Interactions of human islet amyloid polypeptide with lipid structure of different curvatures
2020-12-13LeMeiWenhuiShenXuweiWuJieLiuDechngLiBohuJi
Le Mei, Wenhui Shen, Xuwei Wu, Jie Liu, Dechng Li,*, Bohu Ji
a Biomechanics and Biomaterials Laboratory, Department of Applied Mechanics, Beijing Institute of Technology, Beijing 100081, China
b Institute of Applied Mechanics, Department of Engineering Mechanics, Zhejiang University, Hangzhou 310027, China
Keywords:Lipid membrane Curvature Human islet amyloid polypeptide Molecular dynamics
ABSTRACT Curvature is one of the most important features of lipid membranes in living cells, which significantly influences the structure of lipid membranes and their interaction with proteins.Taken the human islet amyloid polypeptide (hIAPP),an important protein related to the pathogenesis of type II diabetes, as an example, we performed molecular dynamics (MD)simulations to study the interaction between the protein and the lipid structures with varied curvatures. We found that the lipids in the high curvature membrane pack loosely with high mobility. The hIAPP initially forms H-bonds with the membrane surface that anchored the protein, and then inserts into the membrane through the hydrophobic interactions between the residues and the hydrophobic tails of the lipids. hIAPP can insert into the membrane more deeply with a larger curvature and with a stronger binding strength. Our result provided important insights into the mechanism of the membrane curvature-dependent property of proteins with molecular details.©2020 The Authors. Published by Elsevier Ltd on behalf of The Chinese Society of Theoretical and Applied Mechanics.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
The lipid membrane, formed by varied component of lipids,is one of the most important structure in cells that forms a continuous barrier around and inside the cells separating the cytoplasm from the external solution [1–4]. The lipid membrane is not only the barrier keeps the ions, proteins, DNAs and other molecules where they are, but also the zone of many reactions happens [4], such as the production of adenosine triphosphate by F1-ATPase [5], synaptic transmission [6–8], etc. One of the most important physical features of the lipid membranes in living cells is the curvature of the structure, which has attracted much attention because it is involved in many cellular functions,such as membrane fusion and scission, protein sorting, enzyme and protein activation [9, 10]. For instance, the curvature significantly influences the properties of lipid membranes and their interaction with proteins. It was reported that some of proteins are membrane curvature sensors as the proteins have varied affinity with different curvature membranes [11–14]. Previous studies showed that the curvature sensing proteins may interact with the curved membrane through the lipid packing defects[12]. However, the mechanisms of membrane curvature-dependent property of proteins and the molecular details behind these mechanisms are still unclear.
The human islet amyloid polypeptide (hIAPP), a small protein formed by only 37 residues, plays an important role in the pathogenesis of the type II diabetes [15–17]. Normally, the protein hIAPP is soluble and intrinsically disordered in its monomeric state, stored with insulin in theβ-cell secretory granules[15–17]. The misfolding and aggregation of hIAPPs can form the pancreatic islet amyloid, which is a characteristic feature of the type II diabetes [18]. It was proposed that the protein hIAPP can interact with the cell membrane, form oligomers, penetrate into the lipid bilayer, and disrupt the membrane, that leads to theβcell dysfunction and cell death, which is thought to contribute to the pathogenesis of the type II diabetes [19, 20]. Recently, studies showed that the cytotoxic hIAPP prefers to interact with the lipid membrane with a high curvature [21, 22]. However, the curvature effect on the binding of hIAPP to the lipid membrane are still elusive.
