Investigation of Agave cantala-based composite fibers as prosthetic socket materials accounting for a variety of alkali and microcrystalline cellulose treatments
2020-12-14SakuriSakuriEkoSurojoDodyAriawanAdityaRioPraowo
Sakuri Sakuri, Eko Surojo, Dody Ariawan,*, Aditya Rio Praowo,*
a Department of Mechanical Engineering, STT Wiworotomo Purwokerto, Banyumas 53134, Indonesia
b Department of Mechanical Engineering, Universitas Sebelas Maret, Surakarta 57126, Indonesia
Keywords:Cantala fiber Microcrystalline cellulose Prosthetic socket Tensile and flexural characteristics
ABSTRACT This study was aimed to determine the mechanical strength of composites made from Agave cantala with an unsaturated polyester matrix and microcrystalline cellulose. Cantala fiber (CF)was treated with 6% NaOH with immersion times of 0 h (UF), 3 h (AK3), 6 h (AK6), 9 h (AK9), and 12 h (AK12).Thermogravimetric analysis (TGA)analysis shows that treated CF has higher thermal stability than CF without treatment.Cantala fiber was tested by X-ray diffraction. After alkali treatment with a 6-h soaking, it had a crystallinity index of 73.65%. Scanning electron microscopy(SEM) showed that the fibers were cleaner after alkali treatment because hemicellulose, wax, and other impurities were removed. Examination of the contact angle and surface energy showed that treated CF has smaller contact angles and greater surface energy.©2020 The Authors. Published by Elsevier Ltd on behalf of The Chinese Society of Theoretical and Applied Mechanics.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
The use of natural fibers in prosthetic sockets offers advantages such as biodegradability (not characteristic of nonrenewable materials, e.g., aluminum [1]), environmental friendliness,and the availability of materials; they are also lightweight and economical, and have high power and good mechanical resistance. The use of prosthetic sockets with natural fibers as a composite reinforcement is being developed, such as socket prosthetics from banana fibers [2], flax fiber [3], banana fiber [4], cotton fiber [5], and zalacca fiber [6]. The weaknesses found in previous research are low tensile strength, bending, and modulus elasticity compared to glass and carbon fibers. This research develops prosthetic sockets from cantala fiber (CF) composite and microcrystalline cellulose (MCC), which will increase the tensile strength, flexural strength, and elastic modulus. Cantala fiber from this research will be used to develop a prosthetic socket with microcrystalline cellulose, which will increase the tensile strength, bending, and elastic modulus. Cantala fiber is taken fromAgavecantalaplants (Fig. 1a), which are widely available in Yogyakarta, East Java, West Java, and North Sumatra(Indonesia). Cantala is an agave species often used for rope,weaving, bird's nests, and other handicrafts. Picture of cantala fibers is presented in Fig. 1b.
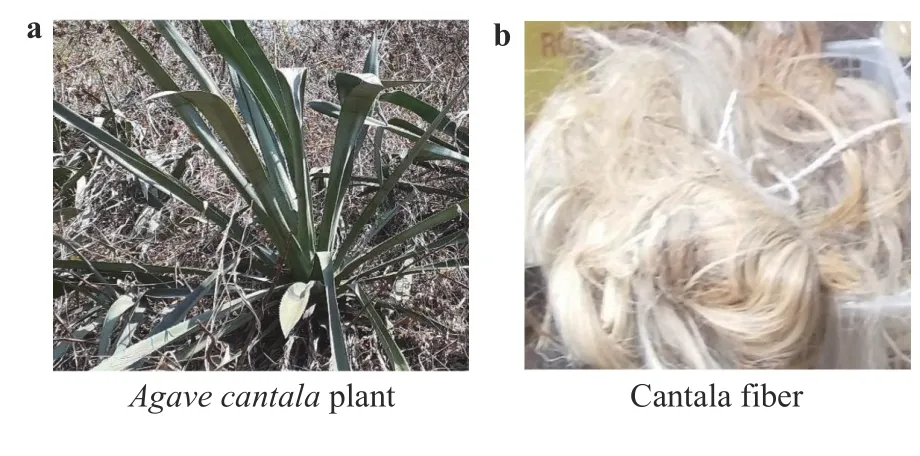
Fig. 1. Characteristics of the research subject:a Agave cantal a plant and b cantala fiber.
Cantala fiber was used as a composite reinforcement because it has a cellulose content of 64.23% [7]. The addition of cantala fiber with alkali treatment as a reinforcement will increase bonding between particles and increase compatibility.The use of cantala fibers as reinforcement was generally carried out after alkaline and silane treatments [8]. Alkalization with Na-OH can increase the flexural strength by 31% [9]. Natural fibers are made into a composite for minimum weight, limited tolerance, production techniques, and simplified operations [10].
