Neoadjuvant chemoradiation is associated with decreased lymph node ratio in borderline resectable pancreatic cancer: A propensity score matched analysis
2021-03-05JunePengGarethMorrisStiffNoamanAliJaneWeySricharanChalikondaKevinElHayekMatthewWalsh
June S Peng , Gareth Morris-Stiff , Noaman S Ali , Jane Wey , Sricharan Chalikonda ,Kevin M El-Hayek , R Matthew Walsh
a Department of Surgery, The Pennsylvania State University, College of Medicine, Hershey, PA 17033-0850, USA
b Department of General Surgery, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, Cleveland, OH 44195-0 0 01, USA
Keywords:Neoadjuvant therapy Lymph node ratio Pancreatic cancer Borderline resectable Vein resection
ABSTRACT Background: Lymph node ratio (LNR) and margin status have prognostic significance in pancreatic cancer.Herein we examined the pathologic and clinical outcomes in patients with borderline resectable pancreatic cancer (BRPC) following neoadjuvant therapy (NAT) and pancreaticoduodenectomy.Methods: Patients who underwent treatment between January 1, 2012 and June 30, 2017 were included.Sequential patients in the BRPC group were compared to a propensity score matched cohort of patients with radiographically resectable pancreatic cancer who underwent upfront surgical resection. The BRPC group was also compared to sequential patients with radiographically resectable pancreatic cancer who required vein resection (VR) during upfront surgery.Results: There were 50 patients in the BRPC group, 50 patients in the matched control group, and 38 patients in the VR group. Negative margins (R0) were seen in 72%, 64%, and 34% of the BRPC, control,and VR groups, respectively ( P = 0.521 for BRPC vs. control; P = 0.002 for BRPC vs. VR), with 24% of the BRPC group requiring a vascular resection. Nodal stage was N0 in 64%, 20%, and 18% of the BRPC, control,and VR groups, respectively ( P < 0.001 for BRPC vs. control or VR). When nodal status was stratified into four groups (N0, or LNR ≤0.2, 0.2–0.4, ≥0.4), the BRPC group had a more favorable distribution( P < 0.001). The median overall survival were 28.8, 38.6, and 19.0 months for the BRPC, control, and VR groups, respectively (log-rank P = 0.096).Conclusions: NAT in BRPC was associated with more R0 and N0 resections and lower LNR compared to patients undergoing upfront resection for resectable disease.
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in the United States with 57 600 new cases projected for 2020 [1].Long-term prognosis is poor due to advanced stage of disease at presentation and aggressive biologic behavior of this cancer. The two main approaches for treatment of localized disease are upfront resection or neoadjuvant therapy (NAT) followed by surgical resection. Borderline resectable pancreatic cancer (BRPC) and locally advanced pancreatic cancer (LAPC) are defined by increasing vascular involvement by the tumor, and determination of the optimal treatment sequence in this setting can be particularly challenging [2].Retrospective series have demonstrated successful resection in one third of patients with BRPC treated with NAT, with comparable survival to patients with initially resectable tumors [ 3 , 4 ].
Pathologic characteristics including tumor size, positive margins, and nodal metastases have long been implicated as predictors of poor prognosis in pancreatic cancer [5]. Higher lymph node ratio (LNR), defined as the number of positive lymph nodes divided by total number of lymph nodes harvested, has also been shown to predict worse disease-free and overall survival based on analyses of both single institution series and a national cancer database [6–8]. The outcomes and value of LNR in patients with BRPC undergoing NAT have not been specifically addressed, and we examined the outcomes for this group of patients in this study.
Methods
Clinical protocol
A neoadjuvant protocol was initiated in 2012 for patients with BRPC as defined by the National Comprehensive Cancer Network(NCCN) [9]. Patients underwent staging diagnostic laparoscopy with peritoneal lavage for cytology to exclude occult metastatic disease prior to treatment. NAT most commonly included gemcitabine, capecitabine or 5-fluorouracil, and concurrent intensity modulated radiation therapy at 5040–5600 cGy over 28 fractions.Patients were restaged by cross-sectional imaging four to six weeks after completion of NAT. Patients with stable local disease and no evidence of distant metastases were scheduled for surgical resection.