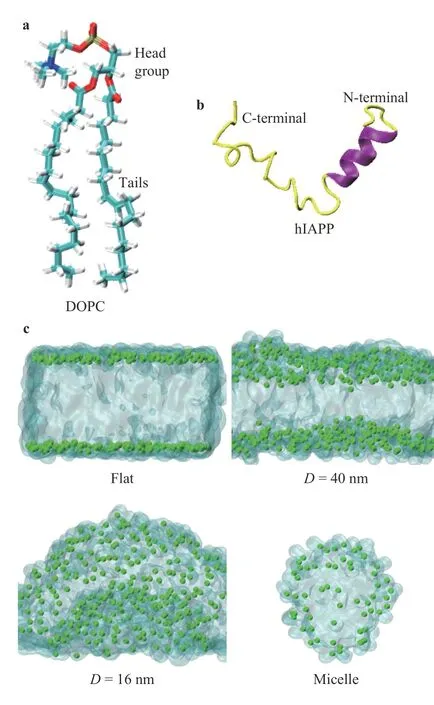
Fig. 1.Illustrations of the simulation models. a Structure of the lipid DOPC. b Structure of protein hIAPP from the PDB ID 5MGQ[26].The helix and the coil-coiled structures were represented by purple and yellow color, respectively. c Simulated lipid models with varied curvatures, i.e., the flat one with zero curvature, the curved membranes with diameters D=40 nm and D=16 nm. In addition,the model of lipid micelle with diameter approximate D=6 nm was also generated to mimic the largest curvature of the lipids can form. For clarify, the tails of the lipids were shown as transparent regions in c,and the phosphorus atoms in the lipid DOPC were shown by green dots.
In this study, we applied molecular dynamics (MD) simulations to study the interactions of hIAPP with curved lipid structures. The lipid bilayers and the lipid micelle formed by dioleoylphosphatidylcholine (DOPC) were generated by the CHARMM-GUI web service [23–25], as shown in Fig. 1. The structure of the protein hIAPP was obtained from the protein data bank (PDB) with ID 5MGQ [26], see Fig. 1b. In vivo experiment, the hIAPP was found to bind with the cristae of the inner mitochondrial membrane, as well as other intracellular and extracellular vesicular structures [27]. The mitochondrial cristae are highly curved membranes with the dimeter in the size of dozens of nanometers [28]. To study the influence of the curvatures, the lipid bilayers with varied curvatures were generated, i.e., the flat membrane with zero curvature, two curved membranes with diametersD=40 nm andD=16 nm, and the lipid micelle with a diameter aboutD=6 nm to model the membrane of the largest curvature that lipids can form, respectively,as shown in Fig. 1c. The simulation box was solvated by TIP3P water molecules [29] and appropriate number of chloride ions were added to neutralize the system.
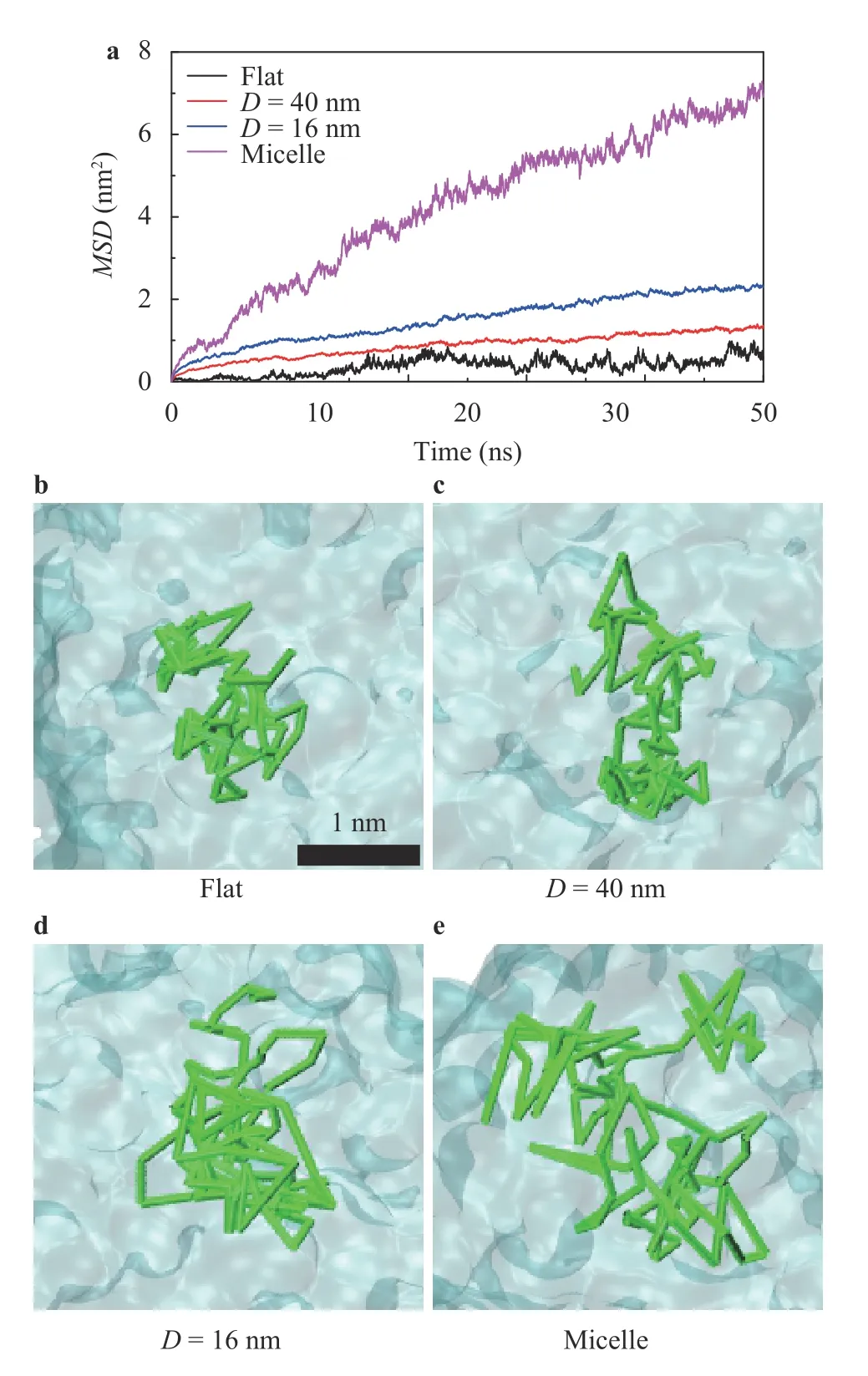
Fig. 2.Mobility of the lipids in the lipid structures. a M SD s of the lipids with different curvatures. b–e Illustrations of the movement trajectories of one DOPC lipid in the flat membrane, the curved ones with D=40 nm and D=16 nm, and in the micelle with D=6 nm, respectively.The position of the selected lipid's phosphorus atom in different time was connected by green rods in b–e, illustrating the trajectories of the lipids. The black bar in b shows the length scale of 1 nm.
