In vitro corrosion resistance,antibacterial activity and cytocompatibility of a layer-by-layer assembled DNA coating on magnesium alloy
2021-03-10LnYueCuiLingGoJingChoZhngZheTngXioLiFnJiChengLiuDongChuChenRongChngZengShuoQiLiKeQinZhi
Ln-Yue Cui,Ling Go,Jing-Cho Zhng,Zhe Tng,Xio-Li Fn,Ji-Cheng Liu,Dong-Chu Chen,Rong-Chng Zeng,∗,Shuo-Qi Li,Ke-Qin Zhi,∗
a College of Materials Science and Engineering,Shandong University of Science and Technology,Qingdao 266590,China
b Department of Oral and Maxillofacial Surgery,The Affiliated Hospital of Qingdao University,Qingdao 266555,China
cKey Lab of Oral Clinical Medicine,The Affiliated Hospital of Qingdao University,Qingdao 266555,China
d School of Materials Science and Energy Engineering,Foshan University,Foshan 528000,China
Received 12 September 2019;received in revised form 11 March 2020;accepted 15 March 2020
Available online 8 August 2020
Abstract A chitosan/deoxyribonucleic acid(CHI/DNA)5 coating was constructed by layer-by-layer(LbL)assembly dip coating method with Mg(OH)2 coating as an inner protective layer on AZ31 alloy.X-ray diffractometry,X-ray photoelectron spectrometry,Fourier transform infrared spectroscopy and field-emission scanning electron microscopy were utilized to represent the chemical compositions and surface morphologies of the coatings.Electrochemical tests and hydrogen evolution measurements were implemented to confirm the good corrosion resistance of the composite coating in artificial body fluid.Antimicrobial activity of the composite coatings was tested via the plate-counting method,and the cytotoxicity of the samples was appraised by MTT assay and Live/dead staining.A double action was put into effect for the composite coating,which the inner Mg(OH)2 coating plays the part of physical barrier,and the outer(CHI/DNA)5 coating is employed as an inducer to fabricate a biocompatible Ca-P corrosion product coating during immersion,making up for its thin thickness.Otherwise,the composite coating is also beneficial for the growth of bone,resulting from the biomineralization effect of the outer polyelectrolyte multilayer.The good antibacterial property of the(CHI/DNA)5/Mg(OH)2 coating is ascribed to the contact-killing strength of CHI.Thus,the obtained(CHI/DNA)5/Mg(OH)2 coating has a wide application prospect in the field of Mg-based bone implantation.
Keywords:Magnesium alloys;Coatings;Layer-by-layer assembly;Biomaterials;Antibacterial properties;Corrosion.
1.Introduction
Magnesium(Mg)and its alloys have aroused tremendous interest as biodegradable orthopedic implant materials,due to their outstanding physical and mechanical properties[1–5].However,the rapid degradation of Mg implants in body fluid gives rise to many adverse effects,such as decline in mechanical strength,massive hydrogen evolution and local alkalization,so that a number of protective coatings in variety has been attempted to advance the corrosion protection of Mg alloys[6–8],including chemical conversion coatings[9–12],micro-arc oxidation(MAO)coatings[13–15],electrodeposited coatings[16–19],polymeric coatings[3,20–22],layer-by-layer(LbL)assembly coatings[23–26],layered double hydroxide(LDH)coatings[27–29]and sol-gel coatings[30].Furthermore,to avoid device-induced bacterial infection,development of antibacterial surface on biomedical Mg alloys is highly required[31,32].But most of the common antibacterial coatings rely on antibiotics and other drugs,which are insecure for the human body[26,33].Thus,it is necessary to form new functional composite coatings to develop corrosion resistance,antimicrobial activity and cytocompatibility of Mg alloys.
Of special interest is LbL assembly coatings with antibacterial groups,serving as a barrier layer as well as antibacterial defender[34].Deoxyribonucleic acid(DNA)can be regarded as naturally negatively charged functional“polymers”,which is extensively used in LbL multilayer formation,playing an important role in the development of localized gene therapy[35],but the possibility of corrosion resistance is ignored.Actually,the DNA molecules,with a special duplex structure,have an extended anionic phosphate backbone,which can be interacted with Ca2+ions.Meanwhile,the nucleation kinetics or crystallinity of mineralized materials may positively affected through secondary structure of folded DNA fragments[36,37].For example,the LbL self-assembled DNA and PLL multilayers on Ti surface were in favor of protein adsorption and biomimetic mineralization[38].Our early study[39]also demonstrated that DNA in LbL assembled multilayer led to Ca-P deposition,and provided certain protection for AZ31 alloy.However,the corrosion resistance of the coating on Mg alloy should be advanced in further and the cytocompatibility of the obtained coating was neglected.And the LbL assembled coating can effectively prevent the probable deactivation and uneven adsorption of DNA for the hydrothermal treatment[37].That is,DNA may be a great option to generate decent corrosion protection behavior of the LbL assembled coatings.
Moreover,chitosan(CHI)is a semi-natural biopolymer with low toxicity[40]and hydrophilic surface to promote cell adhesion and proliferation[41],exhibiting antimicrobial activity based on the contact-killing strategy[42,43].The antibacterial mechanism of CHI can be ascribed to three reasons:(1)interaction between the positively charged amine groups of CHI and negatively charged bacterial membrane led to the disruption and release of intracellular compounds[44];(2)chelation with metal ions,caused by the functional groups of CHI,inhibits bacterial enzyme activity;and(3)CHI segments penetrate cell membrane and inhibit RNA synthesis[45].Thus,CHI has been widely applied for antimicrobial purposes[40],46].
Otherwise,CHI has been utilized for promoting formation of hydroxyapatite(HA)layer[47],enhancing both corrosion protection and biocompatibility of Mg alloys.Both molecular weight and number of layers of CHI in the composite coating influence the degradation performance of the Mg-1Ca alloy[48].In our earlier study[43],an MAO/CHI coating was formed to maintain pH of Hank’s solution at a moderate level(pH≤8.25),and to improve the anti-corrosion performance and anti-bacterial growth activity of Mg-4Li-1Ca alloy.Thereby,the biodegradable CHI can be used as a physical barrier and offers a suitable micro-environment to avoid strong localized alkalinity caused by rapid degradation of Mg alloy.
Note that,LbL assembled multilayer is usually too thin to protect underlying Mg alloys from corrosion[49].Thus,it is considered that forming LbL composite coatings could be a sound approach to restrain the degradation process of Mg alloys in further.Our previous work indicated that silane based LbL composite coating exhibited superior corrosion resistance[24,25].Kumta et al.[34]have formed a sodium alginate(ALG)/Poly-L-lysine hydrobromide(PLL)multilayer in combination with inner MgF2layer to protect Mg alloy AZ31.MAO coating was also be selected as an inner layer to inhibit corrosion in combination with LbL self-assembly technique[50].
In this study,we designed a(DNA/CHI)5multilayer via LbL assembly method with an inner Mg(OH)2layer.The formation of inner Mg(OH)2coating is to prevent the initial and further degradation during the dipping of polyelectrolyte and immersion of artificial body fluids.Polyethyleneimine(PEI)is used as an insulating polymer between the inner Mg(OH)2layer and the outer(DNA/CHI)5multilayer,assisting in molecular electrostatic self-assembly for multi-layer formation and providing a physical barrier for electrons transfer.The obtained composite coating aims to obtain an antimicrobial composite coating suitable for bone implantation,which is biomimetic mineralized during its degradation to overcome the limitation of the LbL assembled coatings.
2.Experimental
2.1.Materials and chemicals
As-extruded AZ31 alloys employed in the experiments,with compositions of Al(2.5–3.0),Zn(0.7–1.3),Mn(>0.20)and balanced Mg,were furnished by Shandong Yin Guang Yu Yuan Light Metal Precision Molding Co.Ltd.,China.PEI(Mw=600,99%)and CHI(Mw=40,000)were obtained from Xiya Reagent Co.Ltd.,China.DNA(300bp/molecule,fish sperm,sodium salt,≥85%)was purchased from Sinopharm Chemical Reagent Co.Ltd.,China.20 mm×20mm×5mm dimension of AZ31 specimens were polished using SiC papers up to 1500 grit,cleaned with deionized(DI)water and alcohol solution,and dried by warm air.
2.2.Coating preparation
As-ground AZ31 specimens together with 2.5M NaOH solution were put into a Teflon-lined stainless-steel pressure vessel with hydrothermal treatment in an electric oven at 170°C for 3h,to form an Mg(OH)2coating on bare AZ31 alloy.Then,the treated AZ31 specimens were cooled down to room temperature,rinsed with DI water and dried with warm air.
