Liver transplantation and BCLC classification: Limitations impede optimum treatment
2021-03-05GerOttoMichelBPittonMriHoppeLotichiusArntWeinmnn
Ger Otto MichelB Pitton Mri Hoppe-Lotichius c Arnt Weinmnn
a (Former) Department of Hepatobiliary and Transplant Surgery, University Medical Center, Mainz, Germany
b Department of Diagnostic and Interventional Radiology, University Medical Center, Mainz, Germany
c Department of General, Abdominal and Transplant Surgery, University Medical Center, Mainz, Germany
d Department of Internal Medicine I, University Medical Center, Mainz, Germany
Keywords:BCLC system Liver transplantation Hepatocellular carcinoma Treatment
ABSTRACT Background: The Barcelona Clinic Liver Cancer (BCLC) system has been endorsed by international guidelines as a staging algorithm of hepatocellular carcinoma. This analysis was performed to assess the outcome of liver transplantation in patients treated against the BCLC recommendations.Methods: The data of 198 patients who underwent liver transplantation for hepatocellular carcinoma were extracted from a prospectively maintained database to classify the patients according to the BCLC system.Results: BCLC staging was as follows: 0, n = 5; A, n = 77; B, n = 41; C, n = 53; and D, n = 22. Accordingly, liver transplantation was performed in the majority of patients against BCLC recommendations.Surgery ( n = 16), radiofrequency ablation ( n = 15) and transarterial chemoembolization ( n = 151) preceded liver transplantation in 182 patients. Sixteen patients were transplanted without pretreatment. The 1-, 5- and 10-year survival rates were 83.8%, 62.4% and 45.9%, and 1-, 5-, and 10-year recurrence rates were 7.7%, 22.7% and 26.7%. The BCLC classification did neither impact survival ( P = 0.796) nor recurrence( P = 0.693). In the Cox analysis, RECIST tumor progression and initial alpha fetoprotein were independent predictors of outcome.Conclusions: Neither the oncological nor the functional stratification imposed by the BCLC system was of importance for outcome. Lack of flexibility and disregard of biological parameters hamper its clinical applicability in liver transplantation.
Introduction
Morphological classification schemes such as Milan criteria,University of San Francisco criteria and Up-to-Seven criteria have been used for more than two decades in patients with hepatocellular carcinoma (HCC) to assess eligibility and prognosis of liver transplantation (LT) [1-3]. At the same time, variables such as microvascular invasion, poor grading, alpha fetoprotein (AFP) level,tumor response to therapeutic interventions and others became increasingly important for our understanding of tumor biology [4-9].
The American and the European (EASL) Guidelines on the Treatment of HCC have endorsed the Barcelona Clinic Liver Cancer(BCLC) system as the standard staging algorithm with prognostic and therapeutic implications [ 10 , 11 ]. That staging system stratifies patients with HCC into five stages (very early stage, 0; early stage,A; intermediate stage, B; advanced stage, C; and terminal stage, D)and assigns each BCLC stage to distinct recommendations of treatment based on oncological criteria (size, number, vascular and extrahepatic invasion), functional capacity of the liver (Child-Pugh classification, CP) and tumor-related health status (ECOG performance status) [ 12 , 13 ]. Depending on the patient’s individual situation, treatment modalities such as LT, liver resection, local ablation,transarterial chemoembolization (TACE), systemic therapy and supportive treatment are recommended.
In this treatment algorithm, LT is proposed for patients in the stages BCLC 0 and A, special situations provided. In our institution,as in many other centers, LT was also performed in BCLC stage B to D to offer numerous patients a chance of curative treatment.Thereby, local tumor treatment preceded LT in most cases. Thisstudy was to analyze the outcome of this strategy and to scrutinize the justification of the strict therapeutic stratification imposed by the BCLC system.