Patients with radiographically resectable pancreatic cancer did not routinely undergo NAT and proceeded to upfront resection.
Patients were routinely reviewed at a multidisciplinary tumor board. Inadequate, poor quality or outdated imaging was repeated to determine resectability status prior to treatment. All patients were typically recommended to undergo adjuvant chemotherapy after surgery, but not all patients received it (either by patient refusal or due to postoperative complications).
Data regarding clinical factors, pathologic, and oncologic outcomes were obtained from a prospective database, supplemented with review of the electronic medical record. Complications reported include occurrences within 90 days of surgery, unless otherwise noted. Survival data were supplemented with publically available death registration data.
Pancreaticoduodenectomy (PD) was performed by six surgeons and intraoperative frozen section examination of the pancreatic and common bile duct margins was included, with additional margins resected if frozen section review was positive for malignancy or atypia. The decision to perform vascular resection was based on intraoperative suspicion of invasion. The resection margins were inked by the surgeon and included marking of the portal and superior mesenteric vein groove, and superior mesenteric artery groove. Pathologic data were reported using standardized templates by pathologists specializing in pancreatic histopathology.Margins were considered negative if the tumor was at least 1 mm from inked margins or edge of the vein cuff. Tumor invasion with positive luminal margin alone was considered negative.
Patient selection
All sequential patients with BRPC based on preoperative imaging, who completed NAT, and underwent PD between January 1,2012 and June 30, 2017 were identified for inclusion. Three groups of patients were identified for inclusion in the study: BRPC, resectable, and resectable with vein resection (VR). The resectable control group was selected using propensity score matching of the BRPC patients to patients treated for radiographically resectable pancreatic cancer who underwent upfront PD during the same time period. BRPC and control patients were matched in a oneto-one ratio using propensity score matching by pathologic tumor size, age, and sex using R-package matching with a caliper of 1 [10].
Due to the concern that differences in clinical outcomes may be due to differences in biologic behavior, vascular involvement, or treatment effect between the BRPC and matched control groups,a VR group was identified which comprised consecutive patients from January 1, 2004 to June 30, 2017 with radiographically resectable pancreatic cancer who required VR at the time of PD but had not undergone NAT. The VR group was meant to represent patients with BRPC or LAPC who were understaged by cross-sectional imaging.
Statistical analysis
Data were described using medians and interquartile ranges(IQR) for continuous variables, and counts and percentages for categorical variables. Univariate analyses were performed using the Chi-square test and Kruskal–Wallis test.
Disease-free survival (DFS) was calculated from the date of surgery to the first radiographic or pathologic evidence of recurrence. Overall survival (OS) was calculated from the date of diagnosis to death or last follow up. For survival analyses, patients who were lost to follow-up were censored at the time of the last normal imaging or clinical visit for DFS and last interaction within the institution’s medical record for OS. Kaplan–Meier survival curves were generated for DFS and OS, and survival was compared using the log-rank test.
Multivariable analyses with Cox proportional hazard models were performed to evaluate for significant predictors of DFS and OS. Due to limited sample size, stepwise selection was not used and the list of variables included in the multivariable model was selected based on significance on univariate comparison of the three groups or thought to be clinically relevant by the authors.
All tests were two-tailed and performed at a significance level of 0.05. All analyses were performed using R software (Version 3.3.1, Vienna, Austria).
Results
Fifty patients with BRPC were included in the study. NAT was comprised of concurrent chemoradiation for 42 patients(21 gemcitabine-based, 18 capecitabine, and three 5-fluorouracil),chemotherapy alone for 4 patients, and chemotherapy followed by chemoradiation for 4 patients.