Multilayers were generated as Fig.1 in a sequence of“ABCBCBCBCBC”,in which solutions A and B were cationic PEI(10 g L−1)and anionic DNA(1 g L−1)dissolved in DI water,respectively.Cationic solution C was prepared with 0.1g CHI added in 99mL DI water and 1mL acetic acid,and pH was adjusted with 2.5M NaOH solution to 6.0.Mg(OH)2coated AZ31 pieces were soaked for 20min in Solution A and 5min for solutions B and C.Five cycles were selected based on the preliminary electrochemical test to obtain the(CHI/DNA)5/Mg(OH)2/Mg samples.
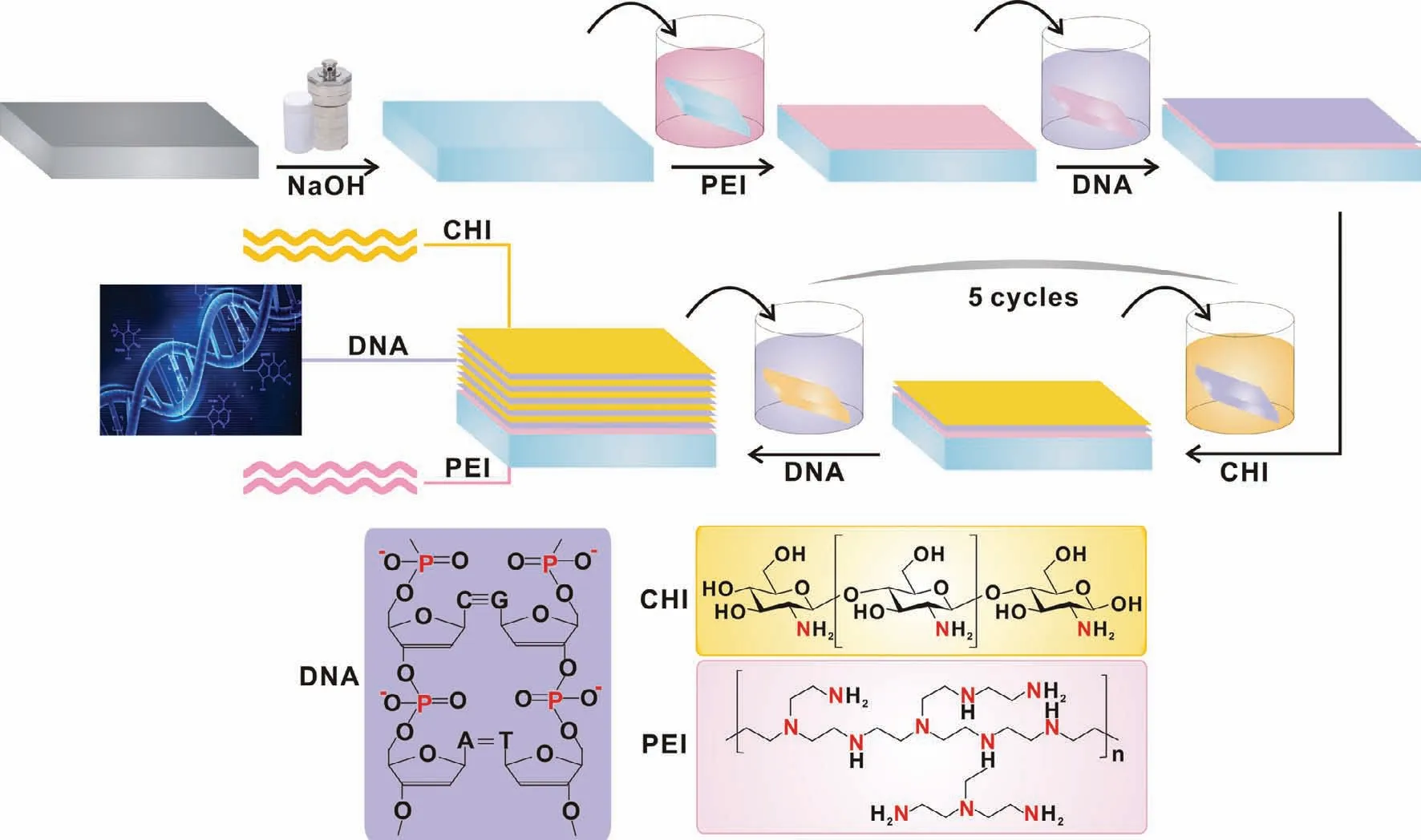
Fig.1.Schematic illustration of preparation of(CHI/DNA)5/Mg(OH)2 composite coating on AZ31 substrate.

Table 1Chemical composition of SBF.
2.3.Surface analysis
Field-emission scanning electron microscopy(FE-SEM,Nova NanoSEM 450,USA)was utilized to describe surface morphologies of the composite coatings.Possible chemical bonding of the composite coating was analyzed through Fourier transform infrared(FTIR)spectrophotometry(Nicolet 380,Thermo Electron Corporation,USA)and X-ray photoelectron spectrometry(XPS,ESCALAB 250,Thermo VG Corporation,USA),respectively.X-ray diffraction(XRD,Rigaku D/MAX 2500PC,Japan)was conducted to determine crystallographic structures.
2.4.Corrosion characterization
Electrochemical analyser(PAR Model 2273,Princeton,USA)with a three-electrode cell set-up was applied to characterize the potentiodynamic polarization and electrochemical impedance(EIS)curves of the samples,in which the prepared samples with an exposed area of 1 cm2,a platinum sheet and a saturated calomel electrode were appointed as the working,counter and reference electrodes.Simulated body fluid(SBF)with a pH value of 7.4 was selected as the corrosive medium(Table 1).Prior to the EIS test,a stable open-circuit potential(OCP)was achieved with 10min.Afterwards,EIS measurements were carried out at the frequency from 100kHz to 0.01Hz for a 10-mV sinusoidal amplitude.The potentiodynamic polarization was performed over a potential range of−2.0 to−1.0V at a scan rate of 1 mV s−1.The obtained EIS curves were matched with appropriate equivalent circuit(EC)models by the ZSimpWin software(Version 3.10,USA).Tafel extrapolation was a means of fitting electrochemical parameters,including corrosion potential(Ecorr),corrosion current density(icorr),anode Tafel slopes(βa)and cathode Tafel slopes(βc).Polarization resistance(Rp)was calculated by the following Stern-Geary equation(Eq.(1))[51,52].Triplicate measurement was necessary for all electrochemical experiments.

An obsequent funnel connected with an acid burette was employed to monitor the hydrogen evolution process in SBF at 37 °C for 432h with full surface exposure[53],and quintuplicate samples were tested to maintain the reproducibility of the obtained data.
2.5.Antibacterial assays
Plate-counting method was utilized to characterize the bactericidal behavior of the composite coating against gramnegative Staphylococcus aureus(S.aureus)and gram-positive Escherichia coli(E.coli).Both microbes were cultured inagar plates at 37 °C all night long.The optical densities(OD)of 0.699 at a wavelength of 650nm and 0.600 at a wavelength of 600nm were adjusted for S.aureus and E.coli,respectively,indicating that a concentration of 109colony-forming units(CFU)·mL−1of the test organism has been approximately reached.Then,the microbe strains were diluted 1000 and 100 times by 0.8% NaCl solution and broth in order.Afterwards,the sample was put into 6mL of broth containing 104CFU mL−1of S.aureus or E.coli.With a treatment of 2h,the suspension was continually diluted for 1000 times with 0.8% NaCl solution,and the suspension involving S.aureus or E.coli was swabbed onto an agar plate.After an 16h incubation at 37 °C,the colonies were then counted to estimate the bacteriostatic ability of the(CHI/DNA)5/Mg(OH)2coating.Each test was performed in triplicate.
2.6.Cytocompatibility tests
2.6.1.Cell proliferation assay
For the in vitro cell culture experiments,mouse MC3T3-E1 pre-osteoblast was selected,culturing with different extracts,to assess the cytocompatibility of the composite coating using indirect contact method.Alpha minimum essential medium(α-MEM)with the addition of 1% penicillin-streptomycin solution and 10% foetal bovine serum(FBS)was used to cultivate cells in a humidified atmosphere with 5% CO2at 37 °C[54].
The extracts were prepared with a sample surface area to α-MEM ratio of 1 mL cm−2in a humidified atmosphere with 5% CO2at 37 °C for 3 days after a sterilization under high temperature and pressure.The fluid supernatant was gathered and stored at-20 °C,and the pH values could be tested by a pH meter(pH-400).A 96-well plate was utilized to culture the seeded cells at a density of 3000 cells per well for 24h to allow attachment.Afterwards,the cell culture fluid was changed to 100μL ofα-MEM supplemented with 10%,25%,50%,75%,100% extracts and 10% FBS.With a culture of 1,3 and 5 days,the obtained extracts were added to each well,superseded with the fresh medium and 10μL of 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide solution(MTT,M8180,Solarbio,China).4h later,the bluepurple crystal was generated and dissolved by dimethyl sulfoxide(DMSO).A microplate reader(Multiskan Go 1510,Thermo Fisher Scientific,China)was used to measure the absorbance at a wavelength of 490nm.The cell viabilities were calculated as follows[51]:
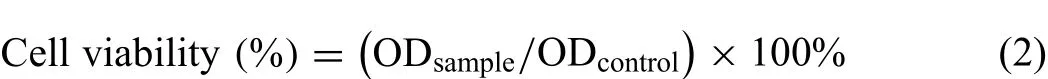
where ODsampleand ODcontrolrepresent the OD values of cells cultured with and without the samples.