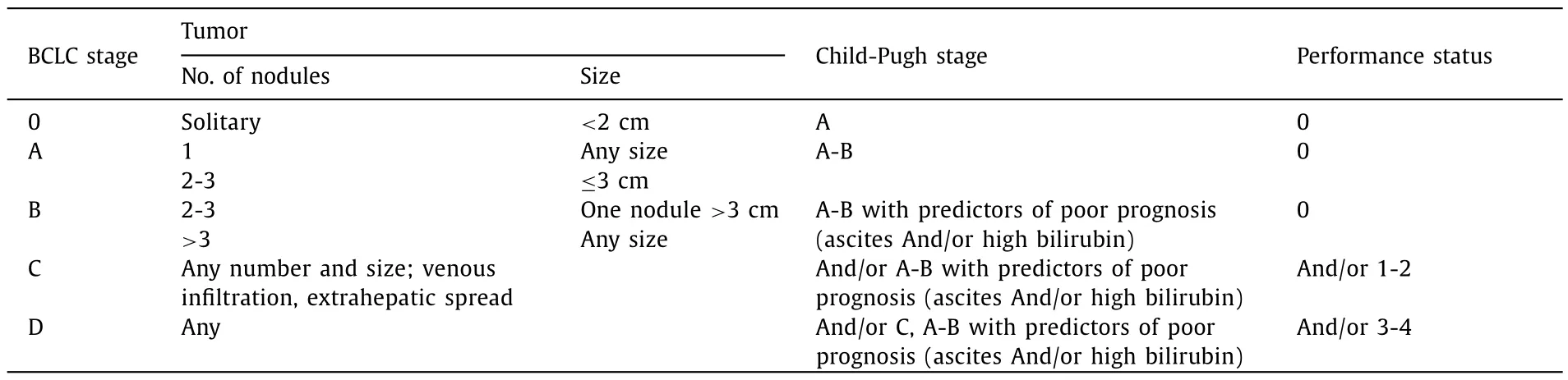
Table. 1 Classification scheme as proposed by the Barcelona Clinic Liver Cancer (BCLC) expert panel [12].
Methods
Between May 1998 and September 2012, 198 patients underwent LT for HCC at our institution. One hundred and thirty-six patients were part of a prospective study on pretransplant TACE focusing on monitoring the response to chemoembolization and its impact on prognosis. The protocol had been approved by the Ethical Committee of the Medical Association of Rhineland Palatinate and the Standing Committee on Organ Transplantation at the German Medical Association as published elsewhere [14]. The remaining 62 patients were pretreated before LT without specific protocols or received no pretreatment at all. All 198 patients included in the present analysis gave informed consent to data collection,scientific workup and publishing.
HCCs with macrovascular infiltration, lymph node or other forms of extrahepatic involvement were excluded from transplantation. Incidentalomas were not considered in this analysis. All livers were transplanted using piggy-back technique. Standard immunosuppression comprised calcineurin inhibitors, mycophenolate mofetil and steroids. Twenty-eight patients received antibody induction. In view of its limited influence on tumor recurrence, 24 patients treated with mammalian target of rapamycin inhibitors were also included in this study [15].
The following data were extracted from our prospectively maintained database: patient’s characteristics, oncological data including AFP, underlying liver disease, severity of cirrhosis, time of diagnosis, modalities and number of pretreatment before transplantation, follow-up data, data required to classify patients according the BCLC algorithm (ECOG performance status [16], CP classification and tumor characteristics). The complete set of data was available in all patients. The scheme adopted for the classification of BCLC stages is indicated in Table 1 .
TACE was performed in intervals of 6 weeks using lipiodol and mitomycin. In the 136 patients participating in the prospective TACE study, LT was performed regardless of tumor response. In all patients, independent of the form of pretreatment, CT or MRI was routinely performed in intervals of 6 weeks. Size and number of tumor nodules were determined from the imaging at primary diagnosis of the HCC and from the last imaging before LT in order to classify the patients according to the response evaluation criteria in solid tumors (RECIST; [17]) and according to the Milan criteria. Even if detailed assessment of vascularization according to the modified RECIST criteria was available in patients included after 2008, the former RECIST system was used in all patients. In patients with a waiting time less than 6 weeks, the initial CT or MRI scan was assumed to be identical to the situation before LT if not a second study was available. For the assessment of the influence of AFP on survival and recurrence, a low cut-off value (100 ng/mL) was used according to a recently published proposal [18].
For pathology work-up, the explant specimens were cut into slices of 1 cm. Histological grading [19]and microvascular invasion were drawn from the pathology reports. In patients without histopathological assessment due to tumor necrosis, grading from biopsies before pretreatment was used. All lesions described on the explant were reclassified according to the latest Union for International Cancer Control (UICC) classification [20]. The United Network for Organ Sharing (UNOS) classification [21]was used to describe the tumor features on the primary imaging focusing on transplant criteria [1].