MCC added to composites can increase the thermal stability,modulus of elasticity, and thermomechanical properties [11].The addition of MCC to high-density polyethylene (HDPE) increases the tensile properties, modulus of elasticity, and Charpy collisions by 1.9‒4.4-fold [12]. Adding MCC to composites will increase the strength, bonding between particles, and mechanical resistance. Research on making composites for prosthetic sockets with cantala fiber material, unsaturated polyester (UPRs),and microcrystalline cellulose is still important for increasing the mechanical strength of prosthetic sockets.
Agave cantalafiber was obtained from CV Rami Jaya Kulonprogo (Yogyakarta, Indonesia). The fiber was extracted from the leaves of theAgave cantalatree using a mechanical retting system, and the fibers were dried below room temperature. Sodium hydroxide (NaOH) with a purity of 98% and Aquades was obtained from PT Merck (Jakarta, Indonesia) for the treatment of fiber alkalis. UPRs of type Yukalac BQTN 157 and methyl ethyl ketone peroxide were obtained from PT Justus Kimia Raya (Semarang, Indonesia). Microcrystalline cellulose with a size of 20 μm and a density of 1.56 g/cm3was obtained from PT Sigma Aldrich (Jakarta, Indonesia).
Cantala fiber was soaked with NaOH at a concentration of 5 wt% for 0 h (UF), 3 h (AK3), 6 h (AK6), 9 h (AK9), of 12 h (AK12)at room temperature. The fiber was rinsed with tap water to clean off the alkaline solution, until a pH of about 7 is reached.The fibers were dried at room temperature for 24 h and heated in an oven at 60 °C for 10 h.
The composite manufacturing process began with chopping cantala fiber into 10-mm pieces for use as a composite fiber material with a matrix made of unsaturated polyester and microcrystalline cellulose. Five types of composite molds were prepared, from UF, AK3, AK6, AK9, and AK12. Each composite type used a 60% volume fraction, 35% fiber, and 5% microcrystalline cellulose. Unsaturated polyester mixed with MCC was stirred at 150 rpm at 60 °C for 30 min using magnetic steers [13, 14]. Then,methyl ethyl ketone peroxide (MEKPO) was mixed into UPRs and MCC. This step was followed by putting the matrix and MCC mixture into the mold using the vacuum infusion method. In the final stage, we removed the composite from the mold after 18 h.
Fourier transform infrared spectroscopy (FTIR) testing functioned to determine the functional CF that is treated and untreated. CF was finely ground and mixed with potassium bromide (KBr) at a ratio of 1:20, with the transmission spectrum recorded at wavelengths between 400 cm-1to 500 cm-1around 24 scanners with 2 cm-1resolution using a Shimadzu IR prestige 21 model (Kyoto, Japan).
Cellulose structures from treated and untreated cantala fiber were analyzed by X-ray diffraction at room temperature using Cu Kα radiation (λ= 1.54 Å). The intensity of Cu Kα radiation was recorded from 2θ= 100° to 900° in 20 steps with a voltage of 30 kV and a current of 30 mA. The crystallinity index (CrI) and degree of crystallinity (%C) were calculated by the Segal method according to Eq. (1) [15]:

whereI(002)is the intensity of the sample peak based on the Miller Index (002) at an angle of 2θranging from 22° to 23° andIamis the minimum intensity of the noncrystal content, which shows a peak at 2θ= 18°.
For scanning electron microscopy (SEM) we used a JSM-610 PLUS/LV model instrument from JEOL Ltd. (Akishima, Japan) to capture two-dimensional images of the cantala fiber surface.Cantala fiber was mounted on a piece of aluminum-coated platinum and observed for 1 min at 2 bar (1 bar=1×105Pa) pressure.
Thermogravimetric analysis was used to test the stability of CF samples with and without treatment. All CF samples were scanned at high room temperatures from 30 °C to 600 °C at 100°C/min in a nitrogen environment. TGA testing used a Perkin Elmer Pyris Diamond TGA 6 Analyzer (Massachusetts, United States).
As German became part of the family he considered it his duty to check every bedroom to be sure each child was snug11 in bed. When he was satisfied that the last person was tucked in, he took up his position by the front door and remained there until the morning.
The surface energy was measured by the angle of contact between the fiber and droplet using the Owens–Wendt method,according to Eq. (2) [16]:
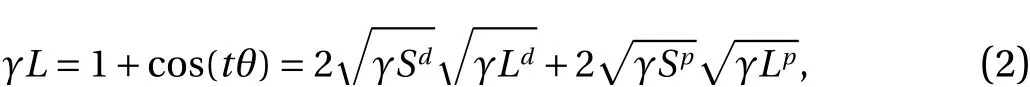
wheredis the energy dispersion (mN·m–1),pis the polar surface energy (mN·m–1),Sis the solid state, andLis the liquid state. For angle measurement and image capture we used a DF Plan 1X-4 macromicroscope.