2.6.2.LIVE/DEAD viability assay
A 24-well plate was used to incubate the osteoblasts with a density of 5000 cells per well.After 6h,the culture medium for osteoblasts was changed to a 500μL mixed media,containing 25%,50%,75%,100% extracts and the rest regular cell culture media.Afterwards,cells were cultured in a humidified atmosphere with 5% CO2at 37 °C.24h later,they were continually cultured in the mixed solution with the composition of calcein-AM and ethidium homodimer(LIVE/DEADR○assay,L3224,Invitrogen,China)for 30min at room temperature in the dark,subsequently,removing the culture fluid and rinsing with PBS solution.The cells were observed by a fluorescence microscope(EVOS,Invitrogen,China),and the cells incubated in fresh media without samples were used as negative controls and each test was conducted in triplicate.
2.6.3.Statistical analysis
Data were presented as the mean±standard deviation(SD)for in vitro cytocompatibility tests.Independent-samples t-test was used to determine the level of significance(∗P<0.05 or∗∗P<0.01),and∗p<0.05 was considered to be statistically significant.
3.Results
3.1.Surface analysis
Fig.2 shows SEM images of the morphologies of bare and coated AZ31 samples.It can be seen that the original surface of AZ31 shows abrasive scratches from mechanical polishing,and a fish-scale-like nanostructure can be seen in the enlarged image(Fig.2a and b).After treatment with(CHI/DNA)5multilayer,the sample surface is consisted of considerable number of micro-cracks due to the interaction between polyelectrolytes and AZ31 substrate during dipping process(Fig.2c and d).Non-uniform hexagonal crystals are formed on the surface of Mg(OH)2coating(Fig.2e and f).For the(CHI/DNA)5/Mg(OH)2composite coating,larger size of hexagonal crystals can be seen,and the coating becomes more compact in comparison with the Mg(OH)2coating,though the outer(CHI/DNA)5multilayer is too thin to cover the original surface contour of the inner Mg(OH)2layer.The inner Mg(OH)2coating is conduced to the deposition of(CHI/DNA)5multilayer by lowering hydrogen evolution.
The cross-sectional morphology of(CHI/DNA)5/Mg(OH)2coating is shown in Fig.3.The whole thickness of the composite coating is about 18μm,while the thickness of the outer(CHI/DNA)5multilayer is too thin to be differentiated.Fig.4 exhibits the XRD patterns of various samples,including bare AZ31,Mg(OH)2and(CHI/DNA)5/Mg(OH)2coatings.Except forα-Mg for the AZ31 substrate,the main composition of the Mg(OH)2and(CHI/DNA)5/Mg(OH)2coatings is Mg(OH)2.Next,XPS analysis will be used to confirm the construction of the outer(CHI/DNA)5multilayer.
To further investigate the LbL assembled outer(CHI/DNA)5multilayer,XPS analysis was employed on the surface of the(CHI/DNA)5/Mg(OH)2coating(Fig.5).Elemental of P,C,N and O can be found at the sample surface.The C1sspectrum has four peaks at 284.1(C–C/C–H),285.6(C–N),286.6(C–O)and 287.1(C=O)eV[55,56].Two peaks at 132.9 and 133.8eV for the P2pspectrum exhibit the presence of P-O−and P-OH groups,respectively,which confirm the assembly of DNA molecules[56].Furthermore,CHI in the multilayers can be demonstrated by the two peaks of the N1sspectrum:one at 398.5eV and another at 399.9eV,corresponding to-NH2and–NH3+groups[55,57].
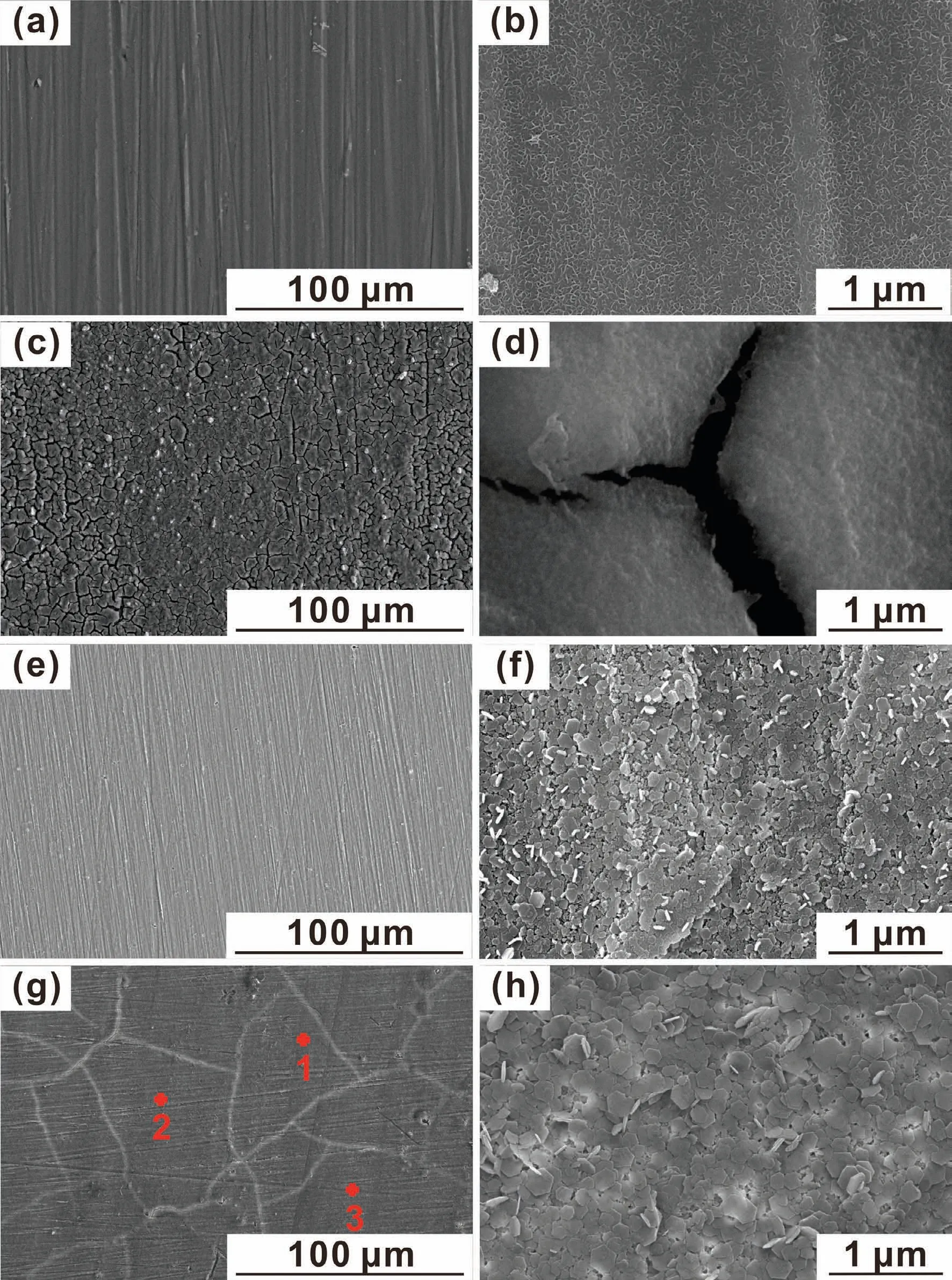
Fig.2.SEM surface morphologies of the(a and b)bare AZ31,(c and d)(CHI/DNA)5,(e and f)Mg(OH)2 and(g and h)(CHI/DNA)5/Mg(OH)2 coatings.
3.2.Electrochemical behaviors
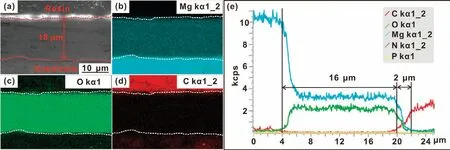
Fig.3.(a)Cross-sectional SEM images,elemental distributions of(b)Mg,(c)O,(d)C and(e)EDS with line scanning of the(CHI/DNA)5/Mg(OH)2 coating.