Statistical analysis
The comparison of categorical and continuous variables was performed with Chi-square test and Mann-Whitney test. The impact of BCLC criteria, oncological data, modalities and results of pretransplant treatment including response criteria on overall survival and recurrence were calculated using Kaplan-Meier estimates and log-rank test. Variables which were significant (P<0.05) in univariate testing were included in the Cox proportional hazard analysis to test their influence on overall survival and recurrence.Additional multivariate analyses restricted to pretreated patients were performed. The prognostic ability of the independent predictors of survival and tumor recurrence was tested and reported as area under the receiver operating characteristic (ROC) curve (AUC).To this end, the two dichotomous variables which proved to be significant in the Cox analysis (RECIST tumor progression yes or no;AFP ≥100 ng/mL or AFP<100 ng/mL) were used to form 4 groups of patients. All statistical calculations were performed using IBM SPSS Statistics (version 23, Chicago, IL, USA).
Results
Demographics
Of the 198 patients, 43 were female and 155 were male, and the median age was 60.0 (33.6–72.8) years. At the time of analysis 113 patients had died, 73 of non-tumor-related reasons and 40 due to tumor recurrence. Four patients with recurrence were alive at 15.5, 17.9, 57.8 and 72.7 months after surgery for isolated needle tract seeding. The median interval between initial diagnosis of HCC and LT was 10.2 (0–72.0) months and median follow-up was 86.2(0.4–227.0) months.
BCLC, oncological data and underlying diseases
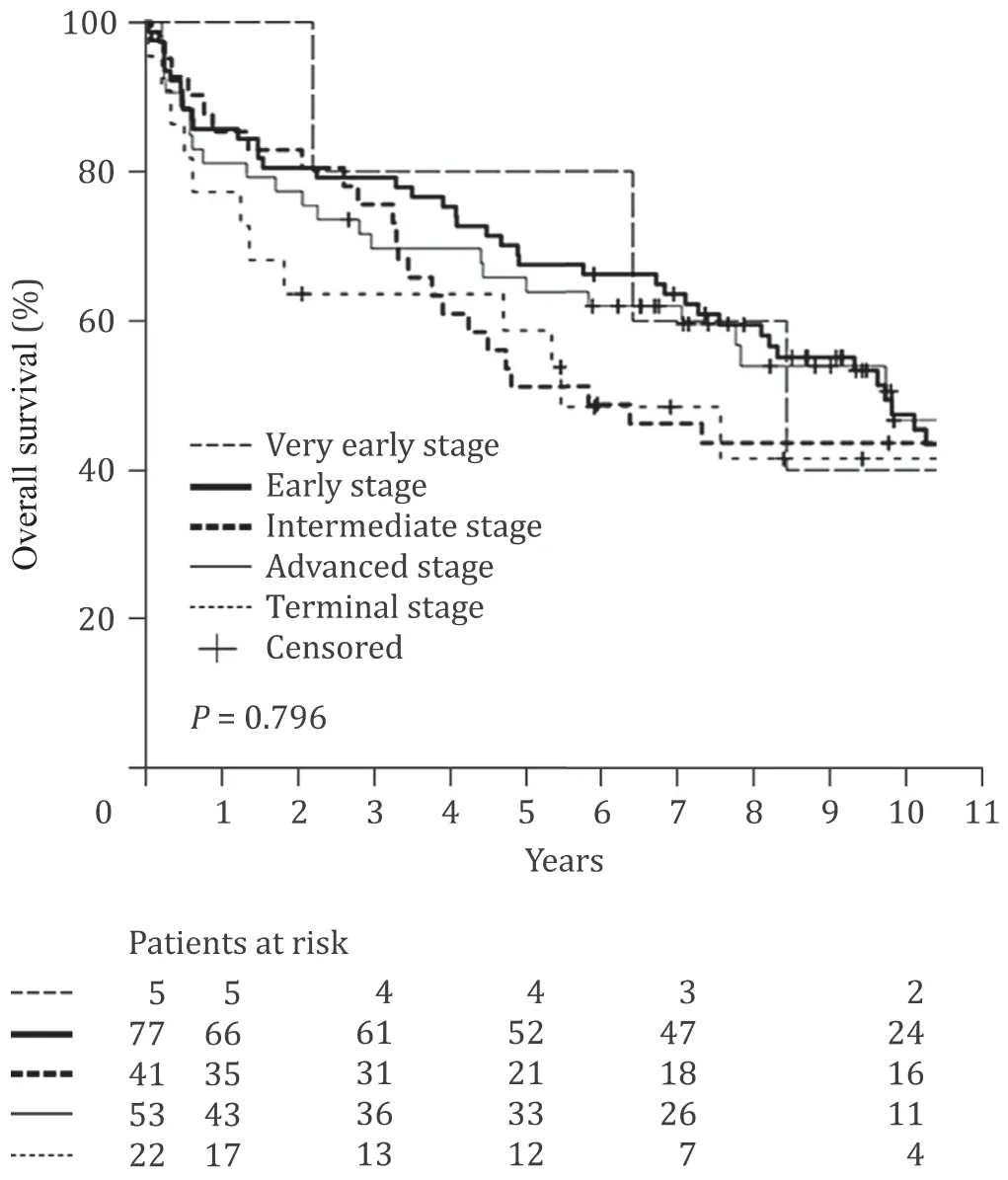
Fig. 1. Overall survival after liver transplantation for hepatocellular carcinoma of 198 patients classified according to the Barcelona Clinic Liver Cancer (BCLC) system.