The MD simulations were performed using GROMACS package [30] with CHARMM36m force field [31]. The NPT ensemble with periodic boundary condition were used in the simulations.The pressure and the temperature were coupled in 1 bar (1 bar=1×105Pa) with Parrinello–Rahman method [32] and 310 K with the V-rescale algorithm [33]. The bond length with hydrogen atoms was restrained by linear constraint solver (LINCS)algorithm to enable a time step to be 2 fs (1 fs=1×10-15s) [34]. The cut-off of the non-bonded interactions was set to 1.2 nm, while the long range electrostatic interaction was calculated by the particle mesh Ewald (PME) method [35]. All the snapshots were analyzed by the visual molecular dynamics (VMD) package [36].
Figure 2 shows the mean square deviations (MSDs) of the lipids in membranes with varied curvatures. TheMSDvalue is a proper parameter for describing the fluidity of the molecules[37], i.e., the higher theMSD, the higher mobility of the lipids is.It showed that a larger curvature of the lipid structures caused a higherMSDof the lipids, as shown in Fig. 2a. Figure 2b–e shows the trajectories of one lipid in membranes with varied curvatures. It confirmed that the lipids in a larger curvature membrane would have a higher mobility, e.g., the larger area of the lipid can visit, as shown in Fig. 2b–e. Previous studies suggested that the packing of lipids would significantly influence the interactions between the protein and the membrane [12]. Figure 3a shows the area per lipid in the membrane with varied curvatures. Obviously, a large curvature causes a large area per lipid, indicating that the lipids pack more loosely in the larger curvature membranes. Figure 3b shows the minimal average distance between the lipids with each other, indicating that a larger curvature of the membrane leads to a larger distance of the lipids. The large distance between lipids indicated that there will be a large gap among lipids because of the loosely packing with a large curvature, through which the residues of proteins may access the hydrophobic tails of the lipids easily, as shown in Fig. 3c, d.