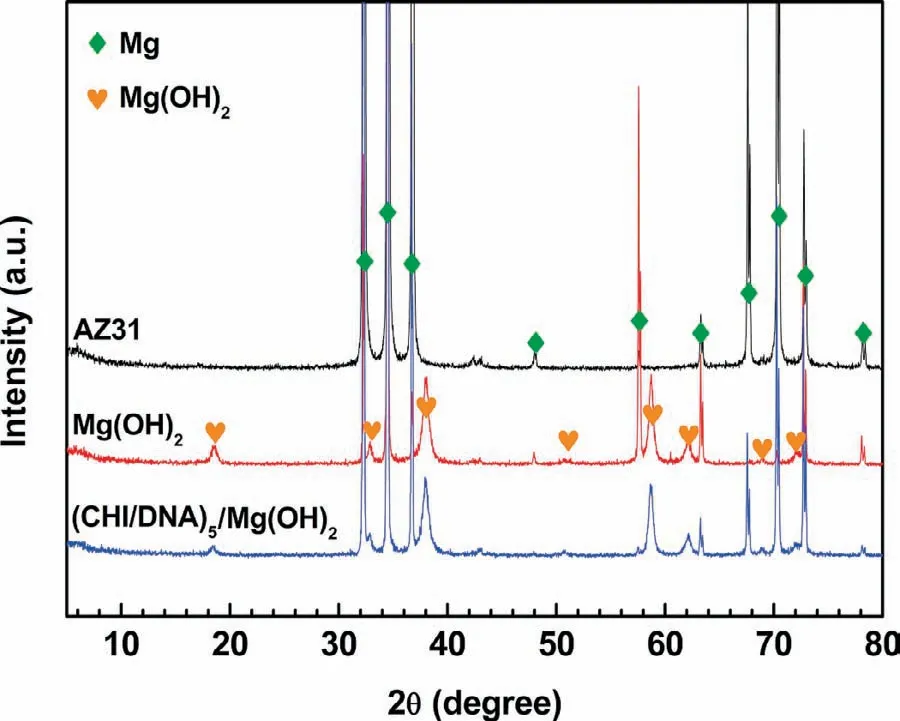
Fig.4.XRD patterns of the bare AZ31,Mg(OH)2 and(CHI/DNA)5/Mg(OH)2 coatings.
The potentiodynamic polarization curves of the three samples are shown in Fig.6,and the corresponding electrochemical parameters are summarized in Table 2.The Ecorrof the AZ31 substrate,which respectively coated with Mg(OH)2or(CHI/DNA)5/Mg(OH)2coatings,is−1.52 or−1.57V,both of which are more positive than that of bare AZ31(−1.74V).The icorrof Mg(OH)2or(CHI/DNA)5/Mg(OH)2coated samples is 1.84×10−7or 1.54×10−7A·cm−2,which decreases by approximate two orders of magnitude than AZ31 substrate(7.63×10−5A·cm−2),indicating that both Mg(OH)2and(CHI/DNA)5/Mg(OH)2coatings enhance the corrosion resistance of AZ31 alloys.Besides,these results also show that the(CHI/DNA)5/Mg(OH)2coated sample has the largest Rpamong all the samples,indicating the best corrosion protection for Mg alloy.It may be ascribed to that most of the holes of the Mg(OH)2coating are sealed by the outer polyelectrolyte multilayer(Fig.2).
Fig.7 displays the EIS curves of the uncoated and coated samples in SBF.Two capacitive loops from the high to low frequency region can be fitted for all samples,attributing to charge transfer reaction and mass transportation during the corrosion process,but the diameters of capacitive loops become obviously larger after the Mg(OH)2and(CHI/DNA)5/Mg(OH)2treatment,demonstrating the good corrosion resistance of the two coatings,especially for the(CHI/DNA)5/Mg(OH)2composite coating,which is in good accordance with polarization results in Fig.6.
As shown in Fig.7c,an EC,composed of solution resistance(Rs),coating resistance(Rf),charge transfer resistance(Rct)and constant phase element(CPE),is used for fitting the obtained experimental data.The CPE is described as:ZCPE=1/Y0(jω)n,where Y0andω(ω=2πf)are the magnitude of capacitance and angular frequency,respectively,and n(n=0~1)is the exponential term of CPE,representing deviation from the ideal capacitance[22].Generally,the higher Rctpresents the better corrosion resistance of the samples[58].The Rctof(CHI/DNA)5/Mg(OH)2coating(3.28×105Ω·cm2)is significantly higher than that of Mg(OH)2coating(6.78×104Ω·cm2)and the bare AZ31(25.43Ω·cm2),demonstrating the reduction of electrochemical degradation rate.Thus,the(CHI/DNA)5/Mg(OH)2coating processes a good corrosion protection for the Mg alloys,which will be confirmed by the next immersion test.
3.3.Degradation behaviors
The obtained samples were soaked in SBF for 432h to monitor the variations of hydrogen evolution volume(HEV)and hydrogen evolution rate(HER)(Fig.8).A(CHI/DNA)5polyelectrolyte coating was constructedon the AZ31 substrate to confirm the beneficial effect on inhibiting corrosion process during immersion.The HEV after 432h immersion can be ranked in a decreasing order as follows:AZ31(8.90±2.49 mL cm−2)>(CHI/DNA)5coating(6.66±0.02 mL cm−2)>Mg(OH)2coating(5.92±0.69 mL cm−2)>(CHI/DNA)5/Mg(OH)2coating(3.72±1.72 mL cm−2).And the differences of HEV between(CHI/DNA)5and Mg(OH)2coating diminish gradually(Fig.8a).In stage I and II,the Mg(OH)2coating exhibits an obvious inhibition of corrosion in comparison of the(CHI/DNA)5coating.Although the HEV of(CHI/DNA)5coating increases signally,the growth trend gradually levels off until it is similar to the Mg(OH)2coating after an immersion of 270h(stage III).For the corresponding HER curves in Fig.8b,a same decreasing order as HEV curves can be seen in the I and II stages.The initial decrease can be ascribed to the dissolution of the alloy substrate or the coating and the formation of corrosion precipitates.The lower HER of the Mg(OH)2coating is explained by physical barrier.While in the III stage,the HER of(CHI/DNA)5coating is become lower than the Mg(OH)2coating due to the deposition of large amount of corrosion products.Based on the dual effect of the Mg(OH)2and polyelectrolyte coatings on protection,the(CHI/DNA)5/Mg(OH)2coating processes a good corrosion resistance in SBF.These results are in good agreement with icorrand Rctfrom electrochemical tests(Fig.6 and 7).Table 3.
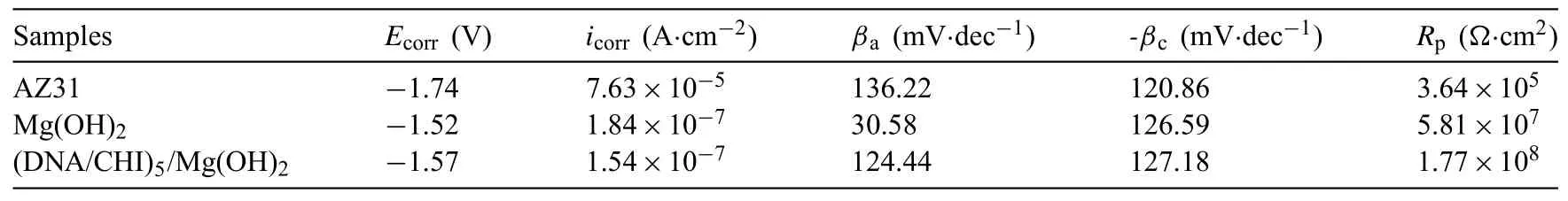
Table 2Electrochemical parameters of the polarization curves for bare AZ31,Mg(OH)2 and(DNA/CHI)5/Mg(OH)2 coatings in SBF.