Interfacial shear strength (IFSS) testing was done by attaching 60 mm long cantala fibers to a mixture of unsaturated polyester, microcrystalline cellulose, and MEKPO. The cantala fiber was glued to a carton with a hole in the center and dried for 120 min. The clamping distance was 50 mm with a pull speed of 250 mm/min, and 30 repetitions were performed. Paper was cut from both sides to get the maximum pull. The fiber was pulled until the bond between UPRs+MCC and the fiber was released(see Fig. 2). The calculation was based on the release of fibers from the matrix. Fiber diameter was measured at the top,middle, and bottom. The tests used a Tenso (Tokyo, Japan)Model 300 type 168 E Newton Unit textile pulling machine.
We tested the tensile and flexural strength using a universal testing machine (UTM) produced by SANS Testing Machine Co.,Ltd., series 4160 (Guangzhou, China). Tensile strength testing was conducted according to ASTM D 638-03 in 2003 and for flexural testing we referred to ASTM 790-03 in 2003.
The FTIR test results of spectrum samples without treatment(untreated fibers, UF) and with alkali treatment showed a shift in peak intensity. The peak intensity without treatment was 3443 cm–1and shifted to a peak between 3429 cm–1to 3432 cm–1after alkali treatment. This peak shift indicated the presence of an‒OH group that was free to contribute to the chemical reaction of CO2[17]. The peak band of 2922 cm–1for UF was characteristic of the stretching of cellulose/hemicellulose vibrations or methyl and methylene groups [18]. A graph of FTIR test results is shown in Fig. 3.
This peak band has a decreased intensity of 2922 cm–1for AK3, a peak intensity of 2882 cm–1for AK6, and a peak of 2900 cm–1for AK12. This showed the existence of stretching ‒OH vibrations between groups of intermolecular hydrogen bonds. The peak intensity of 1738 cm–1at UF showed aromatic skeletal vibrations and carbonate groups where lignin and hemicellulose were still present [19]. After the peak treatment, the intensity was reduced for both AK3 and AK12. The peak intensity at 1623 cm–1was C=C aromatic fiber without treatment and did not change much after treatment. The peak intensity at 1320 cm–1was cellulose Si-O at UF and did not experience many changes after treatment. The peak intensity of 1161 cm–1was the acetyl stretching C‒O; it shifted to 1158 cm–1and disappeared with treatment. The peak intensity of 1060 cm–1showed stretching of Si‒O‒Si and shifting to a peak of 1058 cm–1[20], while the peak intensity of 780 cm–1was due to the silanol group [21].
Cantala fibers form three large peaks at angles of 15°, 22.12°,and 44°. Two peaks at the beginning show areas related to the crystal fields 110 and 002 [22], whereas the final diffraction peaks of plant fibers showed 040 crystal fields [23]. The amorphous portion of the cantata fiber structure is shown by the valley diffractogram between two peaks with an area of about 2θabout 18°. The cellulose structure is shown by diffraction peaks between 22°–23°, which are characteristic of the original cellulose [24]. Observing the X-ray diffraction test results of cantala fiber, the highest- and lowest-intensity peaks can be seen in Fig. 4.
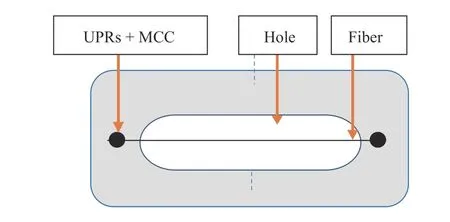
Fig. 2.Interfacial share strength test specimen.
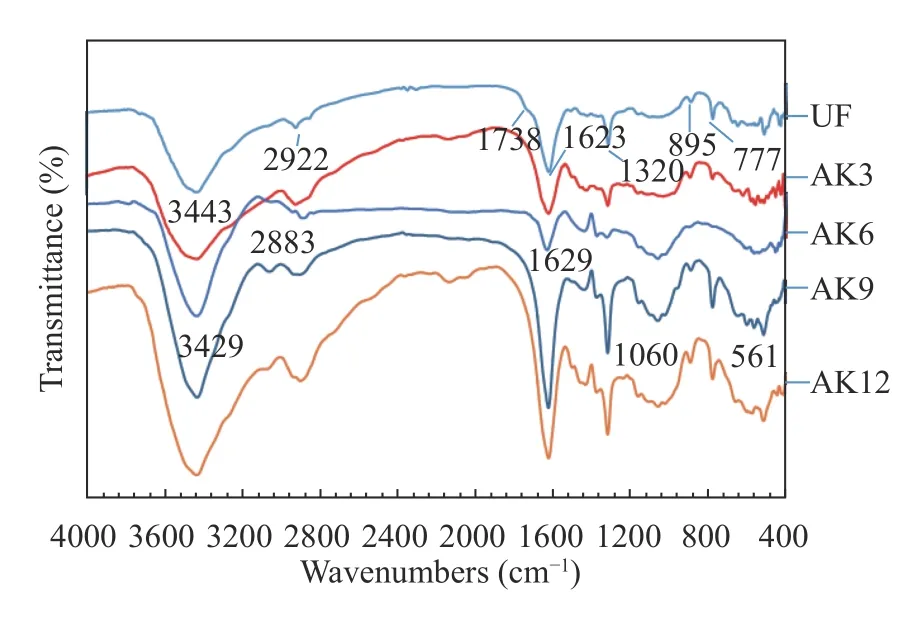
Fig. 3.FTIR spectroscopy results.