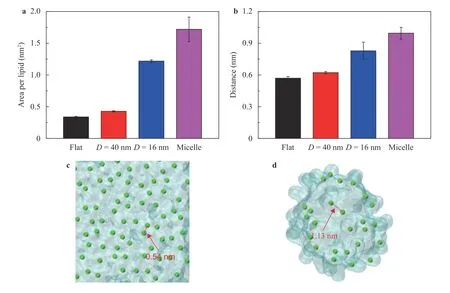
Fig. 3.Packing of lipids with varied curvatures. a Average area per lipid influenced by the curvatures. b Average minimal distance between the lipids in membranes with varied curvatures. c, d Illustrations of the lipid packing in the flat membrane and in the micelle, respectively. The arrows in c, d indicate the gaps between two lipids in the flat membrane and in the micelle, respectively.
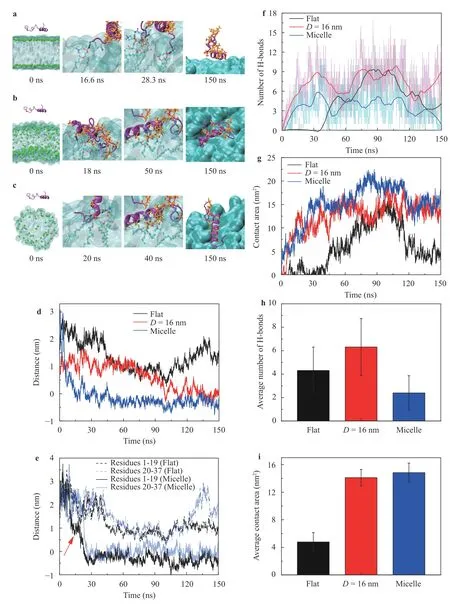
Fig. 4.Binding processes of hIAPP to the lipids with varied curvatures: a the flat membrane, b the curved membrane with D=16 nm, and c the micelle with D=6 nm, respectively. d Distance between the protein hIAPP and the surface of the lipid structures.e Distance of the N-terminal(residues 1-19) and the C-terminal (residues 20-37) to the surface of the lipid structures. The distance was calculated between the center of mass(COM) of the protein segment (e.g.,the residues 1-19 in N-terminal and the residues 20-37 in C-terminal) and the phosphorus atoms in the same leaflet of the lipid bilayer. f, g Evolution of the number of H-bonds and the contact area between the protein and lipids, respectively.h, i Average number of H-bonds and the contact area between hIAPP and lipids when the protein well inserted into the lipid bilayers and the micelle. The average values in h, i were calculated by the simulation data in 120–150 ns.
To understand the mechanism of the membrane curvature effect on the protein binding, we study the binding processes of hIAPP to the membranes with varied curvatures, as shown in Fig. 4. Initially, the protein was set apart from the lipids and the protein diffused randomly in the solution. Occasionally, the protein made a contact with the membrane, forming hydrogen bonds (H-bonds) that anchored the protein on the membrane,as shown in Fig. 4a–c. In the second step, the hydrophobic residues of the anchored protein gradually inserted into the gap between two lipids. Previous study indicated that the packing status and the mobility of lipids are important for the insertion of the proteins into lipid membrane [38]. In the case with zero curvature, the protein was anchored on the lipid surface by the H-bonds, as shown by the snapshots 16.6 ns and 28.3 ns in Fig.4a. However, since the tight packing and the low mobility of the lipids in the flat membrane, the residues were not easy to penetrate the membrane surface. As a result, the protein hIAPP only absorbed on the surface of the flat membrane, as shown by the snapshot 150 ns in Fig. 4a. In contrast, the lipids in the membrane with large curvature will pack loosely and have much mobility, we can see that the protein can insert into the curved membranes deeply when comparing with the flat one, as shown in Fig. 4a–c. Figure 4d shows the distances between the protein hIAPP and the surface of the lipids, confirming that the larger curvature of the lipid membranes leading to a deeper insertion.Previous studies showed that the N-terminal of hIAPP mainly contributed to the interaction with lipid membranes [22, 39, 40].Figure 4e shows that the distance between the residues 1-19 (i.e.,the N-terminal) and the lipid micelle decreased prior to that of the residues 20-37 (i.e., the C-terminal) at the simulation time about 10 ns, indicating that the insertion of the N-terminal residues 1-19 was prior to that of the C-terminal, which is consistent with previous studies [22, 39, 40]. Additionally, the N-terminal inserted more deeply than that of the rest of the protein residues, as shown in Fig. 4e at time 120–150 ns.