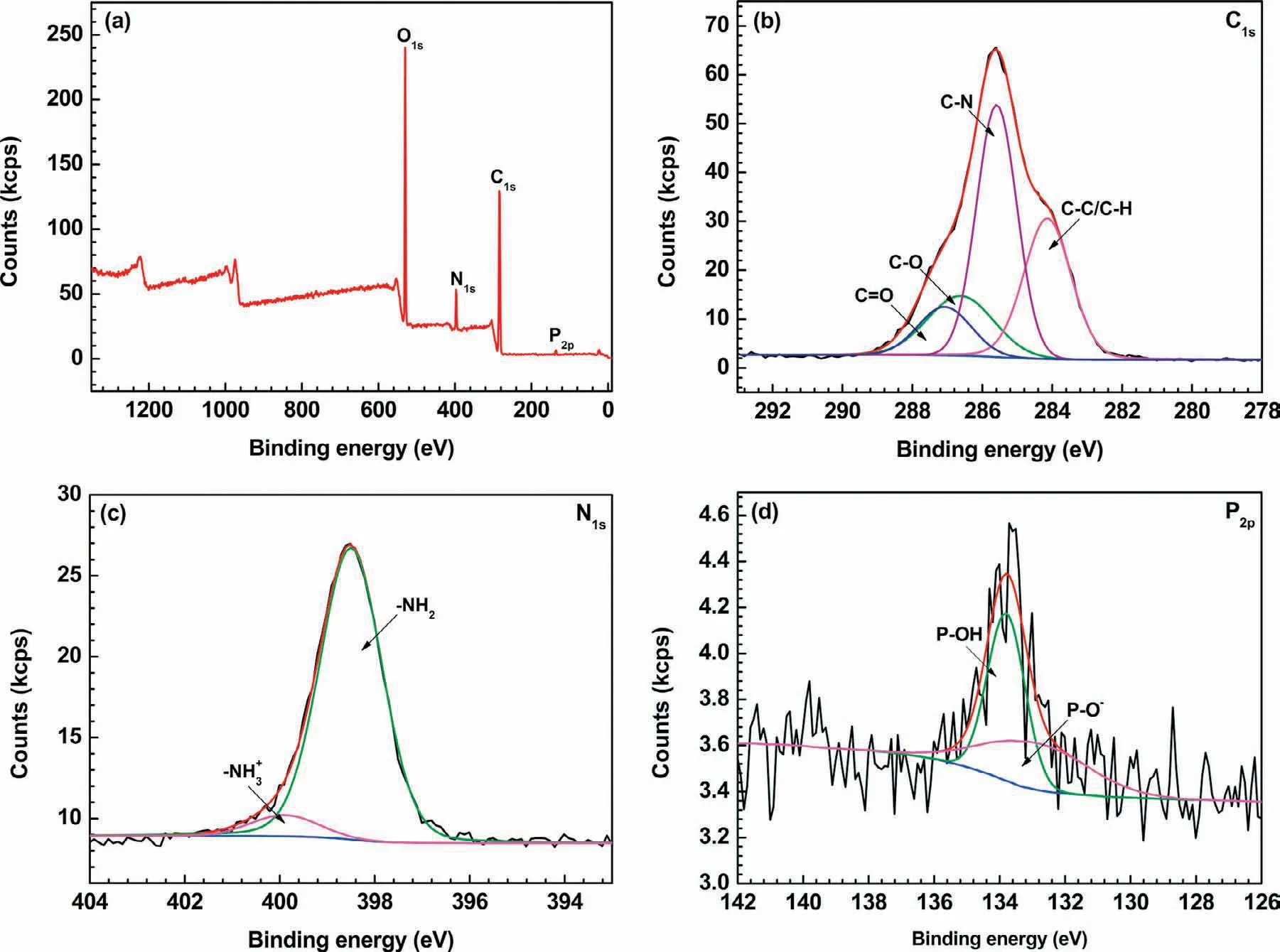
Fig.5.XPS spectra of(CHI/DNA)5/Mg(OH)2 coating:(a)survey spectrum,(b)C1s,(c)N1s and(d)P2p regions.
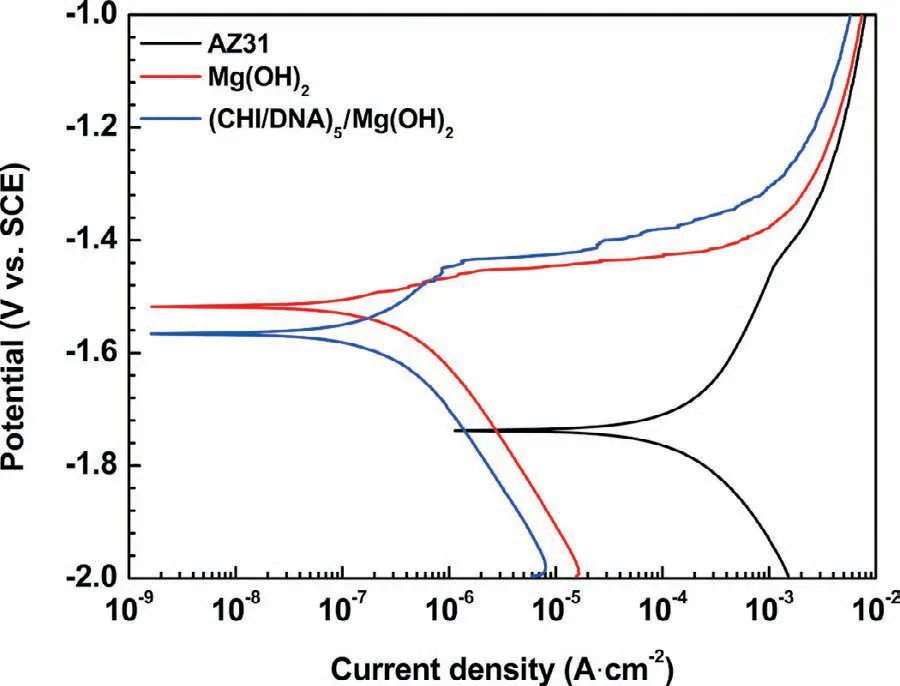
Fig.6.Potentiodynamic polarization curves of uncoated and coated samples in SBF.
Fig.9 exhibits the camera photographs and SEM images of the samples after a 432-h immersion in SBF.Deep networks of pitting and cracks occurs on the bare AZ31,indicating a serious corrosion attack(Fig.9a and e).The Mg(OH)2coating is obviously broken and peeled off(Fig.9b and f).In comparison with the bare Mg,fine microcracks are found on the surface of(CHI/DNA)5coated samples(Fig.9c and g).While for the(CHI/DNA)5/Mg(OH)2coated sample(Fig.9d and h),microcracks can be seen on the composite coating with some cluster of corrosion products.Thus,the obtained(CHI/DNA)5/Mg(OH)2coating provides a good corrosion protection during immersion.
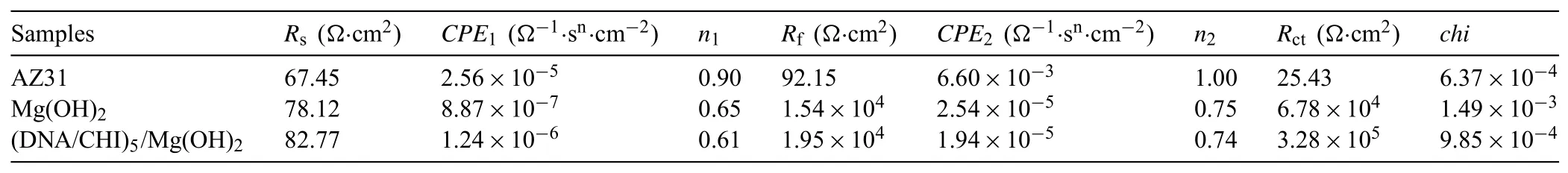
Table 3Electrochemical data obtained by EC fitting of EIS curves.
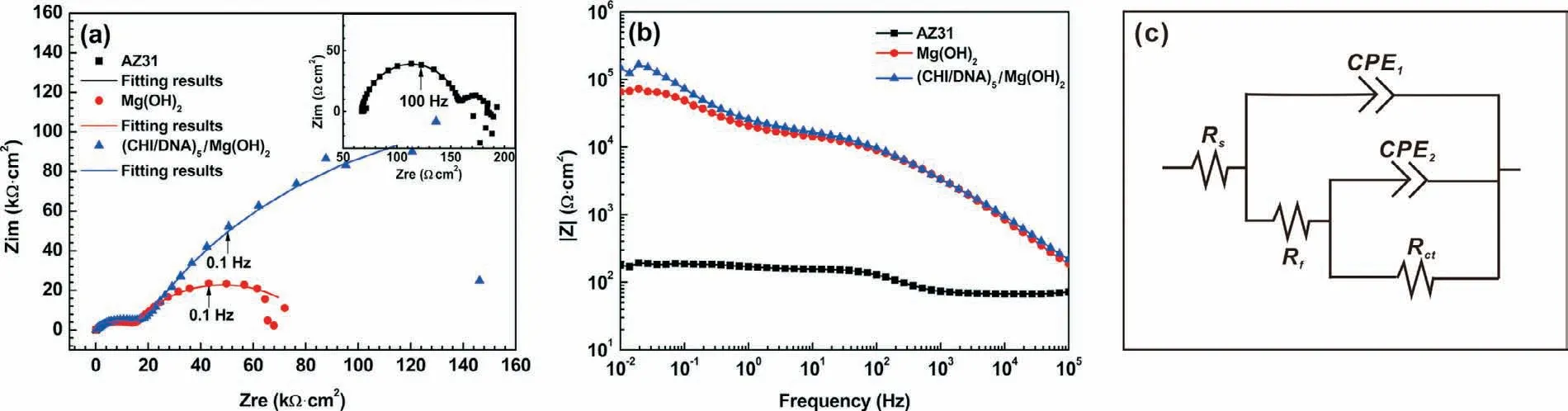
Fig.7.(a)Nyquist,(b)Bode plots and(c)the corresponding EC model of the samples in SBF.

Fig.8.(a)HEV and(b)HER curves of the samples with a 432h immersion in SBF.
