Echinococcus granulosus: A novel parenchymal sparing surgical treatment
2020-09-21DnielKniepeissEminTlkicPeterSchemmer
Dniel Kniepeiss , Emin Tlkic , Peter Schemmer , *
a General, Visceral and Transplant Surgery, Department of Surgery, Medical University Graz, Auenbruggerplatz 29, 8036 Graz, Austria
b Division of General Radiology, Department of Radiology, Medical University of Graz, Auenbruggerplatz 20, 8036 Graz, Austria
Human echinococcosis is a zoonosis caused by Echinococcus (E.)tapeworms.There are different species, but E.granulosus and E.multilocularis have clinical relevance causing cystic and alveolar echinococcosis, respectively.Cystic echinococcosis (CE) is defined as presence of one or more hydatid cysts, most frequently in the liver and the lungs, less usual in the kidneys, bones, spleen, pancreas, heart or brain.The location, the number and size of the cysts determine the severity of the disease [1].
Patients with CE of the liver are often asymptomatic and cysts are discovered accidentally.A few patients show symptoms like pain in the right upper abdomen, often related to active cyst increase.Up to 20% of patients with liver cysts develop mild to severe biliary complications like cysto-biliary fistulas, biliary obstruction and cholangitis [2].Furthermore, bacterial cyst infections and abscess formations can occur as well as cyst ruptures [3 , 4].Large or rapid growing cysts may lead to compression of blood vessels causing thrombosis or Budd-Chiari syndrome.
Treatment of CE is complex and depends on the characteristics and classification of the cysts.The “Guidelines for treatment of cystic and alveolar echinococcosis in humans”published by the WHO Informal Working Group on Echinococcosis [5]recommended the four treatment modalities: (i) drug treatment with benzimidazoles;(ii) percutaneous sterilization techniques; (iii) surgery; and (iv)“watch and wait”strategy.
Concerning surgery there are two major approaches until now [6 , 7].On one hand, there is the partial cystectomy with removal of the pericyste components (endocyst).On the other hand total cystectomy or resection with removal of the parasite-derived cyst components (endocyst) and the entire pericyst (host-derived connective tissue capsule) can be performed.Here we described a novel parenchymal sparing surgical technique for CE of the liver.
From October 2017 to December 2018, 4 patients with CE of the liver underwent partial cystectomy at our department, using the procedure described below.Two patients were male and two female, with a median age of 39 years (range 23-58 years).For diagnosis abdominal ultrasound or CT scan was used.In three patients,the ultrasound showed large (9.5 cm, range 7.5-11.5 cm), partially calcified cysts predominantly in the right lobe of the liver (WHO CE4 type) [8](Table 1).The axial non-contrast CT scan of patient A represented a large (10.5 cm) E.granulosus cyst (WHO CE4 type)with partially calcified wall in subcapsular location, predominantly in the left liver lobe.Adjacent to this lesion was another, smaller,mainly calcified E.granulosus cyst (WHO CE5 type) in posterior location (Fig.1).All patients received a prophylaxis of secondary echinococcosis with albendazole 400 mg twice daily for 4 weeks preoperatively and 3 months postoperatively.
After laparotomy (either reversed L-shape incision in the right upper quadrant or midline incision), the CE is located with intraoperative ultrasound and the liver surface in projection of the CE to be addressed is exposed.Subsequently a 1st layer of wet (normal saline) cloths is placed in the abdomen to protect surrounding tissue from a 2nd layer of cloths soaked with 20% saline placed on top of the first layer.Hyperosmolar saline protects from contamination after potential spilling of hydatid fluid with protoscoleces.
A noncutting 12-mm safety trocar was inserted into the cyst(Fig.2 A) and the parasite-derived cyst content was evacuated completely with a surgical suction (Fig.2 B).Cyst content with high viscosity can be diluted with normal saline injected via the gasconnector of the trocar (Fig.2 C).After complete evacuation of the parasite-derived cyst content, the cyst must be filled with hyperosmolar saline for 15 min to eliminate remnant viable protoscoleces; however, bile fistula must be excluded before with the white test [9].After careful removal of the trocar the inside wall of the cyst can be inspected while Intralipid® 20% or Lipofundin®MCT/LCT 20% or similar infusion is injected via the cystic duct.Identified fistula must be closed.Most of the time there is a need to enlarge the trocar insertion orifice to close a fistula.A purse string suture should be placed to be able to have a tight fit of a buttoned cannula which is used to fill up the cyst with 20% saline(Fig.3 A).After 15 min of exposure the cyst was evacuated again,deroofed, and an omentoplasty was performed (Fig.3 B).