The matched control group included 50 patients who underwent PD for radiographically resectable pancreatic cancer without NAT. The VR group included 38 consecutive patients with radiographically resectable disease who required vascular resection during upfront surgery.
Demographic and perioperative data for the three groups were shown in Table 1 . The three groups were comparable in terms of age, sex, body mass index, and preoperative carbohydrate antigen 19-9 (CA19-9) levels. The BRPC group was less likely to undergo pylorus preservation and the control group had shorter operative time. The BRPC group experienced fewer biochemical leaks although there was no difference in grade B and C postoperative pancreatic fistulas. There were no differences in length of stay,complications, 30-day readmission, or 30-day mortality rates. Adjuvant therapy was administered to 86%, 88%, and 78% of the BRPC,control, and VR groups, respectively. There were no differences in time to adjuvant therapy, or percentage of patients completed adjuvant therapy.
Pathologic outcomes were summarized in Table 2 . Tumor size,stage, and differentiation were comparable. Resection margins were negative (no cancer within 1 mm of the margin) in 72%,64%, and 34% of patients in the BRPC, control, and VR groups, respectively (P= 0.001). Four patients (8%) in the BRPC group had complete pathologic responses. There were no differences in nodal yield.
Examination of the nodal status showed that the BRPC group had the greatest proportion of N0 disease with 32 of 50 patients(64%), compared to 10 of 50 (20%) in the control group, and 7 of 38 (18%) in the VR group (P<0.001). Median LNR was 0, 0.11, and 0.14 in the three groups (P<0.001). Patients with N1 nodal stagewere stratified into subcategories of LNR ≤0.2, 0.2–0.4, and ≥0.4 based on prior literature [8]. Distribution of patients into these discrete categories was overwhelmingly more favorable for the BRPC group (P<0.001), as shown in Table 3 .
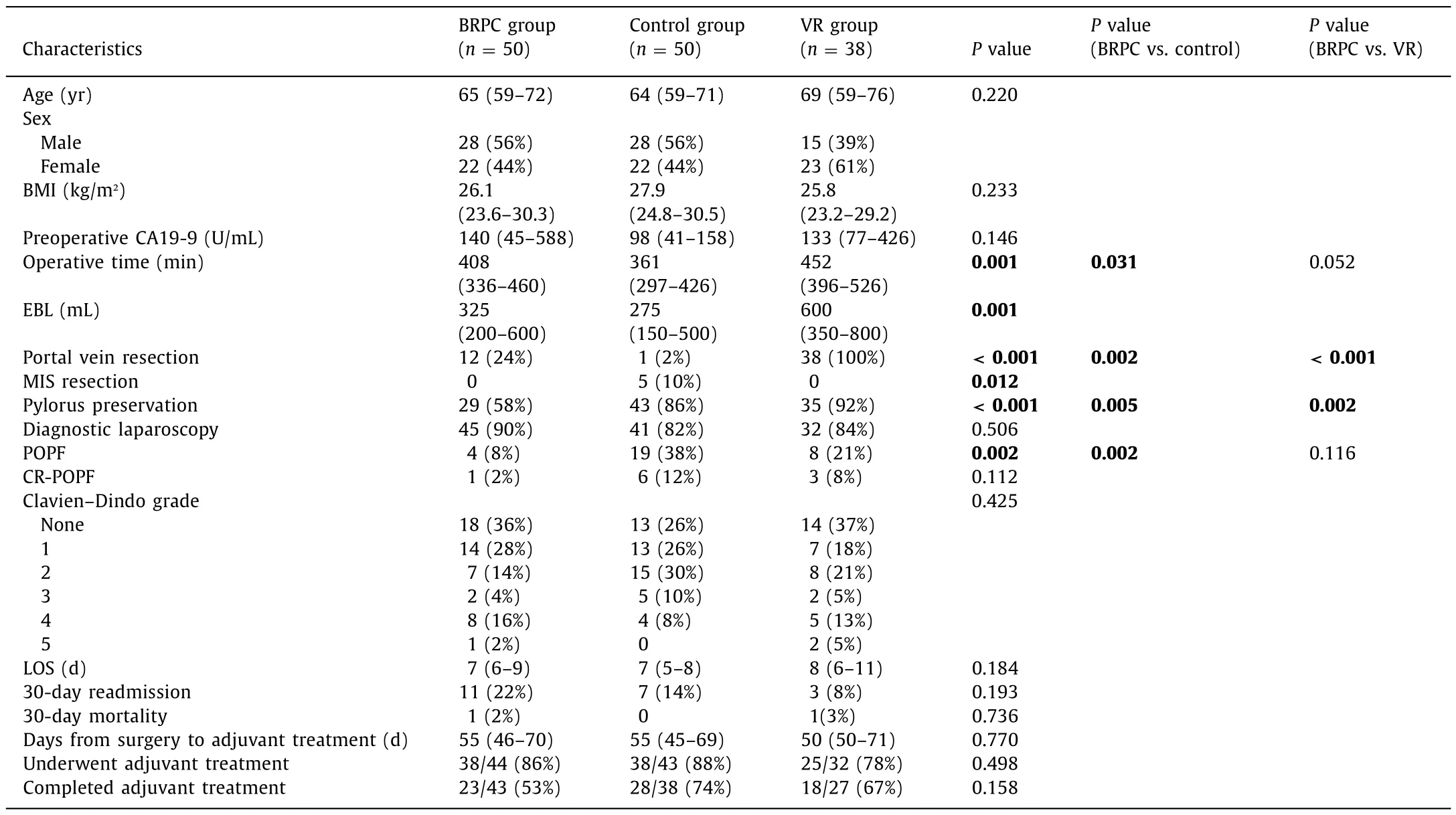
Table 1 Demographic and perioperative characteristics.
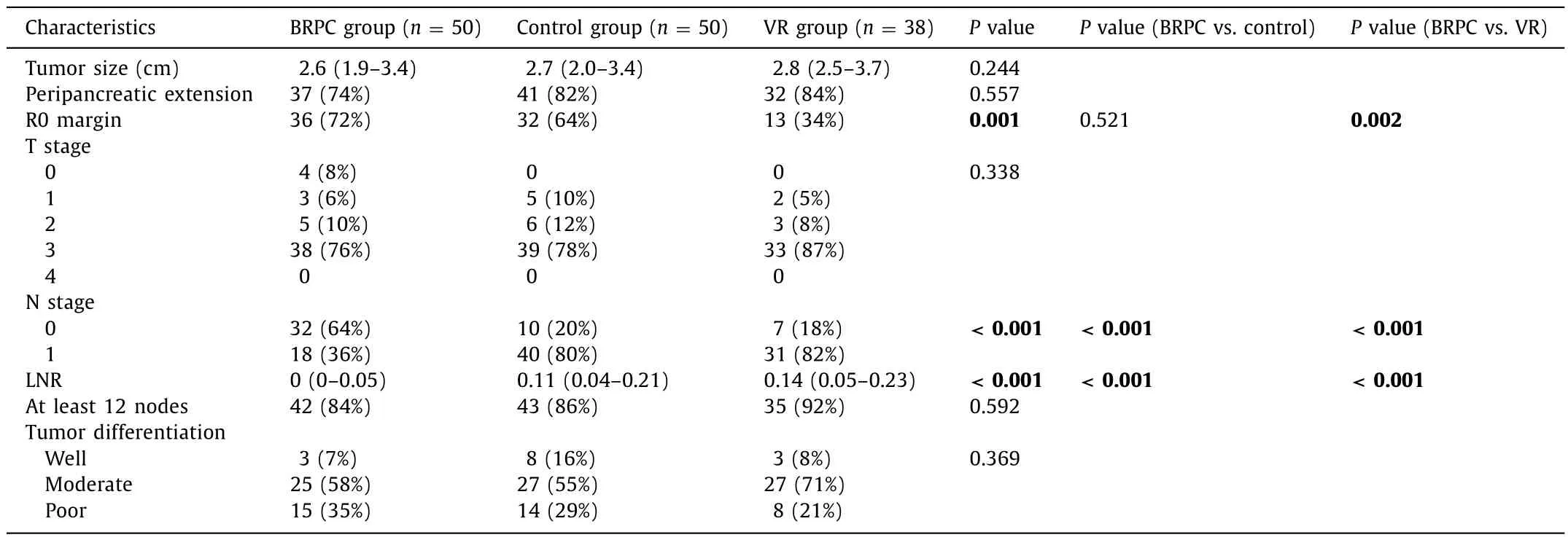
Table 2 Pathologic characteristics.