At the point of diagnosis, the constituents of the BCLC system,BCLC tumor stage, CP classification and performance status were fixed. In none of the patients, performance status was severely impaired due to comorbidity or excessive tumor growth. Overall BCLC staging was as follows: very early stage (0),n= 5; early stage (A),n= 77; intermediate stage (B),n= 41; advanced stage (C),n= 53;and terminal stage (D),n= 22. The detailed results of grouping according to the BCLC classification are shown in supplemental material. Surgery (n= 16), radiofrequency ablation (n= 15) and transarterial chemoembolization (n= 151) preceded liver transplantation in 182 patients.
Additional characteristics of the patients, oncological data and treatment modalities are indicated in Table 2 . The majority of patients suffered from hepatitis C cirrhosis (n= 70), alcoholic cirrhosis (n= 64) or hepatitis B cirrhosis (n= 36). In all patients histopathological confirmation of the tumor was available with the exception of 3 patients who had no initial biopsy and total tumor necrosis on the explant. In those patients (UNOS stage 2 in 2 patients and stage 1 in 1 patient) the diagnosis had been made by initially unequivocal radiological features. The median initial tumor size was 3 (0.7–12) cm. Eighty-two patients had 1 nodule, 84 patients had 2 or 3 nodules (BCLC very early and early tumors) and in 32 patients the number of lesions exceeded 3 (UNOS 4A, BCLC intermediate tumors). In 10 of 82 patients with single tumor, the size of the lesion was more than 5 cm in diameter.
Survival and tumor recurrence
The 1-, 5- and 10-year survival rates of all patients were 83.8%,62.4% and 45.9%. The respective cumulative recurrence rates were 7.7%, 22.7% and 26.7%. The BCLC system and its three constituents were without any influence on survival (P= 0.796) and recurrence(P= 0.693) in the Kaplan-Meier estimates ( Fig. 1 and Table 2 ).Likewise, the modality of treatment preceding LT did not influence survival or recurrence (P= 0.176 andP= 0.090, respectively) and even in 16 patients without pretreatment due to functional impairment, results were comparable to all other patients. Notably, the comparison of very early and early tumors versus intermediate tumors did not result in significant differences. The same was true when patients with stable cirrhosis and performance status (BCLC 0, A and B) were compared to patients with impaired liver function or performance status (BCLC C and D).
As shown in Table 2 , AFP, Milan criteria on the imaging before LT, RECIST tumor progression during pretreatment and tumor grading gained significant importance for overall survival in the univariate analysis.
Sex, AFP, Milan criteria as assessed before LT and on the specimen, RECIST tumor progression, T classification (UICC), tumor grading and microvascular invasion exerted significant influence on recurrence in the univariate analysis.
Multivariate Cox analyses and prognostic stratification
RECIST tumor progression during the waiting time and AFP( ≥100 ng/mL) before pretreatment were independent predictors of reduced overall survival and tumor recurrence in the multivariate Cox regression. In addition, microvascular invasion was a significant risk factor for tumor recurrence. As in 16 of 198 patients transplanted without pretreatment data as to pretreatmentrelated tumor response during the waiting time were not available, the multivariate analyses were repeated after excluding those 16 patients. That analysis yielded two variables impacting survival and recurrence: RECIST tumor progression and AFP ≥100 ng/mL( Table 3 ).