Scanning electron microscopy is a good technique for studying the surface morphology of cantala fibers modified with alkali. Micrographs clearly show the fibers that have been treated and those that have not. The surfaces of the fibers that have not been treated with alkaline look dirty and very smooth (Fig. 5a).The surface of the treated fiber is shown in Fig. 5b–d.
UF indicate fibers were still in the structure of the tissue and fibrils are still bound and wrapped by other substances such as hemicellulose, pectin, lignin, and other waste. After AK3 treatment the fiber was quite clean because the binding had begun to disappear. In the AK6 treatment, the fibers appeared to be cleaner due to the removal of hemicellulose, pectin, wax, oil, and other impurities. Cantala fiber was able to produce a clean surface topography and offered mechanical adhesion properties between the fiber and the matrix, so the mechanical properties improved. Cantala fiber had a surface roughness caused by dissolved amorphous compounds such as hemicellulose, pectin,lignin, and wax [25]. In the 9-h alkali treatment, the SEM results showed that the fiber was damaged in (‒OH) hydroxyl bonds that were lost or broken. The loss of hydroxyl bonds resulted in decreased fiber strength. The 12-h alkaline treatment showed that the fiber was increasingly damaged by the hydroxyl bonds and experienced defibrillation of the fiber, which could result in the fiber breaking.
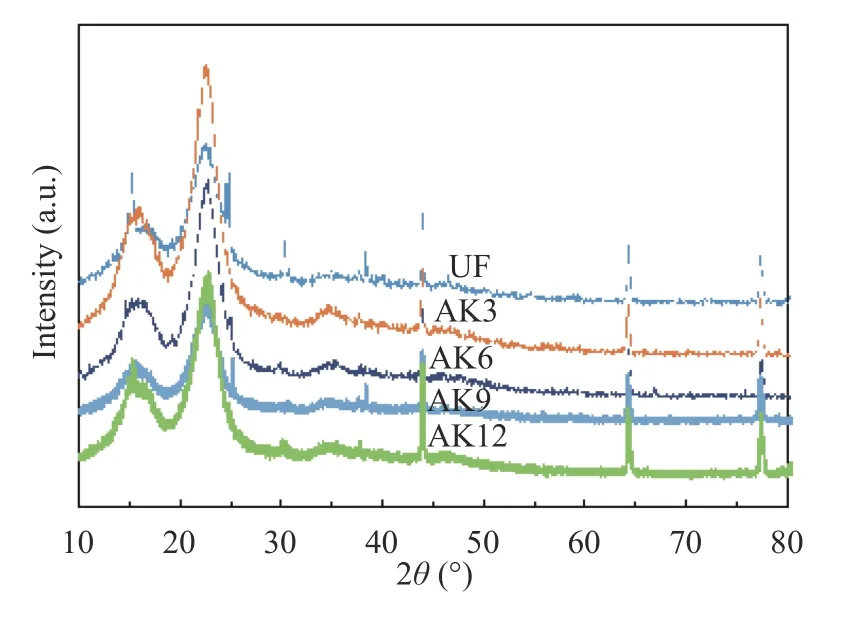
Fig. 4.FTIR results of cantala fiber.
Thermogravimetric analysis results are shown in Fig. 6a with the derivative analysis in Fig. 6b for untreated and treated fibers.CF without treatment showed a weight reduction of 5.7% at temperatures below 100 °C, associated with water evaporation. CF with treatment shows a smaller weight loss, which indicates that the alkali treatment results in the fibers being more hydrophilic.Temperature decomposition at UF occurred at 210 °C with a weight reduction of 5.9%. After treatment there was a shift in the initial decomposition temperature to a higher temperature of 250 °C. The increase in decomposition temperature was caused by the removal of amorphous substances, which are more sensitive to heat than crystal elements [26]. Untreated fibers at a temperature of 300 °C lost 45% of their weight. After the peak treatment, weight loss occurred at a temperature of 348 °C for AK3,350 °C for AK6, and 352 °C for AK9. This was closely related to the removal of most of the amorphous material, which decomposes between 200 °C and 400 °C [27, 28]. Other studies report that cellulose and lignin fibers degrade at 200 °C, while other polysaccharides such as cellulose degrade at higher temperatures [29]. The increase in fiber decomposition temperature by treatment has a considerable effect on the behavior of fiber temperature degradation. The temperature stability of the fibers increases with treatment due to the degradation of hemicellulose,lignin, and silica during the alkali process. Similar results for increasing the thermal stability of fibers by alkali treatment have been reported [30]. Untreated fiber was reduced at a lower rate,i.e., a 68% weight loss, due to the presence of unstable fiber constituents such as hemicellulose and ash, whereas the weight reduction for fiber with treatment was up to 75% for AK6 because the fiber was more stable at that temperature [31]. There was a slight difference in the residual yield remaining after 500 °C.Treatment residues were lower than those without treatment.