Median follow-up was 19.7 months in the BRPC group (range 5.5–66.1 months, first patient censored at 8.5 months), 17.9 months in the control group (range 2.3–55.0 months, first patient censored at 2.3 months), and 17.7 months in the VR group (range 0.8–58.8 months, no patients censored). Kaplan–Meier curves for DFS and OS ( Fig. 1 ) demonstrated no statistically significant differences between the three groups, although there was a trend towards shorter OS in the VR group. The median OS was 28.8 months for the BRPC group, 38.6 months for the control group, and 19.0 months for the VR group (P= 0.096). The median DFS was 14.0 months for the BRPC group, 17.3 months for the control group, and 14.3 months for the VR group (P= 0.234).
DFS and OS were further analyzed stratified by the nodal status(N0, LNR ≤0.2, 0.2–0.4, or ≥0.4). In the BRPC group, the median OS survival was 31.7 months for the N0 group, 29.2 months for the LNR ≤0.2 group, 16.8 months for the LNR 0.2–0.4 group, and no patient had an LNR ≥0.4 (P= 0.956). In the control group,median OS was not reached in the N0 group, 39.2 months for the LNR ≤0.2 group, not reached for the LNR 0.2–0.4 group, and 19.2 months for the LNR ≥0.4 group (P= 0.380). There were also nostatistically significant differences in DFS for the BRPC or control group when stratified by LNR. In the VR group, higher LNR was associated with worse DFS and OS. Median DFS was 25.9 months,14.5 months, 11.6 months, and 8.3 months for the N0, LNR ≤0.2,LNR 0.2–0.4, and LNR ≥0.4 groups, respectively (P= 0.025), and median OS was 33.7 months, 23.2 months, 15.2 months, and 12.0 months, respectively (P= 0.003).
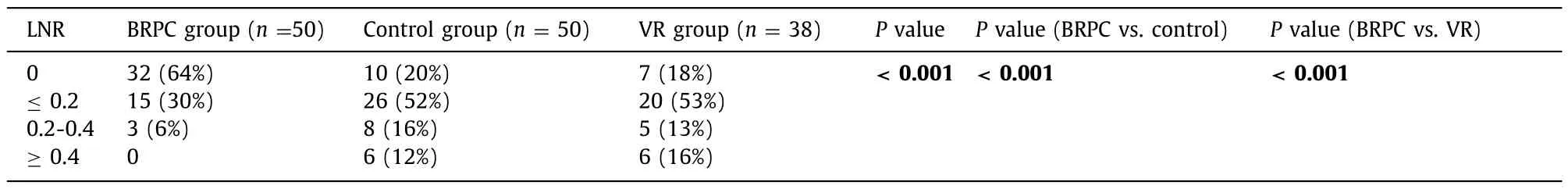
Table 3 Lymph node ratio distribution among the three groups.