When both those independent variables were combined to form 4 groups of patients - regressive disease or stable disease/AFP<100 ng/mL, regressive or stable disease/AFP ≥100 ng/mL, progressive disease/AFP<100 ng/mL, progressive disease/AFP ≥100 ng/mL - a prognostic stratification of patients was possible ( Fig. 2 ). The inclusion of these strata in a ROC yielded an AUC of 0.649 (95% CI: 0.568–0.726;P= 0.001) for overall survival and 0.757 (95% CI: 0.655–0.858;P<0.001) for recurrence. As surmised in Fig. 1 , the inclusion of the BCLC classification (stages 0 to D) in the ROC analysis resulted in AUCs of 0.527 and 0.515 which is without any predictive meaning regarding survival or recurrence.
Discussion
The BCLC system is recommended for staging and treatment allocation in HCC patients. According to the recent EASL guidelines, a novel treatment migration strategy is included in the BCLC system and its evidence is assessed to be high [10]. Local ablative procedures including surgery are primarily recommended to patients in stages 0 and A and LT is restricted to stages 0 and A but to cases with increased bilirubin or ascites [ 12 , 13 ]. As the option for LT in patients with severely impaired liver function but tumor stages 0 and A has recently been embraced by the BCLC system [10], patients in stage C or D, if attributed to those stages due to advanced cirrhosis, are candidates for LT whereas, as a matter of course, patients attributed to these stages due to tumor growth, are not. That issue is noteworthy, as the benefit of LT in stages C or D has been reported to be particularly high [22-24]. Altogether, transplant candidates clearly represent a minority of HCC patients. For patients with multinodular tumors and stable functional parameters (BCLC B), the BCLC system recommends TACE. Patients with tumors involving the great intrahepatic vessels and those with extrahepatic disease (BCLC C) are candidates for sorafenib or newer approved systemic forms of treatment. In BCLC D patients, i.e. patients in the terminal stage of the disease, best supportive care is recommended.