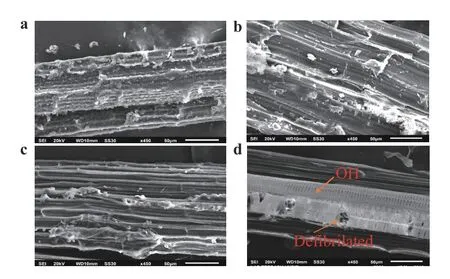
Fig. 5.Results of SEM: a UF,b alkali-treated for 3 h, c alkali-treated for 6 h, and d alkali-treated for 9 h.
Calculation of the contact angle begins with stretching the fiber at two points with a distance of 60 mm between them, and dripping ethylene glycol and water on it using a standard sized 0.05-mL pipette. The results of the contact angle and surface energy tests are in Table 1 and indicate the polar energy, dispersion, and surface energy. The total surface energy was the sum of the polar and dispersed energies. Untreated fibers had a greater contact angle when compared to the treated fibers, so the fiber surface energy without treatment was smaller compared to the fiber surface energy with alkali treatment, both with water droplets and with ethylene glycol. The increase in total surface energy showed a change in the soaking time. The soaking time led to an acid reduction and base increase in fiber and played a very important role in intensifying the wetting of cantala fibers.Surface roughness in cantala fibers will improve the wetting properties and can improve liquid dispersion on the surface [32].The highest surface energy of 76.16 mN/m was obtained by alkaline soaking for 6 h (AK6), and the lowest at AK3. These results indicate that the fiber is hydrophobic and has a low polarity level, which results in the fiber having a low wettability. The low level of wetting was mostly due to the wax and dirt that still clings to the fiber.
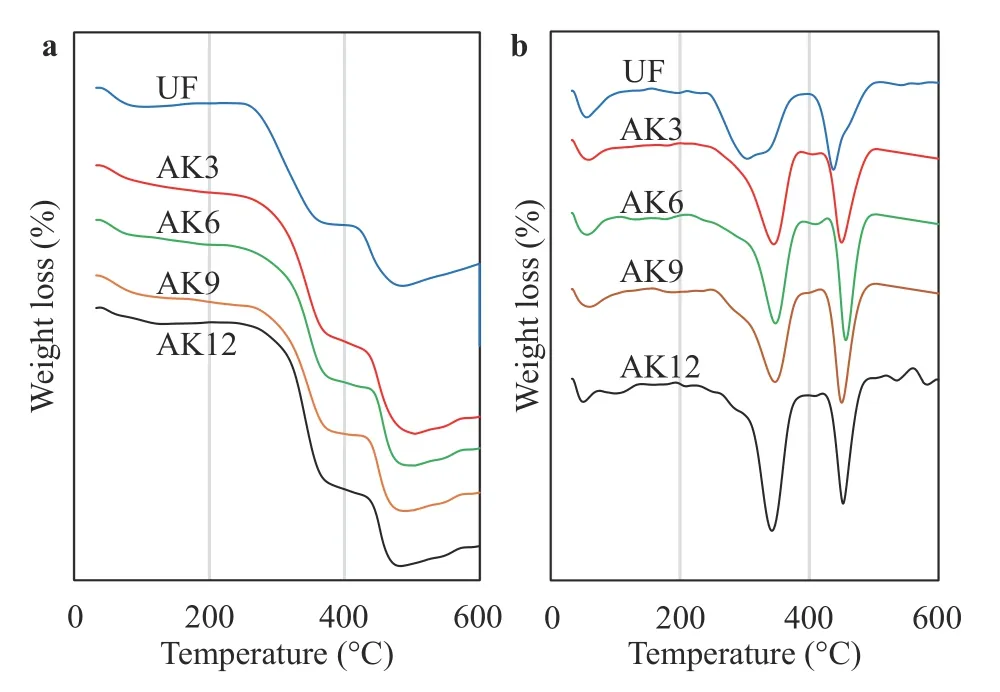
Fig. 6.Testing results:a thermogravimetric analysis and b derivative TGA.