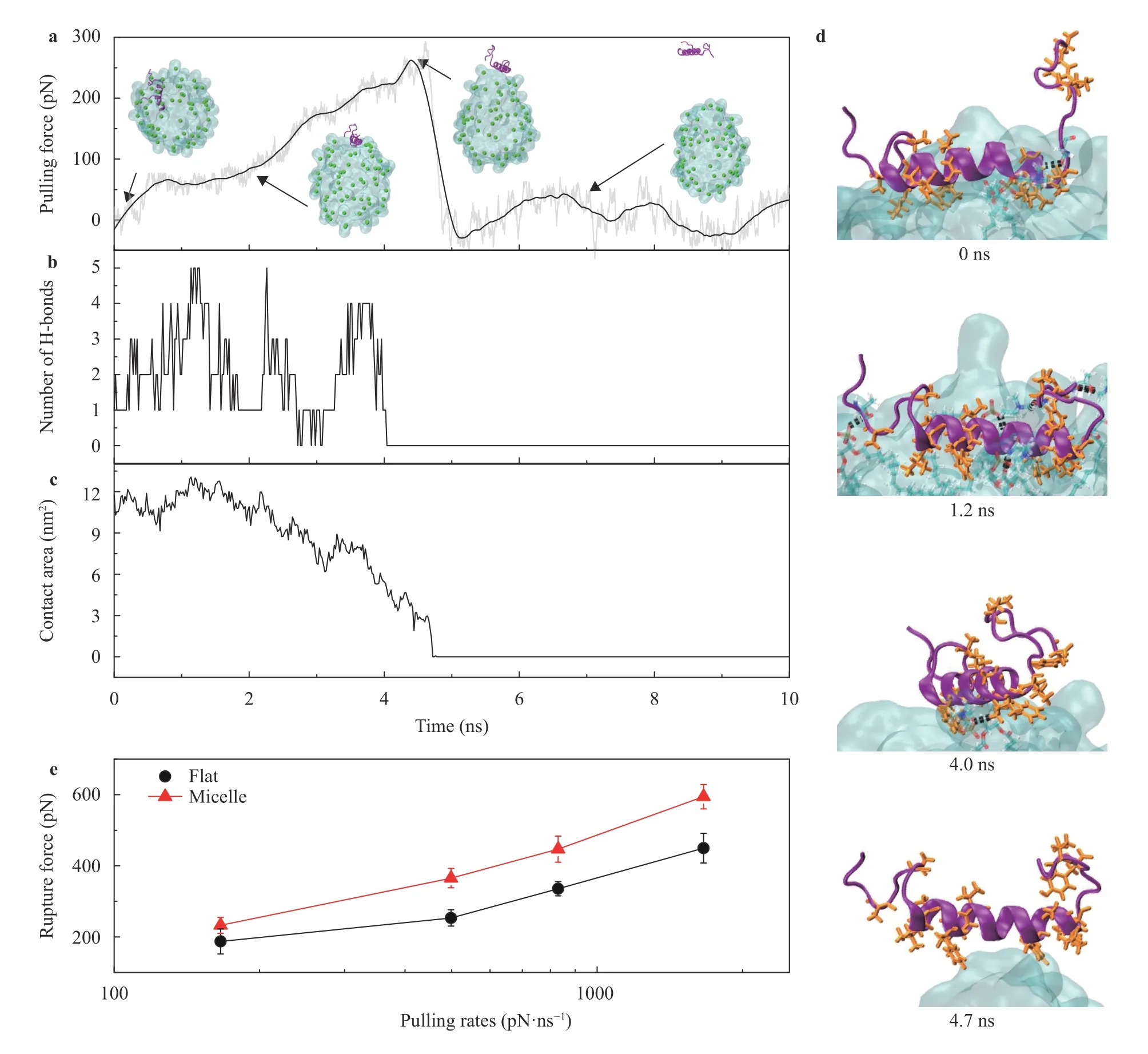
Fig. 5.SMD simulations of pulling the protein hIAPP out of lipid structures with varied curvatures.a Pulling force and the typical snapshots of the protein hIAPP was pulled out of the lipid micelle. b, c Evolutions of the number of H-bonds and the contact area between the protein hIAPP and the lipid micelle during the pulling simulation. d Snapshots of the structure details with simulation time of hIAPP out of the micelle. e Rupture forces to dissociate hIAPP from the flat membrane and lipid micelle under varied pulling rates. The error bars in e show the standard deviations based on 5 independent simulations.