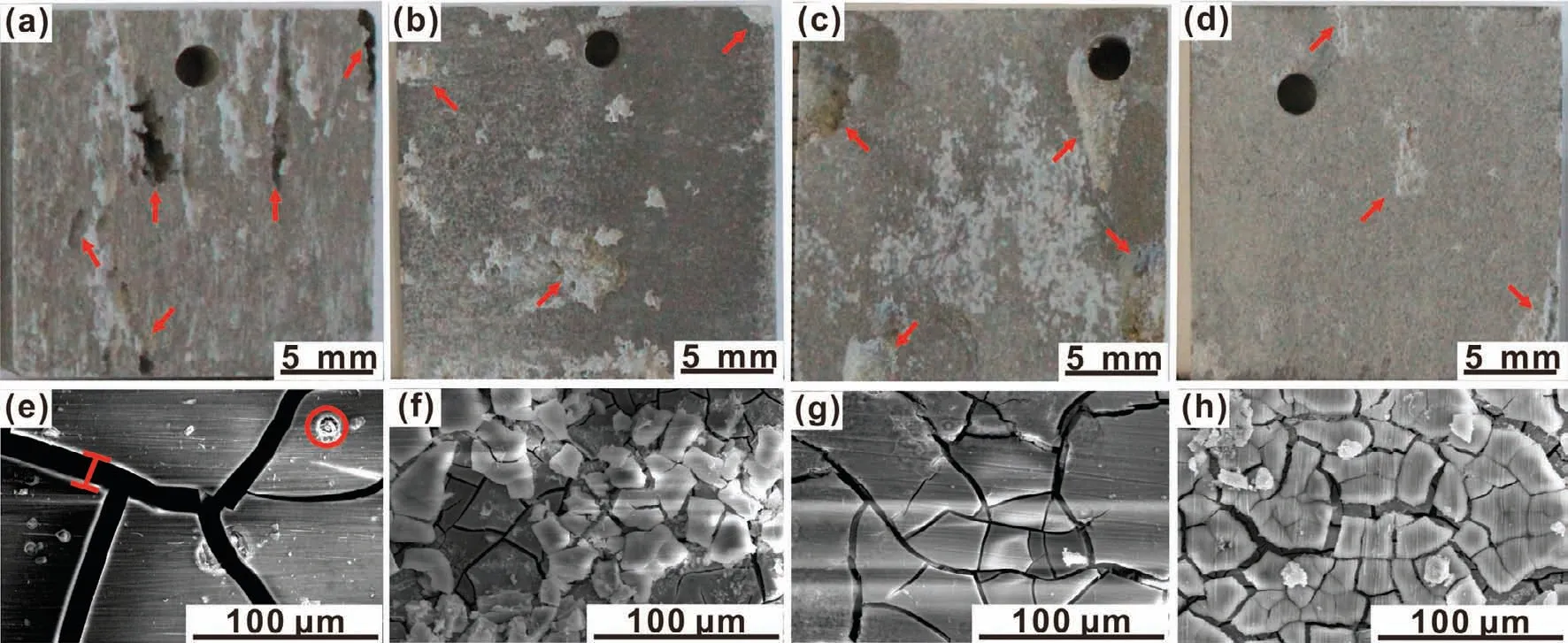
Fig.9.Digital camera photographs and SEM images of the(a and e)AZ31 substrate,(b and f)Mg(OH)2,(c and g)(CHI/DNA)5 and(d and h)(CHI/DNA)5/Mg(OH)2 coatings after an immersion of 432h in SBF.
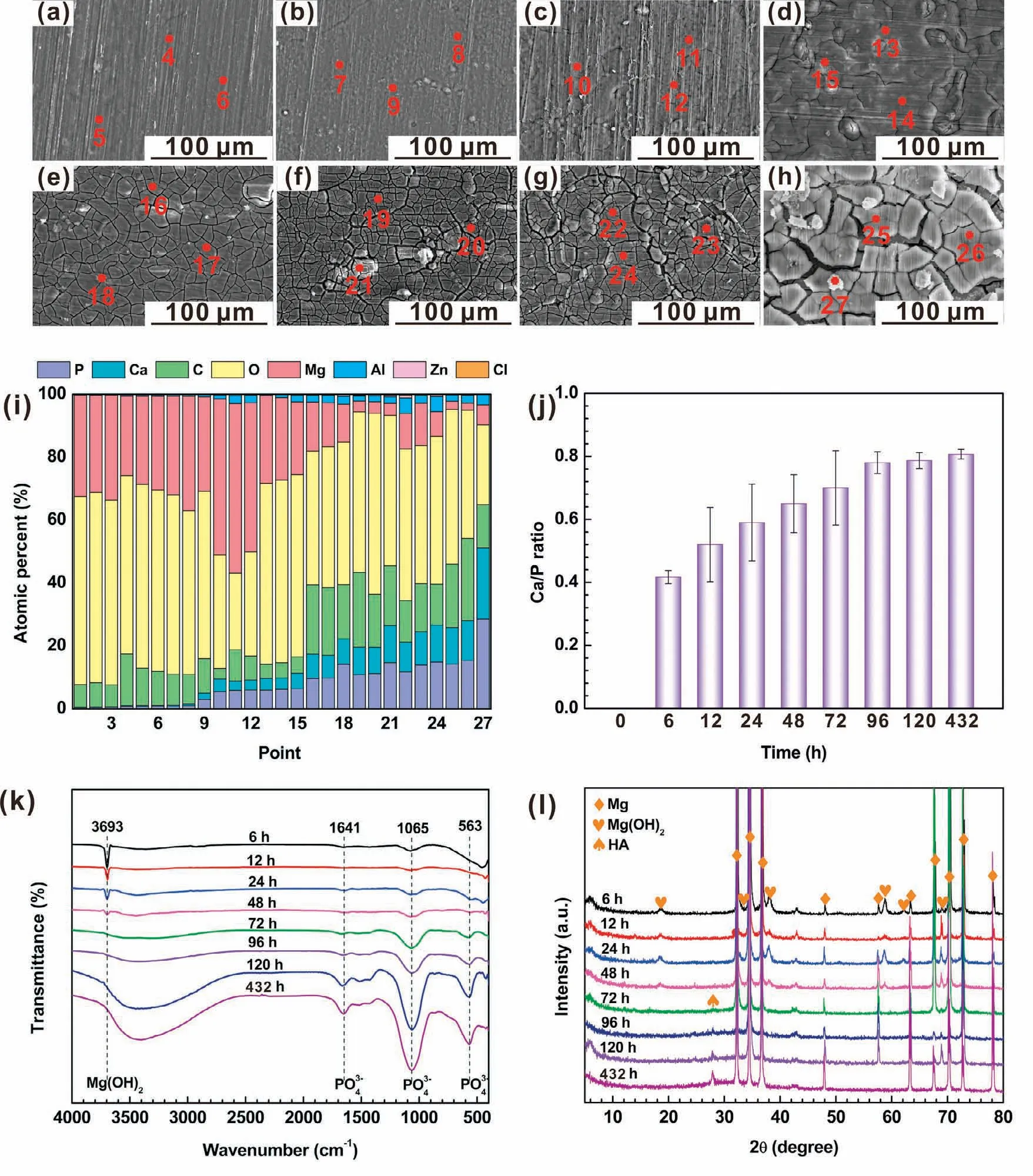
Fig.10.(a-g)SEM images of the(CHI/DNA)5/Mg(OH)2 coating after a(a)6,(b)12,(c)24,(d)48,(e)72,(f)96,(g)120 and(g)432h immersion in SBF;(i)EDS spectra(pointed in Fig.2g and 10a-h),(j)corresponding Ca/P molar ratio,(k)FTIR spectra and(l)XRD patterns of(CHI/DNA)5/Mg(OH)2 coating with different immersion time in SBF.
The SEM images of the(CHI/DNA)5/Mg(OH)2coating with different immersion time are displayed in Fig.10a-h.Corrosion products and cracks were generated on the obtained coating surface after a 24h immersion,which accumulated and enlarged over immersion time.Fig.10i and j exhibit the EDS spectra and corresponding Ca/P ratio of the samples with different immersion time.Elementals Mg,C,O,Ca,P,Al,Zn and Cl present on the sample surface.Within a 96-h immersion,the Ca,P elemental contents and Ca/P ratio increase.After then,they remain relatively stable,which can be ascribed to that a balance achieves between the adsorption of the Ca2+and PO43−ions and the degradation of the Ca-P precipitates.Note that,before an immersion of 6h,there is absence of P can be detected by EDS due to the really thin thickness of the outer(CHI/DNA)5multilayer(Fig.3).The FTIR spectra with different immersion time(Fig.10k)demonstrate that the existential form of P element in the corrosion product is PO43−.Furthermore,the main corrosion precipitate is Ca-deficient hydroxyapatite(HA),which can be confirmed in the XRD patterns(Fig.10l).The decreased intensity of Mg(OH)2peaks and increased intensity of HA peaks in both FTIR spectra and XRD patterns also illustrate the more deposition of the HA precipitate,which can be ascribed to the mineralization effect of the outer(CHI/DNA)5multilayer.