All instruments and devices used during the potential contamination phase which starts with the insertion of the trocar into the cyst and ends with the evacuation of the hyperosmolar saline from the cyst, are stored in 20% saline.
The follow-up was 1, 6 and 12 months after the operation and then once a year and included clinical examination and ultrasound of the liver.

Table 1Radiological classification of CE.
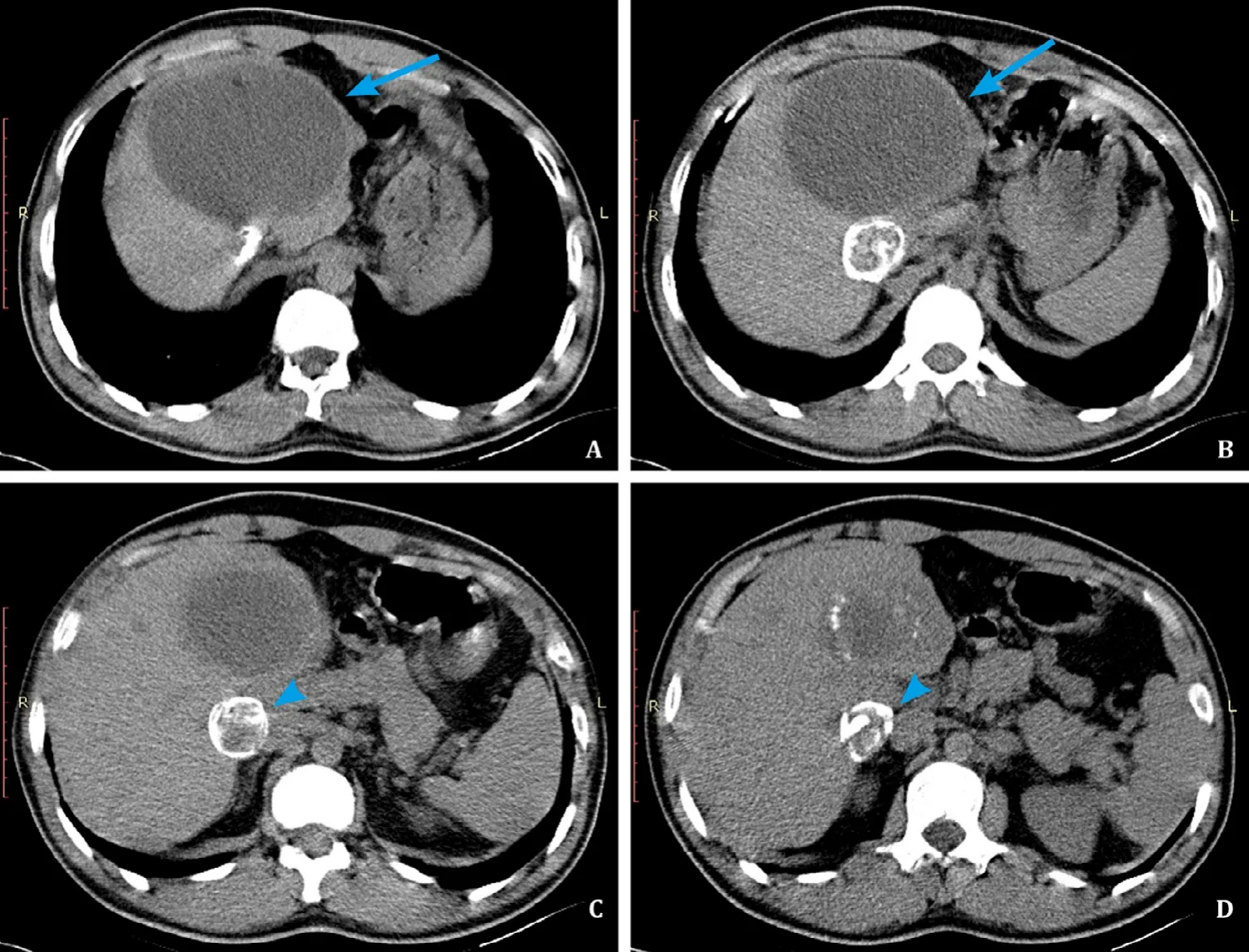
Fig.1.CT scans.Abdominal CT of a 23-year-old male patient.A, B: Axial non-contrast CT shows a large E.granulosus cyst (WHO CE4 type) with partially calcified wall in subcapsular location, predominantly in the left liver lobe (arrow).This large, mainly homogenous cyst may be misinterpreted as type WHO CE1 cyst, which is staged as“active”cyst, but due to the calcifications in the wall this is a case of WHO CE4 type cyst, considered as “inactive”cyst; C, D: Adjacent to this lesion is another, smaller,mainly calcified E.granulosus cyst (WHO CE5 type) in posterior location (arrowhead).Almost completely calcified E.granulosus cyst is a sign of inactivity.
