magnesium alloys,the anode branches showed up the characteristic Tafel curves,which were generally sharp formation.The phenomenon illustrated that the cathodic hydrogen evolution was the main reaction,instead of anodic dissolution reaction[45,46].However,when the potential was more negative than the critical pitting potential,the cathodic branch showed up linear Tafel characteristics.Due to the negative difference effect,the anodic polarization curve of magnesium and its alloy was complex,which aggravated the precipitation of hydrogen during the anodization of the alloy.Therefore,the tangential corrosion current density of the cathode was calculated by Tafel extrapolation.The table in Fig.11 shows the calculated corrosion current density measured in the seawater with respect to the Mg-Nd alloys with different Nd-content.Due to the micro-electrochemical accelerate corrosion effect of precipitated phases,the Diffused precipitated phases in Mg-0.5Nd alloy resulted that the samples in the natural seawater had the largest Icorr.On the other hand,the Icorrof Mg-1.5Nd alloy is the smallest of the three alloys.Therefore,the calculated corrosion current density(Fig.9),the patterns of weight loss rates(Table 4)and corrosion depth(Table 5)of Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd samples are all consistent.On the basis of corrosion potential,tendency toward corrosion is as follows:Mg-0.5Nd>Mg-1.0Nd>Mg-1.5Nd.The results depend on distribution of precipitated phases in the Mg-Nd binary alloys,which changed from the diffused distribution to uniform distribution gradually. Fig.12.Electrochemical impedance spectra of different Mg-Nd alloys samples. The Electrochemical impedance spectra curves of Mg-Nd binary alloys were shown in Fig.12,which suggested different electrochemical corrosion dynamic process.One capacitive reactance at high frequency and one inductive reactance at low frequency constituted the spectra of Mg-Nd binary alloys after immersion in the natural seawater obtained from the South China Sea.The largest electrochemical impedance spectra of Mg-1.5Nd alloy samples indicated that hydrogen evolution of theα-Mg matrix reaction had the largest energy barrier.According to the Nyquist diagrams in Fig.10,the corrosion resistance can be ranked as follows:Mg-0.5Nd The electrochemical impedance spectra curves of Mg-Nd binary alloys were different,which represented distinctive dynamic corrosion process.First of all,the corrosion products produced during the immersion,which formed a double layer in the solution,generating a capacitive reactance on the interface between Mg-Nd alloys and natural seawater.The capacitive dimension of electrochemical impedance spectra curves represented the difficulty level of charge-transfer in the natural seawater,which meant the magnitude of corrosion rate for different Mg-Nd binary alloys[47].The induced reactance was induced by the desorption of the corrosion product film and the movement of magnesium ions with the prolonged immersion time. Fig.14.Schematic related to the corrosion mechanism of Mg-Nd alloys in the exposure site. The tensile strength,yield strength and elongation of Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd alloys measured by universal tensile test machine at the room-temperature are shown in Fig.13(a).The tensile strength and yield strength improved with the increasing of Nd-content.Conversely,the elongation decreased.According to the obtained microstructure