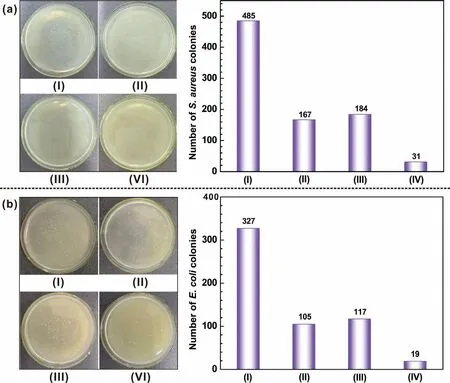
Fig.11.Antibacterial properties of different samples against for(a)S.aureus and(b)E.coli:(I)blank,(II)bare AZ31,(III)Mg(OH)2 coating and(IV)(CHI/DNA)5/Mg(OH)2 coating.
3.4.Antibacterial activity
Fig.11 exhibits the antibacterial ability of the coated and uncoated specimens.The AZ31 substrate and(CHI/DNA)5/Mg(OH)2coating surface present an obviously less number of live bacteria than Mg(OH)2coating.For the bare AZ31,a large amount of Mg2+ions were released resulted from the rapid degradation,leading to an intense bactericidal effect due to the large osmotic stresses originated over the cells[59].In addition,the increase of pH due to the contact reaction between AZ31 substrate and bacterial suspension also causes the death of bacterial[60].For the(CHI/DNA)5/Mg(OH)2coating,the good antibacterial ability can be ascribed to the contact-killing strategy of CHI,which can interact with the negatively charged bacterial cell and leads to bacterial death[42,61,62].
3.5.In vitro biocompatibility
MC3T3-E1 cells are utilized to check the cell viability with presence of extracts for coated and uncoated samples with different concentration.The corresponding pH values are 9.32±0.02 for AZ31,9.27±0.25 for Mg(OH)2coating and 9.13±0.45 for(CHI/DNA)5/Mg(OH)2coating in pure 100% extracts.Fig.12a and b exhibits the OD values and cell viabilities after a 1,3 and 5-days incubation for pre-osteoblasts.The three 100% extracts significantly reduce the viability of MC3T3-E1 cells to 90%,which is acceptable for the samples acted as the bone implant material[63].The reason for the decrease of the cell viability may be ascribed to the high alkalinity resulted from the degradation of the samples and the electrical difference between the positive charged CHI and negative charged cytomembrane of MC3T3-E1 cells.After stepwise dilutions,the cell viability increased to more than 100%,implying the active effect of the(CHI/DNA)5/Mg(OH)2coating for promoting the growth of bone.Meanwhile,Live/Dead staining of osteoblasts after an incubation of 24h could be seen in Fig.12c-q.The cells for all samples perform generally healthy fusiform-like shape,widely spreading with respect to morphology,which display an acceptable and enhanced cytocompatibility to osteoblasts.
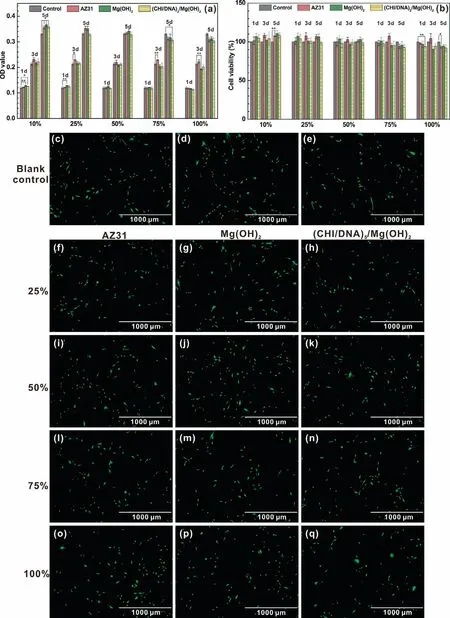
Fig.12.(a)OD values and(b)Cell viability of MC3T3-E1 cultured in different extracts prepared with negative control,AZ31 substrate,Mg(OH)2 and(CHI/DNA)5/Mg(OH)2 coatings for 1,3 and 5 days.Statistically significant differences(∗p<0.05,∗∗p<0.01);Fluorescent images(c-q)of MC3T3-E1 after an incubation for 24h in different extracts of the(c-e)negative control,(f,i,l and o)AZ31 substrate,(g,j,m and p)Mg(OH)2 and(h,k,n and q)(CHI/DNA)5/Mg(OH)2 coatings.
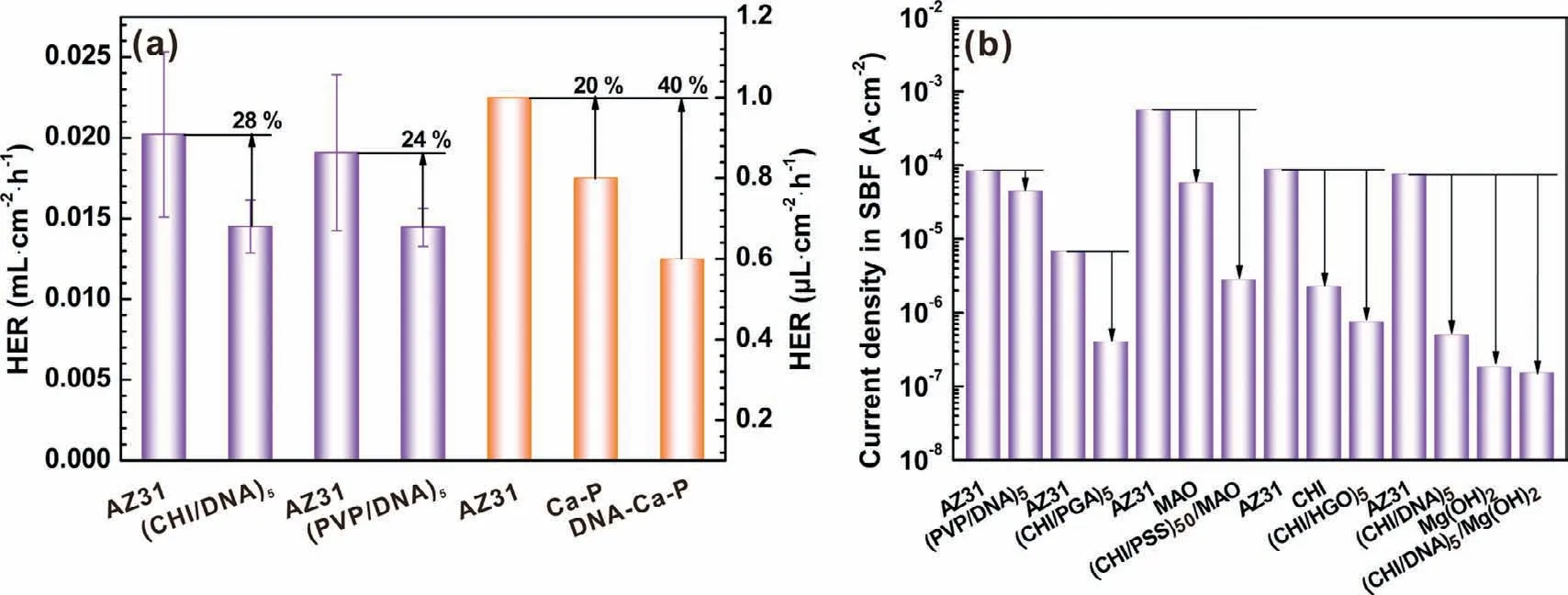
Fig.13.(a)Comparison of HER between(PVP/DNA)5,(CHI/DNA)5 and DNA induced Ca-P coatings,and(b)comparison of icorr among some LbL assembled coatings based on DNA or CHI in SBF[37,39,42,68].