The strategy assigning patients to fixed treatment modalities has been challenged and a revision of the BCLC system wasclaimed [25-31]. That criticism applied particularly to TACE, local ablation and hepatic resection since non-adherence to treatment recommendations has been reported to yield similar or even better results than treatment as per BCLC protocol. In particular, the BCLC treatment strategy in stage B patients may be challenged.Patients with stage B tumors would receive TACE if treated perprotocol even in case of good response to that treatment or other favorable biological factors, and would be excluded from LT if not otherwise stated in those classification guidelines.
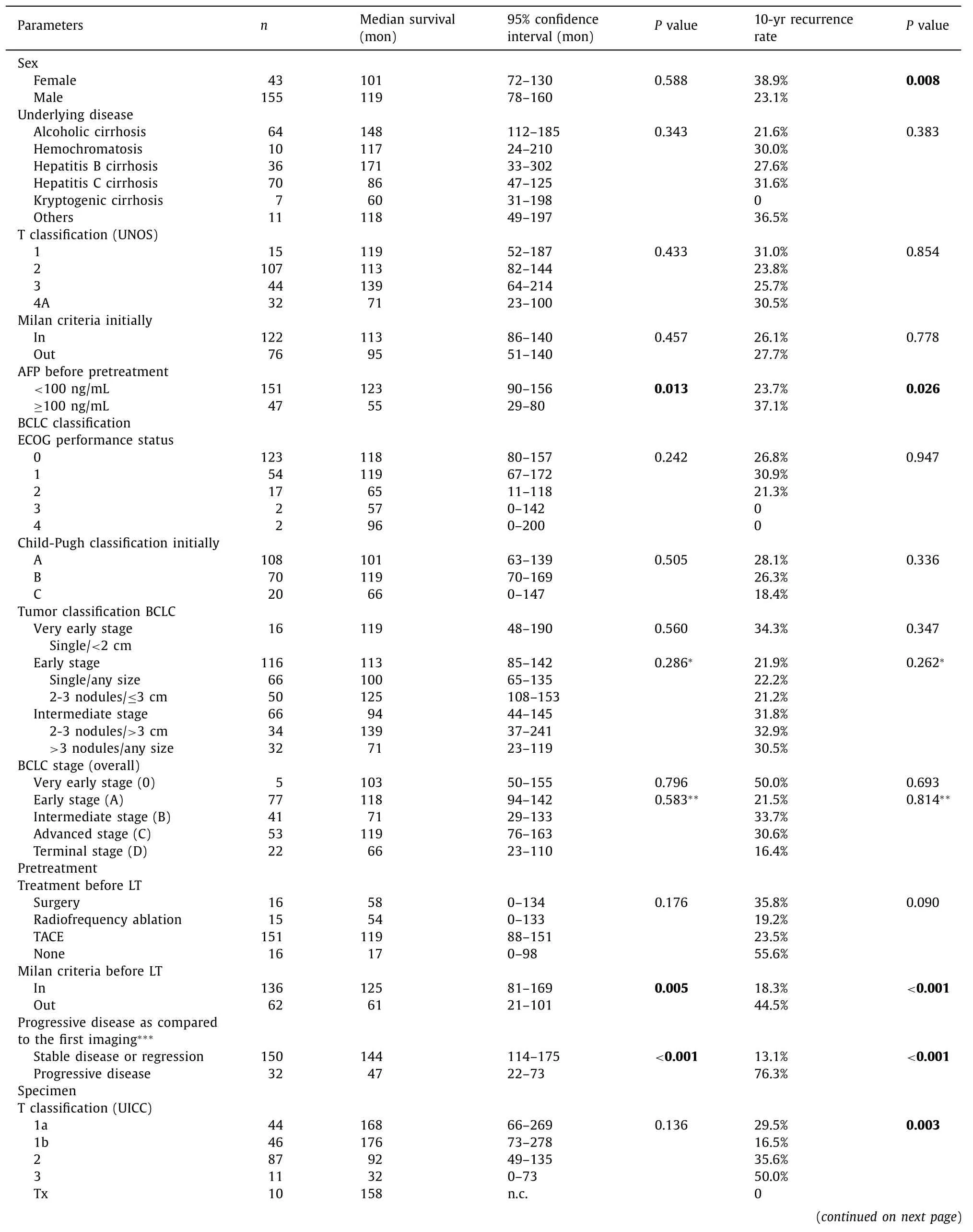
Table. 2 Patients’ and oncologic characteristics, BCLC classification, pretreatment data and explant findings in 198 patients undergoing liver transplantation for hepatocellular carcinoma.
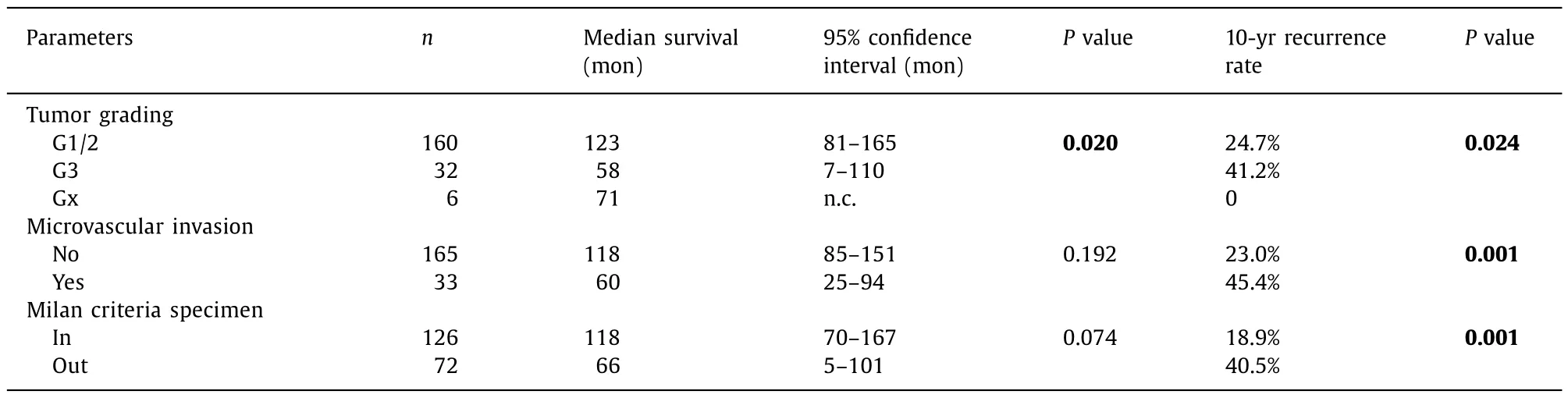
Table. 2 ( continued )
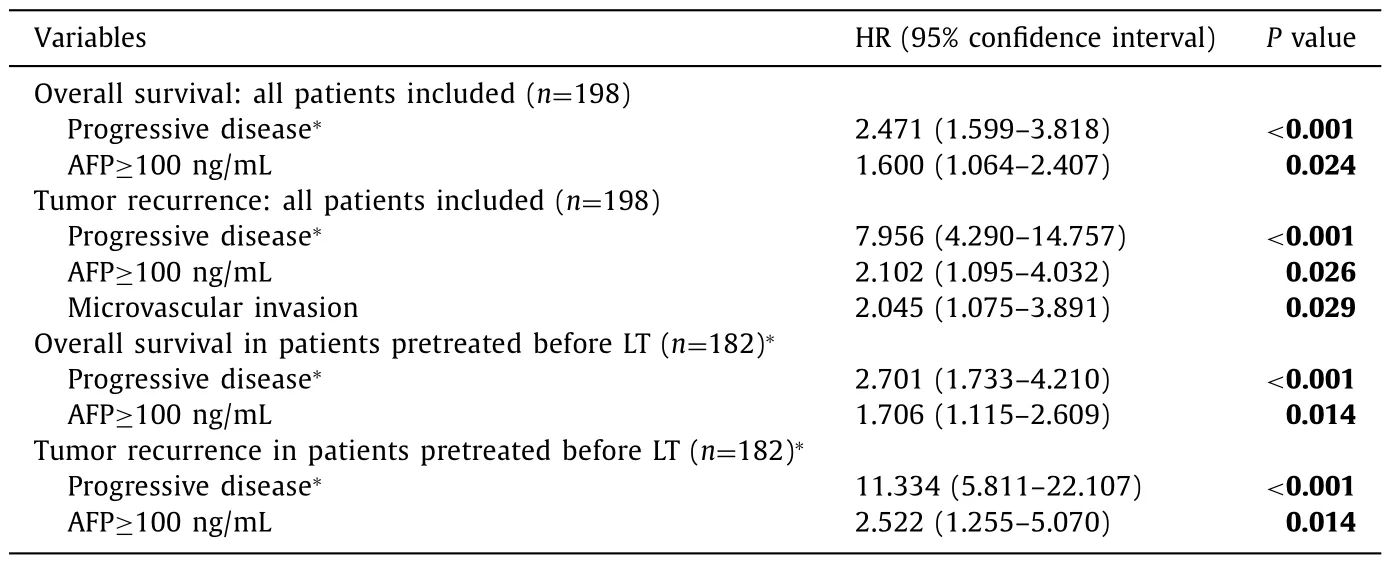
Table. 3 Multivariate Cox analysis calculated in 198 patients undergoing liver transplantation and in 182 patients with pretransplant treatment.
In this study, all but 16 patients were pretreated prior to LT.One hundred and thirty-six of them had been included in a controlled study to test the influence of TACE on outcome of LT. Fortysix of the remaining 62 patients received a bridging treatment before LT without a specific protocol ( Table 2 ). The majority of tumors (n= 132; 67%) were classified as very early and early lesions. Out of those 132 patients, 10 