analysis of Mg-Nd binary alloys and previous research[48–50],this phenomenon could be explained by the strengthening mechanism,indicated that the precipitation hardening effect of Mg12Nd intermetallic phases with a fine dispersed distribution.For the Mg-Nd binary alloys,the alloying element Nd dissolved into magnesium matrix to form the physical bonding,enlarging the binding force betweenα-Mg matrix and precipitated phases.The coherent interface served as an effective barrier for dislocation flow,which reduced ductility and improved tensile strength yet. Generally,the Mg-1.5Nd alloy showed the least attenuation of mechanical performance after exposure in the South China Sea corrosion site.After the exposure test,the average tensile strength of Mg-1.5Nd alloy decreased from 148MPa to 94MPa,indicating that the attenuation ratio was about 36.48%.The average yield strength of Mg-1.5Nd alloy decreased from 115MPa to 72MPa,suggesting that the attenuation ratio was about 37.39%.Moreover,all the Mg-1.5Nd and Mg-1.0Nd alloy samples occurred instantaneous fracture,which resulted that the elongation of alloys were all equal to zero.Moreover,the average attenuation ratio tensile strength of Mg-0.5Nd alloy was reached up to 41.84%.After the exposure test,corrosion pits and micro-cracks formed in the surface of the Mg-Nd binary alloys,leading to the decreasing of cross-sectional area.Therefore,the tensile strength of magnesium alloy declined during the tensile test[51].The corrosion of Mg-Nd binary alloys after exposure in the South China Sea site was comprised of two reaction processes.One process was anodic dissolution ofα-Mg matrix;another process was the cathodic hydrogen evolution.For the Mg-Nd binary alloys,hydrogen evolution had a more important influence in environmental exposure corrosion[52].The poor corrosion resistance of Mg alloys caused corrosion pits to form on the surface of the tensile sample,which resulted in large size cracks in the fracture. The exposure site of South China Sea is a specific high temperature,high humidity,high sea salts and high ultraviolet radiation environment.The schematic related to the corrosion mechanism of Mg-Nd alloys in the exposure site was shown in Fig.12.Generally,when the humidity in the environment ranged from a lower value to a higher value,the corrosion reaction of magnesium alloy matrix was more severe.This phenomenon was attributed to the formation of hydration shell on the surface of Mg matrix when the humidity was over 60%.Sodium chloride in the marine environment deposited on the surface of the magnesium alloys after exposure in the corrosion site.According to previous studies,if the humidity was more than 75% in the atmospheric corrosion environment,Na+and Cl−would pass and dissolve into hydration shell,increasing the conductivity of the hydration shell.The diffusion rate of oxygen atoms in the hydration shell was largely improved due to the with good conductivity.Because the cathode reaction was absorbing oxygen,therefore the reaction rate was effectively improved[53].Simultaneously,the ionic radius of chloridion was merely 130 pm,which resulted to a high mobility in the hydration shell.Therefore,chloridion also could pass into the corrosion product film of Mg-Nd alloys,resulting in its deterioration[54].For the South China Sea corrosion site,the high sea salts(NaCl)one of the typical features.Due to heavy rainfall and high humidity,sodium chloride dissolved into the hydration shell