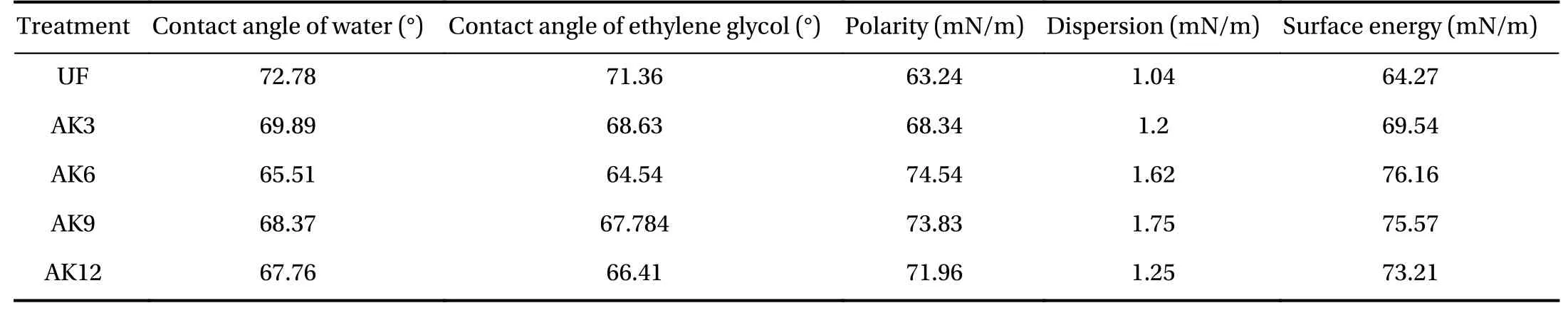
Table 1 Relationship of surface energy and contact angle
The results of the study were based on the ability of the fiber to escape the bonds of the UPRs matrix and microcrystalline cellulose reinforcement mixture. The increased adhesion was due to the increased surface roughness in cantala fibers. Fiber alkali treatment showed that the fibers were cleaner and there was an increase in surface roughness, which indicated that fibers underwent more bonding with the matrix. UF fiber showed an interfacial bonding value of 2.49 MPa, slightly higher than the results in Ref. [33], which showed a value of 2.45 MPa. Fiber with AK3 treatment showed an increase in interfacial bonding by 2.94 MPa. The results of the testing of the shear strength of the interface are shown in Fig. 7.
The highest shear strength was obtained by the AK6 treatment of 3.67 MPa, whereas longer treatment of alkali fibers broke down hydroxyl bonds, thus causing a decrease in the bonds between the fiber face and the matrix. The increase in shear strength of the interface in the treatment had two effects on the fiber, namely, increasing the surface roughness of the fiber, which increased the interlock (bonds) between the fiber and the matrix on the composite, and increasing the exposure of hydroxyl groups on the surface of the fiber, so that the reactive group easily formed chemical bonds in the presence of other compounds [34].
The flexural test measures the force required by bending the beam under the condition of concentrating force at three points.Flexural data are often used to select materials that will support the load without flexing. The flexural modulus can be used as an indication of the rigidity of a material when bending is carried out. The test results showed that the fiber with the treatment had increased flexural strength. Before the UF treatment, the flexural strength was 68.23 MPa. After treating with alkali soaking for 3 h(AK3), the flexural strength increased to 82.32 MPa. The composite test results for flexural strength are depicted in Fig. 8.
The highest flexural strength of 98.24 MPa was obtained at AK6. Kabir et al. argued that alkalization with NaOH can increase flexural strength by 31% [9]. The highest bending condition was for the 93.4 MPa kenaf fiber/composite [3], while the flexural strength decreased after alkali treatment with soaking for 6 h. This was due to an increase in the strength of the interface between the fiber and the matrix. The modulus trend graph of the test results was similar to the flexural strength graph, and the same results were obtained [35] for sisal fiber. Natural fibers with alkali treatment produced higher modulus values because of crosslinking and the bonding of fiber and matrix interfaces[36]. Increased flexural strength was also caused by the addition of MCC to the composite [37].
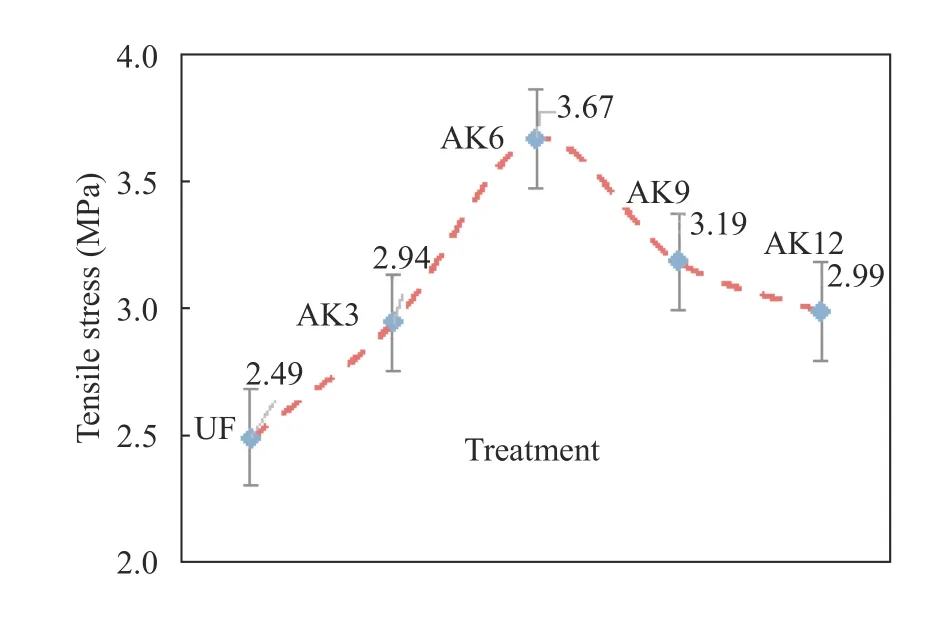
Fig. 7.Interfacial shear strength test results.