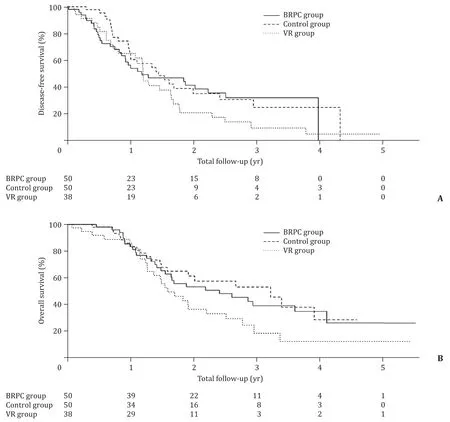
Fig. 1. Kaplan–Meier curve comparing ( A ) disease-free survival, and ( B ) overall survival in BRPC, control, and VR groups. BRPC group: borderline resectable group with neoadjuvant therapy; VR group: vein resection group without neoadjuvant therapy.
Multivariate models were generated to evaluate for predictors of DFS and OS with hazard ratios shown in Table 4 . Increased LNR,positive margin (R1), and not receiving adjuvant therapy were associated with worse DFS and OS.
Discussion
The optimal treatment paradigm for BRPC is still controversial.Options for surgically fit patients include upfront resection or NAT followed by surgery; the optimal agents and sequence of treatments are constantly evolving. Management algorithms for BRPCmust take into account patterns of disease progression, impact of neoadjuvant and adjuvant therapies, quality of life, and healthcare costs. We examined the impact of NAT on pathologic and oncologic outcomes for BRPC. Our analysis revealed that patients with BRPC treated with NAT were more likely to have a negative margin (R0),negative lymph nodes, and lower LNR compared to patients with resectable disease undergoing upfront resection with or without vein resection. Predictors of worse survival in multivariable analysis included higher LNR, positive margin (R1), and omission of adjuvant therapy.
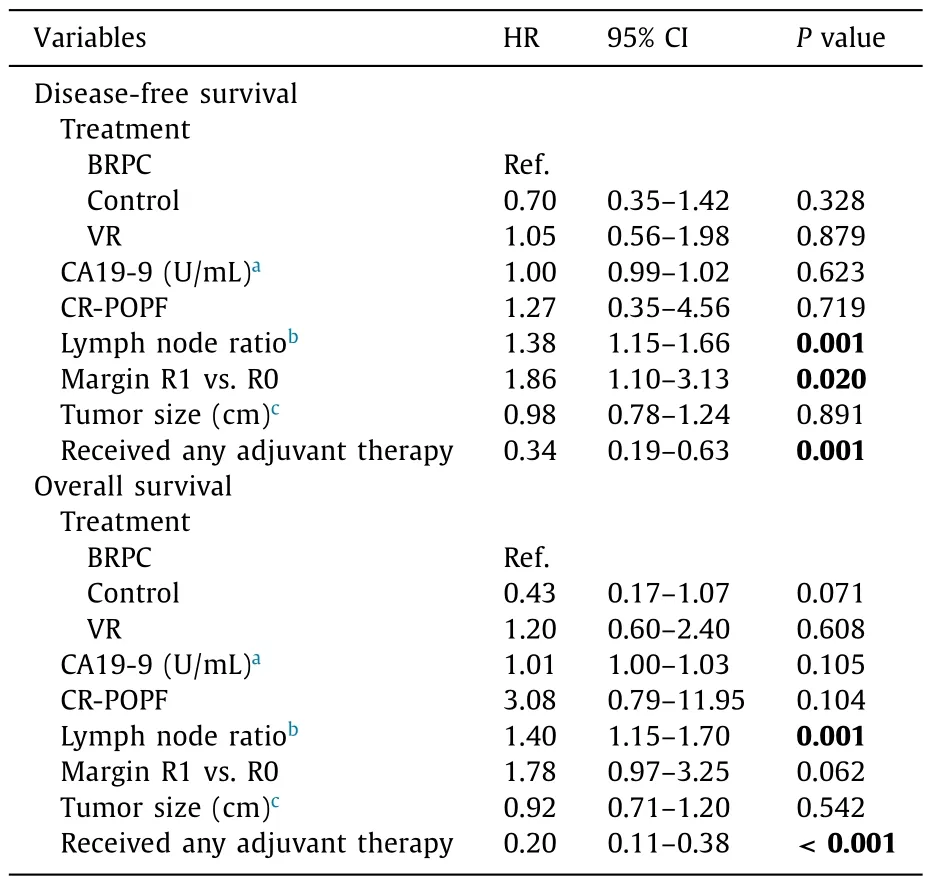
Table 4 Multivariate models for survival.