and became the ionic condition easier.Finally,the corrosion electrochemical reactions of the Mg-Nd binary alloys occurred rapidly and severely. On the other hand,the hydration shell on the surface of Mg-Nd binary alloys was nanoscale,which could not prevent diffusion and transfer of oxygen effectively.Therefore,the corrosion reaction of Mg-Nd binary alloys was continuous and uninterrupted.At a high humidity condition,the corrosion product magnesium hydroxide was contained crystal water,which was relatively fluid and could not prevent the corrosion reactions to take place furtherly[55,56].Finally,strong ultraviolet light causes the repeat of the wet-dry cycle in the exposure site,which led a serious corrosion of the samples.From the discussions above,the influence of high humidity,high temperature,strong ultraviolet light,Na+and Cl−amount on exposure site could well account for serious corrosion the varying exfoliation of Mg-Nd alloys in the harsh South China Sea atmospheric environments. After the exposure in the South China Sea,a film of MgO formed quickly on the surfaces of the Mg-Nd alloys[57-58].Meanwhile,the inorganic salts in the atmosphere deposited on the surface of the samples.Due to the high hygroscopic property of inorganic salts,a uniform thin liquid film is formed on the sample surface at a certain humidity.Therefore,atmospheric corrosion process in the South China Sea was the electrochemical corrosion reaction in the thin layer of electrolyte on the alloy surface.The corrosion mechanism was shown in the figure,which was divided into four steps: (1)Initial stage:adsorption of water vapor:Due to the ability to adsorb water molecules of magnesium alloys,the water vapor condenses on the surface of Mg-Nd alloys to form a very thin water film.At atmospheric temperature,the magnesium alloy mainly occurred the multimolecular physical adsorption,H2O(g)�H2O(l),which was a exothermic reaction,followed by the adsorption process△H=−44.01 kJ•mol−1. (2)Dissolution of corrosive gas and inorganic salts in water film:During the corrosion process,O2,CO2,Na+and Cl−in the south China sea environment interact with the magnesium alloy surface.After the formation of water film on the surface of magnesium alloy,O2and CO2molecules dissolve into water film and formed H2CO3.At the same time,soluble inorganic salts such as NaCl were deposited on the surface of the alloy and dissolved in the water film to form a thin film electrolyte. (3)The electrochemical reaction on the surface of magnesium alloys[59]:After a thin film electrolyte formed on the surface,electrochemical corrosion reaction occurred.The process was as follows: Anodeα-Mg dissolved: Reduction of H and O occurs at the cathode: Formation of corrosion product: The CO2and NaCl in atmospheric environment were adsorbed and hydrolyzed in the thin film solution to form anion diffusion to the anode region,which reacted with Mg(OH)2to form MgCO3and MgCl2.The continuous dissolution of CO2reduced the pH value in the anode region and accelerated the dissolution.Stable corrosion products Mg(OH)2andMgCl2were precipitated when the ion concentration in the film liquid was saturated. (1)Surface wet and dry alternating process:When the relative humidity in the atmospheric environment was low,the thin film liquid on the sample surface evaporates,leading to the precipitation and deposition of the corrosion products Mg(OH)2and MgCl2.And then the corrosion pits formed,which results in the reduction