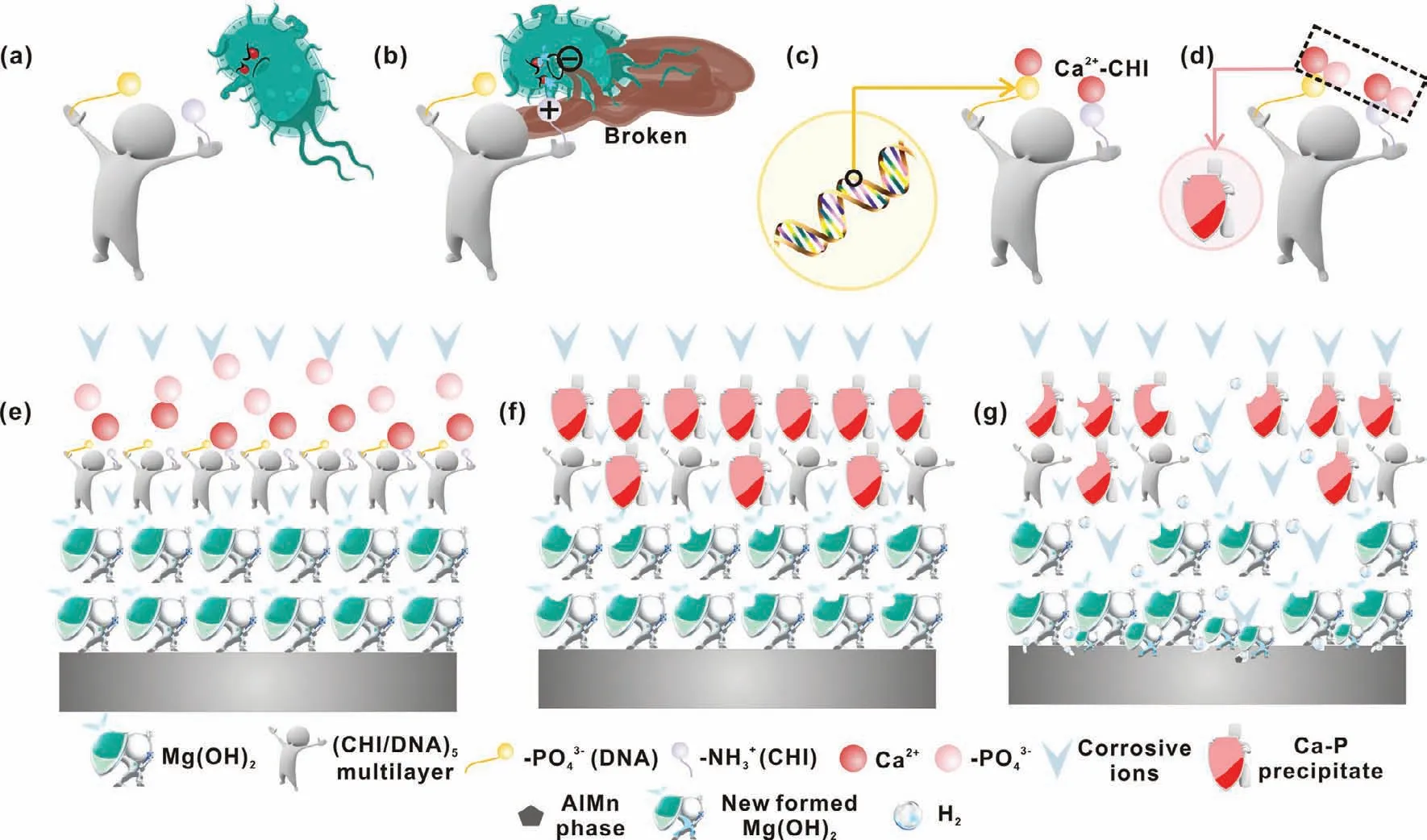
Fig.14.Schematic representation of the antibacterial and degradation mechanism of the(CHI/DNA)5/Mg(OH)2 coating:(a and b)contact-killing strategy of the CHI,(c and f)Ca-P nucleation and growth,(g)electrochemical corrosion of the substrate.
4.Discussion
In our previous study,a similar(polyvinylpyrrolidone(PVP)/DNA)5multilayer was formed using dip coating method[39].A comparison of HER between(PVP/DNA)5and(CHI/DNA)5coatings is shown in Fig.13a.The decreased HER of the(CHI/DNA)5coating is slightly higher than that of(PVP/DNA)5coating,which may be ascribed to the better mineralization effect of the outermost polyelectrolyte CHI layer.CHI attachment increases hydrophilicity of Mg substrates and offers amino groups as active binding or chelating sites for Ca2+and PO43−ions[64–66].The mineralization effect also depends on the amount and nature of chemical groups and surface charges,which should be explored in further studies[67].In comparison of the Ca/P ratio(0.78)for(PVP/DNA)20coating after an immersion of 48 h[39],the Ca/P ratio of(CHI/DNA)5/Mg(OH)2coating increases to 0.81 with a 96h immersion,which is attributed to the reduction in Mg2+release resulted from the physical barrier of inner Mg(OH)2coating.Another comparison of reduced HER between(CHI/DNA)5and DNA induced Ca-P coatings illustrates that DNA in(CHI/DNA)5coating exhibits a good protection for the AZ31 alloy(Fig.13a),due to the molecular structure of DNA in Ca-P coating may be changed and inactivated during the hydrothermal process[37].The irregular morphology of the DNA induced Ca-P coating may be attributed to the heterogeneous adsorption in the initial hydrothermal reaction[37].And the surface of the LbL assembled(CHI/DNA)5coating is more homogeneous after a total soaking of 11 times.
Fig.13b displays a comparison about the current density of some LbL assembled coatings based on DNA or CHI in SBF.It is obvious that CHI is a better positive charged polyelectrolyte than PVP to advance the corrosion resistance of Mg alloys,which also is a good choice for constructing a antibacterial LbL assembled coatings[39].For the coatings with similar CHI layers,all negative charged polyglutamic acid(PGA)[42],heparin-functionalized graphene oxide(HGO)[68]and DNA enhance the anti-corrosion property greatly,and DNA exhibits the greatest improvement of corrosion resistance for Mg alloys,which can be ascribed to its high molecular weight and double helix structure.Note that,when the LbL assembly dip coating method is used,forming composite coating is the most important means to develop the corrosion resistance of Mg alloys,especially for the inorganic coating as the inner layer to decrease the degradation rate from initial dipping process.Layer number becomes another factor for the properties of the composite coatings.
The antibacterial property of the samples can be ascribe to the contact-killing strategy between the positive charged CHI layer and the negative charged bacterial cytomembrane[42,61](Fig.14a and b).The degradation mechanisms of the(CHI/DNA)5/Mg(OH)2coating are shown in Fig.14c-g.During immersion,Mg(OH)2coating is a physical barrier,which can prevent the penetration of corrosive ions.Although the(CHI/DNA)5coating is really thin,it can be acted as an inducement to construct a HA corrosion product film for longtime protection(Fig.14c and d)[69].The induction mechanism can be attributed to four reasons:(1)the phosphate groups in DNA molecules lead to the nucleation and growth of heterogeneous Ca-P precipitates[39];(2)nucleation kinetics or the crystallinity of mineralized material can be positively affected through secondary structure of folded DNA fragments[36];(3)the molecular recognition of-NH2in CHI generates the Ca2+-CHI coordination compound[65];(4)the reticular structure of the LbL assembled coating blocks the Ca2+and PO43−ions from SBF solution.Furthermore,the new formed Mg(OH)2corrosion products also can be used as physical barrier to protect Mg alloy from corrosion.Of course,the galvanic corrosion betweenα-Mg and AlMn phase initiates the pitting corrosion of the Mg alloys.Note that,the good biomineralization effects of the DNA and CHI make up for the deficiency of the thin thickness of the outermost polyelectrolyte multilayers,especially during the degradation process in SBF,which is also beneficial to the growth of human bone.
5.Conclusions
The obtained(CHI/DNA)5/Mg(OH)2coating shows uniform hexagonal crystal surface with improved corrosion resistance,antibacterial activity and cytocompatibility.
(1)The(CHI/DNA)5/Mg(OH)2coating possesses a good long-time corrosion protection for Mg alloy based on a dual effect including:the Mg(OH)2coating is a physical barrier,which can prevent the penetration of corrosive ions;the(CHI/DNA)5coating can be acted as an inducer to construct a HA corrosion product film for long-time protection.
(2)The(CHI/DNA)5/Mg(OH)2coating possesses significant antibacterial effects due to the antibacterial activity of CHI,which can interact with the negatively charged bacterial cell and leads to bacterial death based on the contact-killing strategy.
(3)The good biomineralization effects of the DNA and CHI make up for the deficiency of the thin thickness of the outermost polyelectrolyte multilayers,exhibiting an active effect for promoting the growth of bone,which is promising to be used as bone implants.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(51571134),Shandong Provincial Natural Science Foundation(ZR2017BEM002),and Shandong University of Science and Technology Research Fund(2014TDJH104).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Tailoring the degradation rate of magnesium through biomedical nano-porous titanate coatings
- Modified embedded-atom method interatomic potentials for Mg–Al–Ca and Mg–Al–Zn ternary systems
- Dual alloying improves the corrosion resistance of biodegradable Mg alloys prepared by selective laser melting
- The corrosion behavior of Mg–Nd binary alloys in the harsh marine environment
- In vitro and in vivo evaluations of Mg-Zn-Gd alloy membrane on guided bone regeneration for rabbit calvarial defect
- Relieving segregation in twin-roll cast Mg–8Al–2Sn–1Zn alloys via controlled rolling