The concept of LNR has been explored for pancreatic cancer and several groups have reported on its significance in prognosis and staging. Berger et al. [11]reviewed 128 patients undergoing PD in a single institution series; when LNR was stratified into three groups(LNR = 0,<0.15, or>0.15), the higher LNR groups had shorter DFS and OS. Pawlik et al. [7]examined the impact of nodal yield and LNR in a single institution series of 905 patients undergoing PD; LNR was stratified into four groups based on sensitivity analyses (LNR = 0, 0 to 0.2, 0.2 to 0.4, or>0.4). The group reported an OS of 25.3 months in the LNR = 0 group and stepwise decrement of OS to 12.2 months in the LNR>0.4 group. Slidell et al.[8]examined 3836 patients in the Surveillance, Epidemiology, and End Results (SEER) database which included all types of pancreatic resections, and found that a higher LNR was strongly predictive of poor median survival (18 months for LNR = 0 vs. 10 months for LNR>0.4,P<0.001).
In a systematic review, higher LNR was associated with worse OS in 17 of 19 studies [12]. Several studies have demonstrated superiority of LNR over the American Joint Committee on Cancer (AJCC) nodal staging system in predicting outcomes [ 13 , 14 ].However, many studies [ 6–8 , 10–12 ]evaluating LNR have either included BRPC within the resected cohort or excluded this group of patients, as some of these studies predated the current definition of borderline resectability and routine use of neoadjuvant therapy,eliminating the ability to evaluate the impact of NAT on LNR in BRPC. The impact of NAT was addressed in a recent series which reported patients who underwent NAT followed by resection, and showed that patients with an LNR<0.2 had significantly improved DFS and OS compared to patients with an LNR ≥0.2 [15]. Although not specified, this study likely included patients with both resectable and borderline resectable tumors.
The current study uniquely focused on patients with more advanced borderline resectable disease who had a negative diagnostic laparoscopy and then underwent NAT. With this treatment paradigm, we found that only 24% of the BRPC group required VR at surgery. The BRPC group was also significantly more likely than the matched control group to have node negative disease (64% vs.20%) and lower LNR, but comparable rate of R0 resection. Despite more favorable pathology in the BRPC group, DFS and OS were slightly worse in the BRPC compared to resectable control group,although not statistically significant. Median DFS was similar between the BRPC group and the resectable group, which is essentially equivalent when we consider a 10 to 12-week lead time to administer NAT in the BRPC group. Median OS was also not significantly different between the BRPC group and the control group,which may be due to small sample size and event rates, but may hint towards more aggressive disease biology in BRPC. The difference in median OS between the BRPC group and the VR group may be clinically significant despite failure to reach statistical significance, and may reflect the impact of the NAT. Survival analysis in the BRPC cohort stratified by LNR showed a median OS in the N0 group clinically longer than that in LNR 0.2–0.4 group, but this difference failed to reach statistical significance likely due to small number of patients and low event counts in the higher LNR group.
Negative margin status has also been established to correlate independently with improved survival [ 16 , 17 ]. Our study demonstrated a higher percentage of margin negative resections in the BRPC group despite a small percentage requiring VR, which highlights the value of high-quality preoperative staging and suggests a significant benefit of NAT in tumors with vascular involvement.The recently published, randomized PREOPANC trial showed that NAT results in a higher rate of margin negative resections in all patients, and in the borderline resectable group (defined as arterial abutment<90 °or venous involvement between 90 °–270 °without occlusion), leading to improved OS and DFS [18].