Tensile strength testing used ASTM D638-03 2003. The test results showed that the fiber before treatment had a tensile strength of 53.65 MPa. The tensile strength increased after AK3 treatment, to 68.81 MPa, and reached 79.82 MPa in the AK6 treatment; after the AK6 treatment, the tensile strength decreased. The highest tensile strength of kenaf fibers with alkaliunsaturated polyester was 90.8 MPa [34]. The decrease in tensile strength was supported by the SEM results for AK9 where the fiber is damaged at –OH and defibrillated, leading to fibers breaking. The increase in tensile strength was due to the cleaner fiber, because hemicellulose, pectin, lignin, and wax decreased.Increased tensile strength was due to the surface of the fiber being rougher, so there was an increase in the strength of the interface between the fiber and the matrix. The graph of modulus test results showed results similar to the tensile strength testing [35].The increased tensile strength and modulus were due to the addition of microcrystalline cellulose. The addition of microcrystalline cellulose to polyvinyl alcohol (PVA) as a composite resulted in an increase in the tensile strength and modulus [38]. The results of the test are shown in Fig. 9. As a comparison with the results of the composite UPRs-MCC-Cantala Fiber test, tensile strength data for the prosthetic socket material produced by OttoBock (New South Wales, Australia) [39] are presented in Fig. 10.
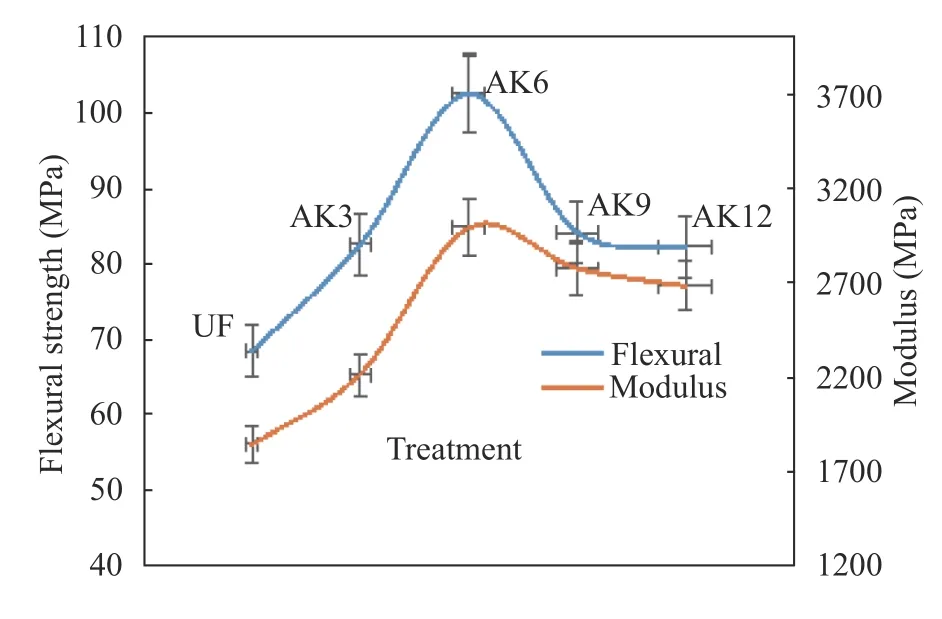
Fig. 8.Flexural testing results.