of the critical relative humidity for the condensation of water vapor on the surface and the reduction of the potential barrier for the formation of water film.When the relative humidity increased,the surface of the alloy was more likely to form water film.After the surface of Mg-Nd alloys dried,the microcrack occurred[60].According to the theory of surface chemistry,water vapor,corrosive gas and water-soluble inorganic salt are easy to condense and dissolve at the microcrack during capillary condensation,leading to the increase of corrosion rate in this area.As the reaction continued,the corrosion product thickens continuously under the effect of alternating wet and dry,resulting in micro-cracks and other defects,which aggravated the local corrosion. (1)The microstructure and corrosion behavior of Mg-0.5Nd,Mg-1.0Nd and Mg-1.5Nd binary alloys were researched.The quantity and distribution status of Mg12Nd precipitated phases in the microstructure were analyzed.The research found that the amount of the Mg12Nd precipitated phases increased and distributed more evenly due to the addition of Nd-content. (2)The weight loss rate of Mg-0.5Nd alloys was 0.0436 g•cm−2•y−1(0.0837 mm•y−1),whereas this of Mg-1.5Nd alloys was 0.0294 g•cm−2•y−1(0.0517 mm•y−1)after 2 years exposure corrosion.The electrochemical measurement in the laboratory also obtained the same conclusion.The Mg-1.5Nd alloy had the best corrosion resistance than Mg-1.0Nd alloy and Mg-0.5Nd alloy.According to the research,the corrosion resistance can be ranked as follows:Mg-0.5Nd (3)In the corrosion environment,sodium chloride in the marine environment deposited on the surface of the magnesium alloys after exposure in the corrosion site.When the humidity was more than 75% in the atmospheric corrosion environment,Na+and Cl−passed and dissolved into hydration shell,increasing the conductivity of the hydration shell.For the South China Sea corrosion site,the high sea salts one of the typical features.The ionic radius of chloridion was merely 130 pm,which resulted to a high mobility in the hydration shell.Finally,the corrosion electrochemical reactions of the Mg-Nd binary alloys occurred rapidly and severely. Acknowledgement The authors gratefully acknowledge the Fundamental Research Project of Technology Program of Qingdao(No.17–1-1–76-JCH),Shandong Provincial Natural Science Foundation(ZR201910230421)and the National Natural Science Foundation of China for Exploring Key Scientific Instrument(No.41827805)for providing support.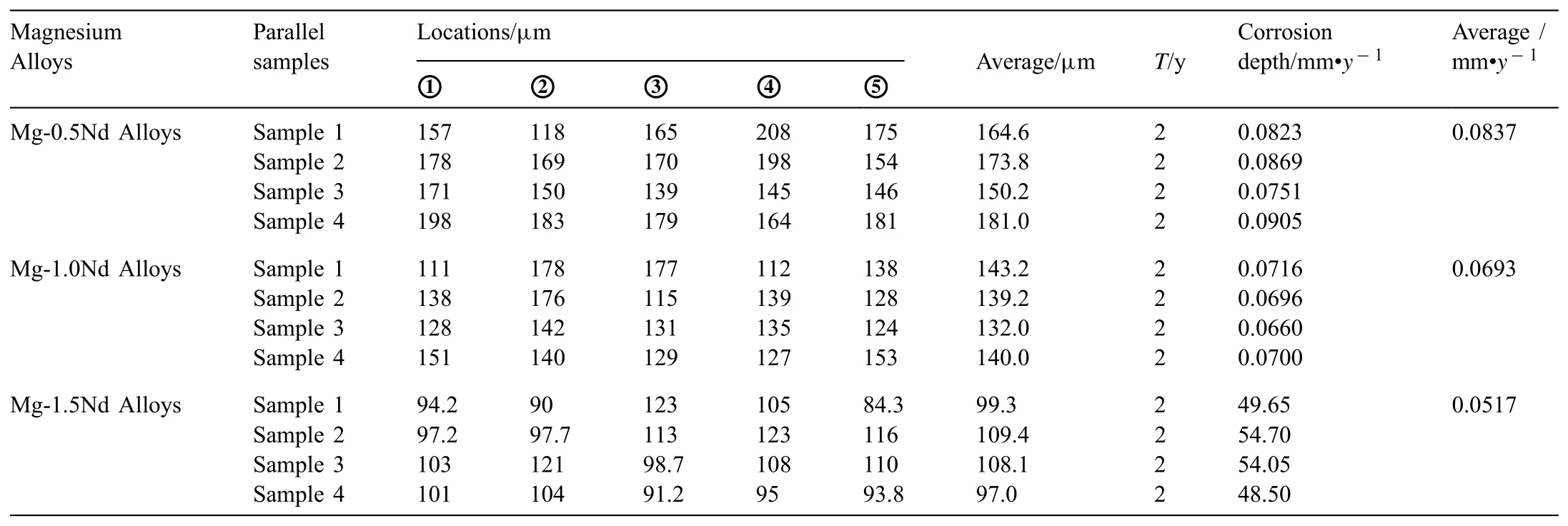
3.3.Electrochemical characterizations in the seawater


3.4.Mechanical property
3.5.Atmospheric corrosion mechanism in the exposure site

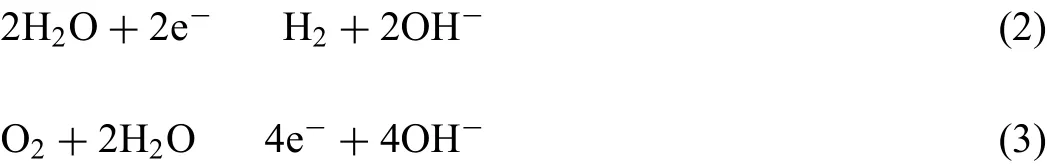

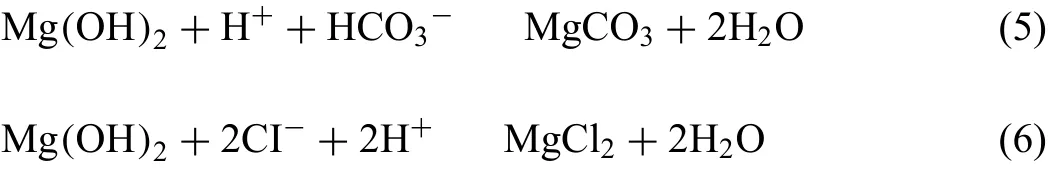
4.Conclusion
杂志排行
Journal of Magnesium and Alloys的其它文章
- Tailoring the degradation rate of magnesium through biomedical nano-porous titanate coatings
- Modified embedded-atom method interatomic potentials for Mg–Al–Ca and Mg–Al–Zn ternary systems
- Dual alloying improves the corrosion resistance of biodegradable Mg alloys prepared by selective laser melting
- In vitro and in vivo evaluations of Mg-Zn-Gd alloy membrane on guided bone regeneration for rabbit calvarial defect
- In vitro corrosion resistance,antibacterial activity and cytocompatibility of a layer-by-layer assembled DNA coating on magnesium alloy
- Relieving segregation in twin-roll cast Mg–8Al–2Sn–1Zn alloys via controlled rolling