The exact role of NAT for BRPC is still debated but is increasingly utilized in practice. A consensus statement by the International Study Group of Pancreatic Surgery supports upfront surgical resection for BRPC with isolated, reconstructable venous involvement [19]while the NCCN recommendations currently advocate NAT for BRPC [9]. We routinely use NAT for BRPC rather than upfront resection, due to concern for occult metastatic disease, as well as poor pathologic outcomes with upfront resection and greater need for vascular resection. There are multiple ongoing randomized trials to address the best approach in this setting [ 20 , 21 ].
There are several limitations of this study. The series was relatively small, retrospective in nature, with potential for bias in treatment decisions by patients and physicians. We attempted to mitigate these biases by using a matched control cohort who underwent treatment during the same period by the same surgeons and oncologists. The choice of chemotherapy and radiation regimens was not strictly prescribed on protocol, and the favored regimen changed during the time course of the study.Treatment was therefore heterogeneous in this study, which can make the results challenging to interpret. NAT during the time frame of this study was comprised primarily of single agent chemotherapy and standard fractionation radiotherapy, which may limit the ability to apply the results of this study to patients who receive multi-drug regimens and radiation techniques un-der clinical trials. The adjuvant treatment regimen favored during the time frame of this study was single agent gemcitabine,which was supplanted by combination with gemcitabine and capecitabine [22]and 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLRINOX) [23]shortly after the time frame of this study.The small number of patients undergoing treatment for BRPC, the low numbers of patients with higher LNR, and low survival event rates limited the sample sizes in this study, and may account for failure to detect survival differences.
In conclusion, our analysis indicated that NAT was associated with more favorable pathologic outcomes in BRPC after NAT compared to upfront resection. Survival analysis showed equivalent DFS between the three groups, with a trend towards better OS in the BRPC compared to the VR group. These results highlight the necessity of accurate preoperative staging to determine vascular involvement, and although there is no demonstrated survival benefit in this study, we advocate the use of NAT in BRPC to avoid unnecessary vascular resection at surgery, and to avoid surgery in patients with occult metastatic disease or aggressive tumor biology.
Acknowledgments
We thank Colin O’Rourke and Chao Tu for assistance with statistical analysis and John McMichael for assistance with data management.
CRediT authorship contribution statement
June S Peng:Conceptualization, Data curation, Formal analysis,Writing - original draft, Writing - review & editing.Gareth Morris-Stiff:Conceptualization, Writing - review & editing.Noaman S Ali:Conceptualization, Writing - review & editing.Jane Wey:Conceptualization, Writing - review & editing.Sricharan Chalikonda:Conceptualization, Writing - review & editing.Kevin M El-Hayek:Conceptualization, Writing - review & editing.R Matthew Walsh:Conceptualization, Data curation, Formal analysis, Supervision.
Funding
None.
Ethical approval
This study was conducted in compliance with theDeclaration ofHelsinkiand with local and national regulations. The study was exempted from informed consent and was approved by the Institutional Review Board of Cleveland Clinic Foundation.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Liver transplantation and BCLC classification: Limitations impede optimum treatment
- Do the existing staging systems for primary liver cancer apply to combined hepatocellular carcinoma-intrahepatic cholangiocarcinoma?
- Overlap of concurrent extrahepatic autoimmune diseases is associated with milder disease severity of newly diagnosed autoimmune hepatitis
- Aggressive surgical approach in patients with adrenal-only metastases from hepatocellular carcinoma enables higher survival rates than standard systemic therapy
- Integrating transcriptomes and somatic mutations to identify RNA methylation regulators as a prognostic marker in hepatocellular carcinoma
- The utility of two-dimensional shear wave elastography and texture analysis for monitoring liver fibrosis in rat model