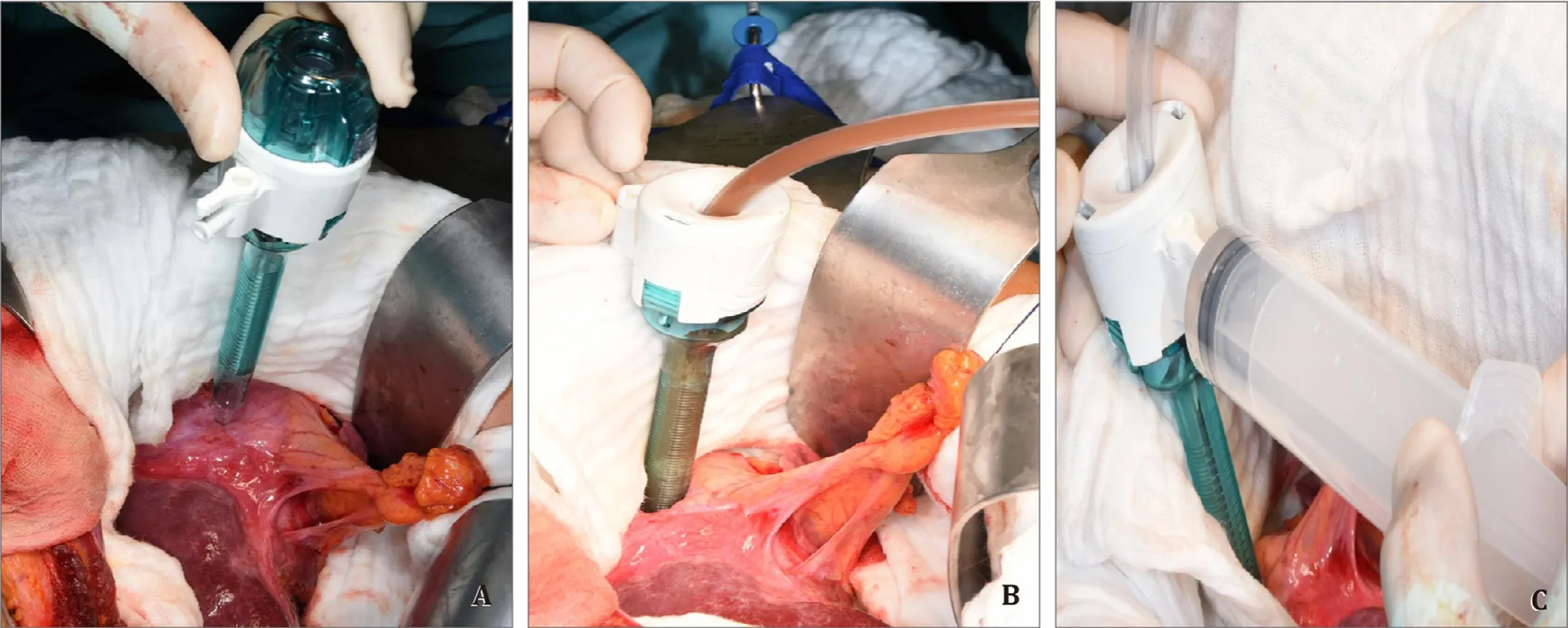
Fig.2.A: A noncutting 12-mm safety trocar is inserted into the cyst; B: The parasite-derived cyst content is evacuated completely with a surgical suction placed into the cyst via the trocar; C: The cyst content with high viscosity can be diluted with normal saline injected via the trocar's gas-connector.