solitary tumors exceeded the Milan criteria (diameter>5 cm) and could not have been listed- similarly to the 66 patients with intermediate tumors - if they were not part of our prospective study on down-staging. Due to impaired hepatic function and/or performance status, just 82 of 132 patients were in the BCLC stages 0 or A, i.e. in stages justifying LT. According to overall BCLC staging of the remaining 116 patients, 41 classified as stage B and 75 assigned to stages C and D for functional reasons were transplanted, strictly speaking, against the BCLC rules. Excluding those 116 patients from LT would hardly have been justified as neither their intermediate tumor stage nor their poor “functional reserve” (BCLC stages C and D) resulted in any significant inferiority in the Kaplan-Meier estimates.
None of the constituents of the BCLC system or the form of pretreatment used before LT gained significant influence on survival or recurrence. In the multivariate Cox analysis, survival and recurrence depended on biological variables, namely tumor progression(RECIST criteria) during waiting time, AFP and –when recurrence in all 198 patients is considered - microvascular invasion. Accordingly, in the total of patients as well as in the 182 patients undergoing any pretreatment, two independent predictors - tumor progression and AFP - were crucial for prognosis. It should be noted, that excluding the patients, who met those negative predictors, from LT beforehand would have halved the 10-year recurrence rate in our study, i.e. the 10-year recurrence rate would have been 13%. These results corroborate the importance of biological criteria whereas a rigid consideration of tumor size and number - at least in the limited extent of this study - and the functional hepatic reserve or the performance status gained no influence on outcome.