Figure 4f, g shows the number of H-bonds and the contact area between the protein and the membrane as the functions of curvature, respectively. It shows that the protein hIAPP can form multiple H-bonds with the lipid membrane. Interestingly, comparing with the flat membrane, there were more H-bonds when hIAPP binds with the membrane with a larger curvature, e.g.,D=16 nm, as shown in Fig. 4f–h. In contrast, when bound with the lipid micelle, the number of H-bonds decreased significantly, comparing with the flat membrane case and the curved one withD=16 nm. It should be note that the H-bonds were mainly formed by the head groups of the lipids and the protein.In the case of the flat membrane, the protein was only absorbed on the lipid surface with a few H-bonds while the C-terminal extended to the solution (see Fig. 4a). When bound with the curved membrane withD=16 nm, the protein well inserted into the membrane that the whole protein including the N-terminal and C-terminal made a close contact with the lipid head groups,forming more H-bonds. However, in the case of the lipid micelle,the lipids with the largest curvature pack more loosely and have higher mobility so that the protein hIAPP inserted more deeply into the hydrophobic region of the membrane. Accordingly, the residues will be buried in the hydrophobic region of the lipids so that block the formation of H-bonds with the lipid head groups.The penetration of the protein needs to overcome the barrier by the H-bonds between the protein and the membrane surface.The contact area increased with the increasing of the curvatures,confirming that the larger curvatures led to a deeper insertion of the protein, as shown in Fig. 4g.
To study the interaction between the protein hIAPP and the membranes with varied curvatures, we performed steered MD(SMD) simulations [41–48] to dissociate the inserted hIAPP from the lipids. Figure 5a shows the typical dissociation process of the protein hIAPP pulled apart from the lipid micelle. Since initially the protein was inserted deeply into the lipid structure, when the protein was pulled out from the hydrophobic region of the lipid micelle the residues of protein can form more and more Hbonds with the lipid head groups at the micelle surface. As a result, the number of H-bonds increased with the pulling process at the beginning, as shown in Fig. 5b. In contrast, the contact area decreased gradually showing that the protein was pulled apart from the lipid micelle, as shown in Fig. 5c. Figure 5d shows the snapshots of hIAPP being pulled out from the lipid micelle. The rupture force of the SMD simulations is a well parameter to represent the binding strength between two molecules [42, 49]. As shown in Fig. 5e, the rupture forces of the protein separating from the membrane significantly depend on the membrane curvature. The rupture forces of hIAPP in the lipid micelle under varied pulling rates were larger than those in the flat membrane,indicating that the binding strength of hIAPP with the membrane of larger curvature is stronger.
In summary, we studied the interaction between the protein and the lipid structures with varied curvatures through MD simulations. We found that the lipids in the membrane with high curvature preferred to pack loosely with high mobility, which promotes the insertion of hIAPP into the lipid membrane. The protein hIAPP initially forms H-bonds with the membrane surface that anchored the protein on the membrane, and then hIAPP inserts into the membrane through the hydrophobic interactions between the residues and the hydrophobic region of the lipids. Moreover, the protein hIAPP can insert into the membrane of larger curvature more deeply with a stronger binding strength. Our result provided the molecular details of the mechanism for the membrane curvature-dependent property of proteins.
Acknowledgement
D. Li and B. Ji were supported by funds from the National Natural Science Foundation of China (Grants 11932017,11772054, 11772055, and 11532009). D. Li was supported by the Fundamental Research Funds for the Central Universities (Grant 2019QNA4060).
杂志排行
Theoretical & Applied Mechanics Letters的其它文章
- A modified Lin equation for the energy balance in isotropic turbulence W.D. McComb*
- Influence of wing flexibility on the aerodynamic performance of a tethered flapping bumblebee
- Efficient model for the elastic load of film-substrate system involving imperfect interface effects
- Evolution of vortices in the wake of an ARJ21 airplane: Application of the liftdrag model
- Analytical and numerical studies for Seiches in a closed basin with bottom friction
- Ergodic sensitivity analysis of one-dimensional chaotic maps