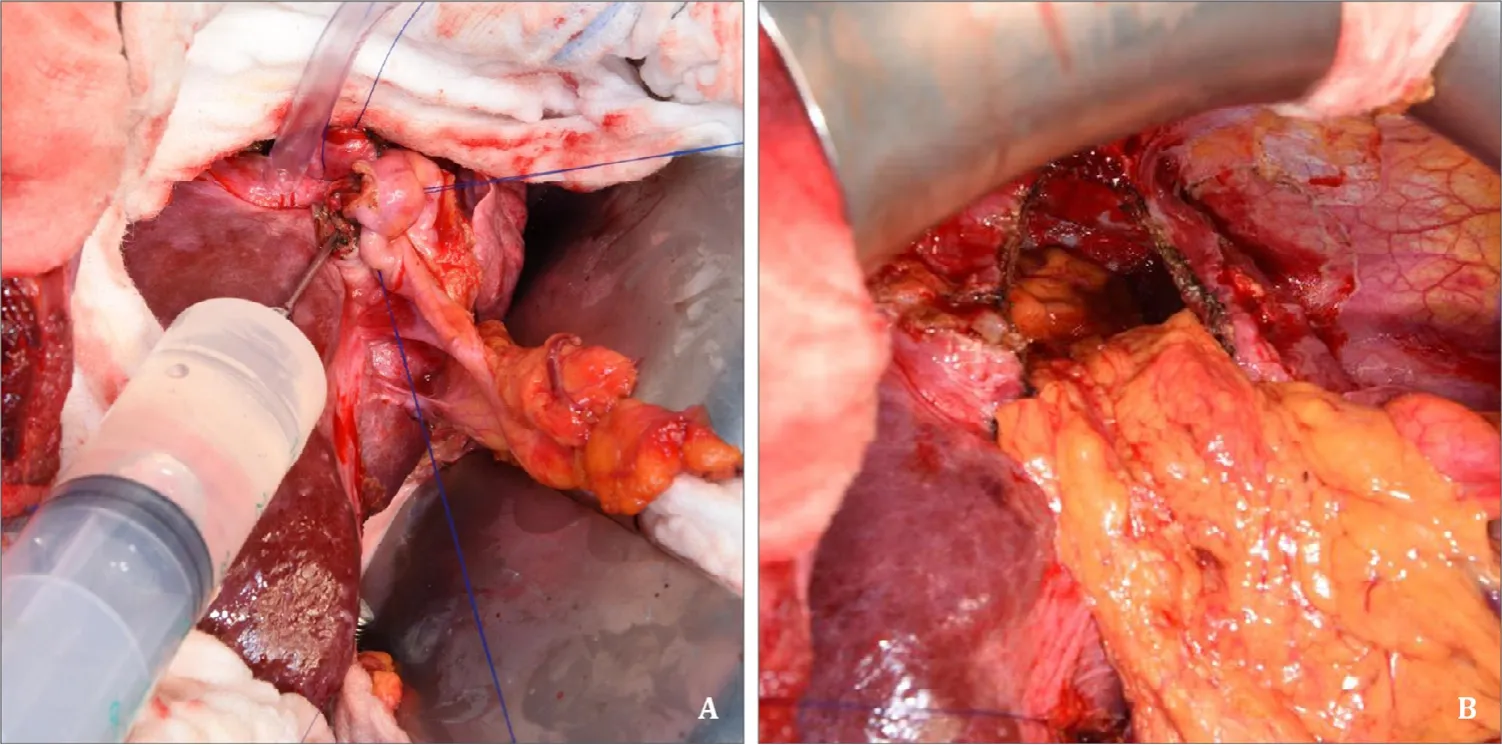
Fig.3.A: Sterilization of the cyst.Hyperosmolar 20% saline is injected via a buttoned cannula that is placed through the former trocar orifice that is closed with a purse string suture.After 15 min of hyperosmolar saline exposure the cyst is evacuated, deroofed and (B) filled with the greater omentum.

Table 2Patients' characteristics.