Notably, the description of tumor features on the specimen highlighted as gold standard to predict prognosis in many publications [ 1-3 , 32 ]failed to reach significance in our multivariate analysis. Tumor dynamics imposed by pretreatment during the waiting time are, obviously, more relevant for predicting recurrence(and survival) than the allegedly most reliable description of lesions. Those biological properties, entailing tumor regression due to pretreatment or progression despite pretreatment, appear to be predictive for the outcome. Accordingly, the small number (n= 16)of patients without pretreatment included in this study may represent the potential “average response” and the outcome is, indeed,comparable to all other patients. Moreover, in comparison to findings on the explant, both independent predictors for recurrence -tumor progression and AFP - are known before LT and, therefore,available to be considered in the process of selecting patients for transplantation.
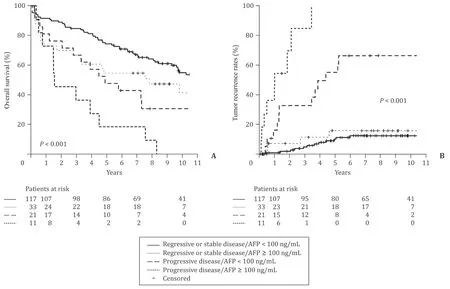
Fig. 2. Stratification of overall survival ( A ) and tumor recurrence ( B ) in 182 patients with pretreatment before liver transplantation in 4 risk groups according to the classification in the legend.
In light of these results and of numerous other studies which resulted in similar statements [ 5-8 , 14 , 33-35 ]and particularly in view of the very recently published UNOS data [36], dynamic and biological aspects should be considered in a classification system claiming consequences for treatment. Flexibility and the need to switch to a different treatment modality should, therefore, be explicitly stated in the BCLC system. Based on that, optimum treatment for many patients who are now potentially excluded from LT could be guaranteed.
This study has the typical limitations of a retrospective evaluation in patients treated in a single institution. Due to the long period of recruitment, the attitude towards selecting pretreatment modalities and the decisions to select patients for transplantation may have changed over time. In addition, homogeneity of our study cohort may be challenged as alterations of pretransplant tumor features were not only TACE-related but also - in a limited number - caused by tumor ablation. It would clearly be beyond the scope of this study to assess the impact of different pretreatment modalities on outcome, and even if the number of patients included in the available large analyses [ 7 , 8 , 36 ]is considerable, the significance of different pretreatment modalities needs to be scrutinized in future prospective studies.
In conclusion, practical clinical use of the BCLC algorithm requires flexibility in selecting candidates for LT. Rigid application of BCLC staging excludes many patients with HCC from LT although they may substantially benefit from this treatment. This applies particularly to patients with intermediate tumors. Patients with favorable tumor biology may evolve into excellent candidates in the course of pretreatment. Therefore, changes in the initial treatment strategy are justified and should be an integral part of the BCLC system.
Acknowledgments
None.
CRediT authorship contribution statement
Gerd Otto:Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing.Michael B Pitton:Data curation, Investigation, Methodology.Maria Hoppe-Lotichius:Data curation, Project administration.Arndt Weinmann:Conceptualization, Data curation, Formal analysis.
Funding
None.
Ethical approval
The patients were part of a previous study. That protocol was approved by the Ethical Committee of the Medical Association of Rhineland Palatinate and the Standing Committee on Organ Transplantation at the German Medical Association. All patients gave their informed consent to be included in this evaluation.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.hbpd.2020.12.009 .
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Do the existing staging systems for primary liver cancer apply to combined hepatocellular carcinoma-intrahepatic cholangiocarcinoma?
- Overlap of concurrent extrahepatic autoimmune diseases is associated with milder disease severity of newly diagnosed autoimmune hepatitis
- Aggressive surgical approach in patients with adrenal-only metastases from hepatocellular carcinoma enables higher survival rates than standard systemic therapy
- Integrating transcriptomes and somatic mutations to identify RNA methylation regulators as a prognostic marker in hepatocellular carcinoma
- The utility of two-dimensional shear wave elastography and texture analysis for monitoring liver fibrosis in rat model
- Impact of referral pattern and timing of repair on surgical outcome after reconstruction of post-cholecystectomy bile duct injury: A multicenter study