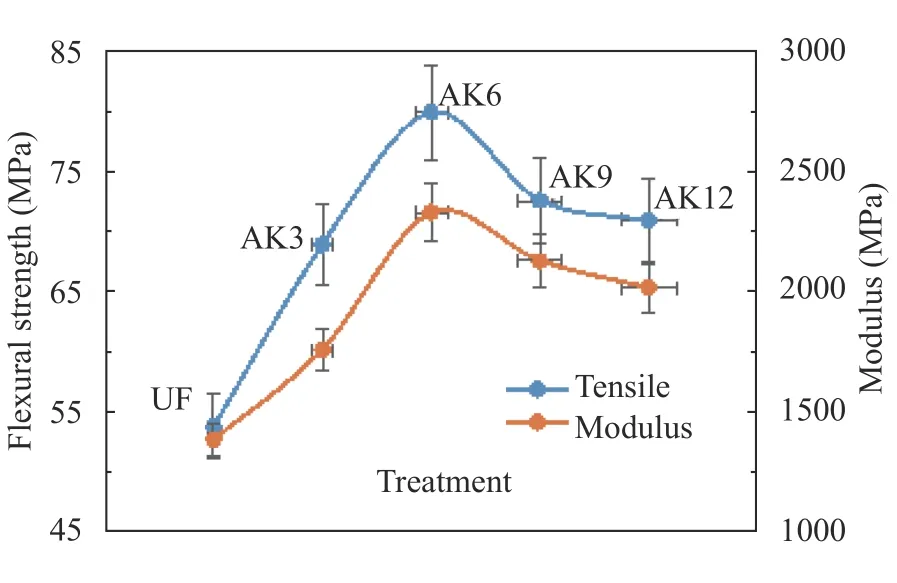
Fig. 9.Tensile strength test results.
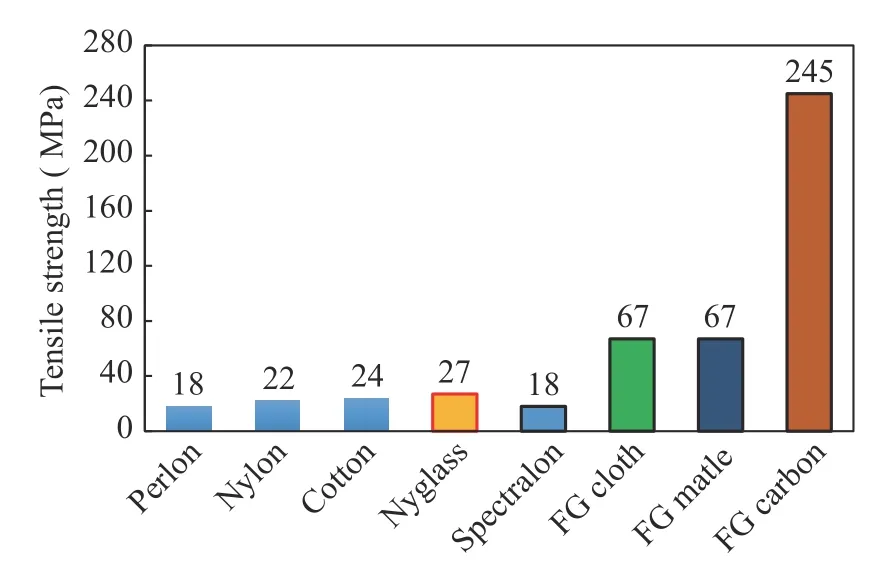
Fig. 10.Ottobock prosthetic material.
Socket test results using polyethylene terephthalate glycol(PETG), ThermoLyn rigid and Orfitrans stiff for the socket had tensile strength values of 53.7 MPa, 15.5. MPa, and 15.2 MPa, respectively [40]. Thus, the results of composite testing with natural fiber material, cantala fiber [41], and unsaturated polyester matrix with the addition of microcrystalline cellulose showed strength above the tensile strength of the materials currently used in prosthetic sockets on the market. Future development of the bio-resources is promising, especially with designated focus to partially replace conventional materials, e,g., steel and aluminum as technical material in real-world structure and engineering product [6, 42–49].
Alkaline treatment results in the removal of impurities and cementing the material in cantala fibers. TGA analysis showed a CF thermal stability increase after treatment. X-ray diffraction showed amorphous content and an increase in the crystallinity index. Micrograph SEM provided concrete evidence of the reduction of cementing components and impurities. Alkaline treatment can reduce the contact angle, increase adhesion, and increase surface energy in the fiber. Alkaline treatment increases the IFFS between the fiber and the matrix. Tensile strength, flexural strength, and the elastic modulus in composites increased with alkali treatment and the addition of microcrystalline cellulose. Optimal values of tensile strength, flexural strength, and elastic modulus were obtained by soaking cantala fiber for 6 h in a 6% NaOH solution. This product exceeds the tensile and flexural strength of materials commonly used in the prosthetic sockets that are on the market.
杂志排行
Theoretical & Applied Mechanics Letters的其它文章
- A modified Lin equation for the energy balance in isotropic turbulence W.D. McComb*
- Influence of wing flexibility on the aerodynamic performance of a tethered flapping bumblebee
- Efficient model for the elastic load of film-substrate system involving imperfect interface effects
- Interactions of human islet amyloid polypeptide with lipid structure of different curvatures
- Evolution of vortices in the wake of an ARJ21 airplane: Application of the liftdrag model
- Analytical and numerical studies for Seiches in a closed basin with bottom friction