Median operation time was 149 min with a range of 110-225 min.Median uneventful hospital stay was 9.5 days with a range of 9 to 14 days.Complications like postoperative biloma or fluid collection of other origin did not occur at any time point after the procedure.Most importantly there was no recurrence of CE within a median follow-up of 17 months (range 13-24 months).Patients' characteristics are shown in Table 2.
Our novel parenchymal sparing surgical approach as described here especially comprises (i) evacuation of paracyte-derived cyst content including endocystectomy, (ii) sterilisation of the cyst wall(host), and (iii) deroofing of the cyst (partial cystectomy).
As it is an open approach, there is an excellent view and control of the parasite-derived material, which reduces the risk of intraoperative contamination of the abdomen through spillage.Careful handling with potentially contaminated instruments, devices and gloves is of upmost importance.The spilling prevention of the sac fluid is crucial.Therefore a 12-mm noncutting trocar is gently placed with the tightly surrounding liver capsule.Further as a safety measure to prevent contamination of the abdomen the trocar surrounding area was protected by two layers of towels.Lower layer of towel was soaked with normal saline to protect the patients tissue from the upper layer of towels soaked with 20%CE-toxic saline solution.
The results of this novel parenchymal sparing surgical technique showed a limited risk for bleeding with an intact host-derived posterior cyst wall.Thus no complications, i.e.bleeding, postoperative biloma or fluid collection, occurred.There is no evidence for disadvantages so far during the whole length of the follow-up period.
In contrast to this novel parenchymal sparing surgical treatment, total cystectomy is associated with a high risk of surgical complications and may even include a significant loss of parenchyma of the liver with associated prolonged hospital stay,morbidity of up to 60.0% and even mortality of up to 7.8% [10-12].The experiences of different surgical techniques (unroofing procedure, hepatectomy, peri-cystectomy) within one centre showed complications like fever, surgical site infection, bile leak and anaphylaxis in up to 46% [13].Recurrence occurred in up to 18% of the patients, in another study [14]in up to 40% in cases of open surgery.
While the laparoscopic approach is performed in many centers,there is a great potential for intraoperative contamination with recurrence rates up to 11.1% and a higher risk for anaphylaxis [15].Furthermore, laparoscopic surgery is restricted to superficial cysts that can be easily reached minimal invasive and are away from major vessels.Conversion to open laparotomy due to anatomical limitations or inaccessible locations was necessary in 4.92% with an overall mortality of 0.22% and morbidity of 15.07% [15].
While we have not observed any recurrent disease with our method, there is up to 40% recurrence described in the literature with other surgical strategies [13 , 14].
During a median follow-up period of 17 months (range 13-24 months) disease-free survival was achieved with our novel parenchymal sparing surgical approach.However, further follow-up of patients is needed to achieve a 5-year disease-free survival considered as gold standard for cure.
The best treatment for CE of the liver is still controversial.Surgical standard methods (peri-cystectomy, unroofing procedures)have high complication and recurrence rates.Radical surgery(hemihepatectomy) offers lower recurrence rates, but is associated with a higher intraoperative risk [16].
Our novel parenchymal sparing surgical approach is safe, does not require special equipment, and no recurrence occurred within a follow-up period of up to 24 months.Therefore, this surgical approach could be an alternative to radical surgical methods.
Acknowledgments
None.
CRediT authorship contribution statement
Daniela Kniepeiss:Writing - original draft, Data curation.Emina Talakic:Data curation, Writing - review & editing.Peter Schemmer:Supervision, Conceptualization, Writing - review &editing.
Funding
None.
Ethical approval
Not needed.
Competing Interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Endoscopic papillary large balloon dilation with or without sphincterotomy for large bile duct stones removal: Short-term and long-tem outcomes
- Two-in-one: A pooled analysis of primary hepatic neuroendocrine carcinoma combine d/collide d with hepatocellular carcinoma
- A ten-year experience of inferior vena cava reconstruction for malignancy: The importance of a multidisciplinary approach with hepatobiliary surgery
- New variation of median arcuate ligament compression causing hepatic arterial hypoperfusion during liver transplantation
- Serum chitinase-3-like protein 1 is a biomarker of liver fibrosis in patients with chronic hepatitis B in China
- Role of selected criteria and preventive chemotherapy in tumor recurrence after liver transplantation
